Association of High Ratio of CSF/Plasma HIV-1 RNA with Central Nervous System Co-Infection in HIV-1-Positive Treatment-Naive Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. Laboratory Assessments
2.4. CNS Co-Infection Assessments
- Tuberculous meningitis: (i) The CSF with lymphocytic pleocytosis, low glucose, and elevated protein; (ii) the brain CT/MRI showing enhancement of the meninges and the periphery with the tuberculoma lesions; (iii) the culture or CSF smear or CSF-PCR; (iv) the successful response by a specific treatment. The diagnosis complied with (iii) or any two of the other three criteria.
- Toxoplasma meningitis should have been consistent at the same time: (i) progressive neurological deficits; (ii) contrast-enhancing mass lesion(s) on the CT/MRI; (iii) successful response within 2 weeks of the specific treatment.
- Cryptococcal meningitis needed to meet any one of these criteria: (i) visualizing the fungus in the CSF using India ink; (ii) detecting the cryptococcal antigen by the latex agglutination assay in the CSF; (iii) positive CSF culture for C. neoformans.
- Diagnosis of neurosyphilis should have been consistent at the same time: (i) epidemiological history; (ii) clinical manifestations; (iii) positive serum TPPA; (iv) CSF leukocyte count of ≥20 × 106/L; CSF protein of ≥500 mg/L; (v) CSF TPPA positive.
- Viral encephalitis included CMV (cytomegalovirus) and JC virus (JCV). Diagnosis of CMV encephalitis via clinical appearance, positive PCR in CSF, and other pathology was excluded. Diagnosis of Progressive Multifocal Leukoencephalopathy, which is caused by JCV, depends on the evidence of JCV-deoxyribonucleic acid (JCV-DNA) in CSF and compatible clinical-radiological picture.
- Multiple co-infections referred to the combination of more than two types of co-infection
- Detection of co-infectious pathogens mentioned in our manuscript by Next-Generation Sequencing (NGS) testing.
2.5. Statistical Methods
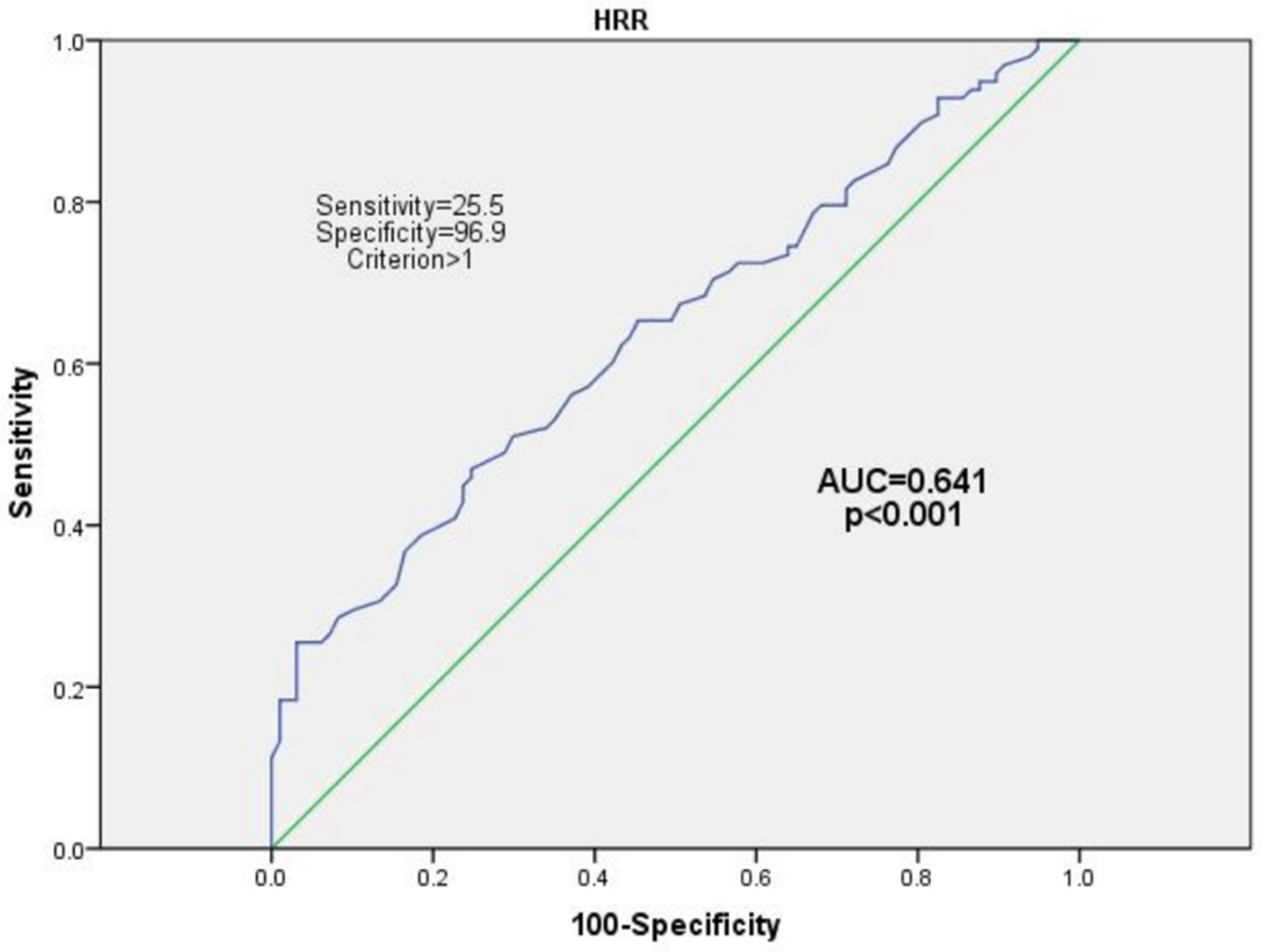
3. Results
Multivariate Analyses between the HRR and CNS Co-Infection
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ellis, R.J.; Gamst, A.C.; Capparelli, E.; Spector, S.A.; Hsia, K.; Wolfson, T.; Abramson, I.; Grant, I.; McCutchan, J.A. Cerebrospinal fluid HIV RNA originates from both local CNS and systemic sources. Neurology 2000, 54, 927–936. [Google Scholar] [CrossRef]
- Churchill, M.; Nath, A. Where does HIV hide? A focus on the central nervous system. Curr. Opin. HIV AIDS 2013, 8, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Ash, M.K.; Al-Harthi, L.; Schneider, J.R. HIV in the Brain: Identifying Viral Reservoirs and Addressing the Challenges of an HIV Cure. Vaccines 2021, 9, 867. [Google Scholar] [CrossRef]
- Marra, C.M.; Lockhart, D.; Zunt, J.R.; Perrin, M.; Coombs, R.W.; Collier, A.C. Changes in CSF and plasma HIV-1 RNA and cognition after starting potent antiretroviral therapy. Neurology 2003, 22, 1388–1390. [Google Scholar] [CrossRef]
- Spudich, S.S.; Nilsson, A.C.; Lollo, N.D.; Liegler, T.J.; Petropoulos, C.J.; Deeks, S.G.; Paxinos, E.E.; Price, R.W. Cerebrospinal fluid HIV infection and pleocytosis: Relation to systemic infection and antiretroviral treatment. BMC Infect. Dis. 2005, 5, 98. [Google Scholar] [CrossRef]
- Ronald, J.; Ellis, M.; Hsia, K.; Spector, S.A.; Nelson, J.A.; Heaton, R.K.; Wallace, M.R.; Abrason, I.; Atlinson, J.H.; Grant, I.; et al. Cerebrospinal fluid human immunodeficiency virus type 1 RNA levels are elevated in neurocognitively impaired individuals with acquired immunodeficiency syndrome. Ann. Neurol. 1997, 42, 679–688. [Google Scholar]
- Childs, E.A.; Lyles, R.H.; Selnes, O.A.; Chen, B.; Miller, E.N.; Cohen, B.A.; Becker, J.T.; Mellors, J.; McArthur, J.C. Plasma viral load and CD4 lymphocytes predict HIV-associated dementia and sensory neuropathy. Neurology 1999, 52, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Canestri, A.; Lescure, F.X.; Jaureguiberry, S.; Moulignier, A.; Amiel, C.; Marcelin, A.G.; Peytavin, G.; Tubiana, R.; Pialoux, G.; Katlama, C. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin. Infect. Dis. 2010, 50, 773–778. [Google Scholar] [CrossRef]
- Dravid, A.N.; Natrajan, K.; Kulkarni, M.M.; Saraf, C.K.; Mahajan, U.S.; Kore, S.D.; Rathod, N.M.; Mahajan, U.S.; Wadia, R.S. Discordant CSF/plasma HIV-1 RNA in individuals on virologically suppressive antiretroviral therapy in Western India. Medicine 2018, 97, e9969. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, S.; Geretti, A.M.; Beloukas, A.; Fisher, M.; Winston, A.; Else, L.; Nelson, M.; Taylor, S.; Ustianowski, A.; Ainsworth, J.; et al. Discordant CSF/plasma HIV-1 RNA in patients with unexplained low-level viraemia. J. Neurovirol. 2016, 22, 852–860. [Google Scholar] [CrossRef]
- Soulie, C.; Grudé, M.; Descamps, D.; Amiel, C.; Morand-Joubert, L.; Raymond, S.; Pallier, C.; Bellecave, P.; Reigadas, S.; Trabaud, M.A.; et al. Antiretroviral-treated HIV-1 patients can harbour resistant viruses in CSF despite an undetectable viral load in plasma. J. Antimicrob. Chemother. 2017, 72, 2351–2354. [Google Scholar] [CrossRef] [PubMed]
- Winston, A.; Antinori, A.; Cinque, P.; Fox, H.S.; Gisslen, M.; Henrich, T.J.; Letendre, S.; Persaud, D.; Price, R.W.; Spudich, S. Defining cerebrospinal fluid HIV RNA escape: Editorial review AIDS. AIDS 2019, 33 (Suppl. 2), S107–S111. [Google Scholar] [CrossRef] [PubMed]
- Eden, A.; Fuchs, D.; Hagberg, L.; Nilsson, S.; Spudich, S.; Svennerholm, B.; Price, R.W.; Gisslen, M. HIV-1 viral escape in cerebrospinal fluid of subjects on suppressive antiretroviral treatment. J. Infect. Dis. 2010, 202, 1819–1825. [Google Scholar] [CrossRef]
- Cysique, L.A.; Waters, E.K.; Brew, B.J. Central nervous system antiretroviral efficacy in HIV infection: A qualitative and quantitative review and implications for future research. BMC Neurol. 2011, 11, 148. [Google Scholar] [CrossRef] [PubMed]
- Bavaro, D.F.; Calamo, A.; Lepore, L.; Fabrizio, C.; Saracino, A.; Angarano, G.; Monno, L. Cerebrospinal fluid compartmentalization of HIV-1 and correlation with plasma viral load and blood-brain barrier damage. Infection 2019, 47, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Decloedt, E.H.; Rosenkranz, B.; Maartens, G.; Joska, J. Central nervous system penetration of antiretroviral drugs: Pharmacokinetic, pharmacodynamic and pharmacogenomic considerations. Clin. Pharmacokinet. 2015, 54, 581–598. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, R.J.; Rohlwink, U.; Misra, U.K.; van Crevel, R.; Mai, N.T.H.; Dooley, K.E.; Caws, M.; Figaji, A.; Savic, R.; Solomons, R.; et al. Tuberculous meningitis. Nat. Rev. Neurol. 2017, 13, 581–598. [Google Scholar] [CrossRef] [PubMed]
- Howlett, W.P. Neurological disorders in HIV in Africa: A review. Afr. Health Sci. 2019, 19, 1953–1977. [Google Scholar] [CrossRef]
- Muccini, C.; Crowell, T.A.; Kroon, E.; Sacdalan, C.; Ramautarsing, R.; Seekaew, P.; Phanuphak, P.; Ananworanich, J.; Colby, D.J.; Phanuphak, N. Leveraging early HIV diagnosis and treatment in Thailand to conduct HIV cure research. AIDS Res. Ther. 2019, 16, 25. [Google Scholar] [CrossRef]
- Robertson, E.J.; Najjuka, G.; Rolfes, M.A.; Akampurira, A.; Jain, N.; Anantharanjit, J.; von Hohenberg, M.; Tassieri, M.; Carlsson, A.; Meya, D.B.; et al. Cryptococcus neoformans ex vivo capsule size is associated with intracranial pressure and host immune response in HIV-associated cryptococcal meningitis. J. Infect. Dis. 2014, 209, 74–82. [Google Scholar] [CrossRef]
- Chang, C.C.; Kangethe, R.; Omarjee, S.; Hiramen, K.; Gosnell, B.; Sojane, K.; Moosa, M.S.; Lewin, S.R.; French, M.A.; Ndung’u, T. Relationship of Human Immunodeficiency Virus Viral Load in Cerebrospinal Fluid and Plasma in Patients Co-infected with Cryptococcal Meningitis. Open Forum. Infect. Dis. 2017, 4, ofx032. [Google Scholar] [CrossRef] [PubMed]
- Eggers, C.; Arendt, G.; Hahn, K.; Husstedt, I.W.; Maschke, M.; Neuen-Jacob, E.; Obermann, M.; Rosenkranz, T.; Schielke, E.; Straube, E. HIV-1-associated neurocognitive disorder: Epidemiology, pathogenesis, diagnosis, and treatment. J. Neurol. 2017, 264, 1715–1727. [Google Scholar] [CrossRef] [PubMed]
- Merlini, E.; Iannuzzi, F.; Calcagno, A.; Bai, F.; Trunfio, M.; d’Arminio Monforte, A.; Bonora, S.; Giulia, M. Peripheral and cerebrospinal fluid immune activation and inflammation in chronically HIV-infected patients before and after virally suppressive combination antiretroviral therapy (cART). J. NeuroVirol. 2018, 24, 679–694. [Google Scholar] [CrossRef] [PubMed]
- EACS European. European AIDS Clinical Society (EACS) GUIDELINES Version 10.0 November 2020; EACS European: Warsaw, Poland, 2020. [Google Scholar]
- Portegies, P.; Solod, L.; Cinque, P.; Chaudhuri, A.; Begovac, J.; Everall, I.; Weber, T.; Bojar, M.; Martinez-Martin, P.; Kennedy, P.G.E. Guidelines for the diagnosis and management of neurological complications of HIV infection. Eur. J. Neurol. 2004, 4, 297–304. [Google Scholar] [CrossRef]
- Pozniak, A.L.; Coyne, K.M.; Miller, R.F.; Lipman, M.C.I.; Freedman, A.R.; Ormerod, L.P.; Johnson, M.A.; Collins, S.; Lucas, S.B.; on behalf of the BHIVA Guidelines Subcommittee. British HIV Association guidelines for the treatment of TB/HIV coco-infection 2011. HIV Med. 2011, 12, 517–524. [Google Scholar] [CrossRef]
- Beguelin, C.; Vazquez, M.; Bertschi, M.; Yerly, S.; de Jong, D.; Rauch, A.; Cusini, A. Viral escape in the CNS with multidrug-resistant HIV-1. J. Int. AIDS Soc. 2014, 17, 19745. [Google Scholar] [CrossRef]
- Rawson, T.; Muir, D.; Mackie, N.E.; Garvey, L.J.; Everitt, A.; Winston, A. Factors associated with cerebrospinal fluid HIV RNA in HIV infected subjects undergoing lumbar puncture examination in a clinical setting. J. Infect. 2012, 65, 239–245. [Google Scholar] [CrossRef]
- Antinori, A.; Perno, C.F.; Giancola, M.L.; Forbici, F.; Ippolito, G.; Hoetelmans, R.M.; Piscitelli, S.C. Efficacy of Cerebrospinal Fluid (CSF)–Penetrating Antiretroviral Drugs against HIV in the Neurological Compartment: Different Patterns of Phenotypic Resistance in CSF and Plasma. HIV/AIDS 2005, 41, 1787. [Google Scholar] [CrossRef][Green Version]
- Margolis, A.M.; Heverling, H.; Pham, P.A.; Stolbach, A. A review of the toxicity of HIV medications. J. Med. Toxicol. 2014, 10, 26–39. [Google Scholar] [CrossRef]
- Anderson, A.M.; Munoz-Moreno, J.A.; McClernon, D.R.; Ellis, R.J.; Cookson, D.; Clifford, D.B.; Collier, A.C.; Gelman, B.B.; Marra, C.M.; McArthur, J.C.; et al. Prevalence and Correlates of Persistent HIV-1 RNA in Cerebrospinal Fluid During Antiretroviral Therapy. J. Infect. Dis. 2017, 215, 105–113. [Google Scholar] [CrossRef]
- Calcagno, A.; Romito, A.; Atzori, C.; Ghisetti, V.; Cardellino, C.; Audagnotto, S.; Scarvaglieri, E.; Lipani, F.; Imperiale, D.; Di Perri, G.; et al. Blood Brain Barrier Impairment in HIV-Positive Naive and Effectively Treated Patients: Immune Activation Versus Astrocytosis. J. Neuroimmune Pharmacol. 2017, 12, 187–193. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, S.M.; Rotta, I.; de Pereira, A.P.; Tang, B.; Umlauf, A.; Ribeiro, C.E.L.; Letendre, S.; Ellis, R.J. Neurobehavioral Research Center Group.; Cerebrospinal fluid pleocytosis as a predictive factor for CSF and plasma HIV RNA discordance and escape. J. Neurovirol. 2020, 26, 241–251. [Google Scholar] [CrossRef] [PubMed]
- De Luca, A.; Ciancio, B.C.; Larussa, D.; Murri, R.; Cingolani, A.; Rizzo, M.G.; Giancola, M.L.; Ammassari, A.; Ortona, L. Correlates of independent HIV-1 replication in the CNS and of its control by antiretrovirals. Neurology 2002, 59, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Letendre, S.L.; Brande van den, G.; Hermes, A.; Woods Paul, S.; Durelle, J.; Beck, J.M.; McCutchan, J.A.; Okamoto, C.; Ellis, R.J. Lopinavir with Ritonavir Reduces the HIV RNA Level in Cerebrospinal Fluid. Clin. Infect. Dis. 2007, 45, 1511–1517. [Google Scholar] [CrossRef]
- Letendre, S.; Marquie-Beck, J.; Capparelli, E.; Best, B.; Clifford, D.; Collier, A.C.; Gelman, B.B.; McArthur, J.C.; McCutchan, J.A.; Morgello, S.; et al. Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch. Neurol. 2008, 65, 65–70. [Google Scholar] [CrossRef]
- Ferretti, F.; De Zan, V.; Gerevini, S.; Turrini, F.; Boeri, E.; Gianotti, N.; Hasson, H.; Lazzarin, A.; Cinque, P. Relapse of Symptomatic Cerebrospinal Fluid HIV Escape. Curr. HIV/AIDS Rep. 2020, 17, 522–528. [Google Scholar] [CrossRef]
- Coughlan, R.; Cameron, S. Key data from the 17th International Workshop on Co-morbidities and Adverse Drug Reactions in HIV. Antivir. Ther. 2016, 21, 75–89. [Google Scholar] [CrossRef]
- Saag, M.S.; Gandhi, R.T.; Hoy, J.F.; Landovitz, R.J.; Thompson, M.A.; Sax, P.E.; Smith, D.M.; Benson, C.A.; Buchbinder, S.P.; Del Rio, C.; et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2020 recommendations of the International Antiviral Society–USA panel. JAMA 2020, 324, 1651–1669. [Google Scholar] [CrossRef]

| CNI Group (N = 98) | Non-CNI Group (N = 97) | p-Value | |
|---|---|---|---|
| Age (mean ± SD) | 46.74 ± 12.65 | 49.74 ± 16.15 | 0.239 |
| Male gender, n (%) | 76(77.55) | 75(77.32) | 0.969 |
| Disease characteristics | |||
| CD 4 (cell per µL), median (IQR) | 20 (39–72) | 41 (19–106) | 0.365 |
| Ratio of CD4/CD8, median (IQR) | 0.08 (0.13–0.20) | 0.145 (0.08–0.24) | 0.326 |
| Serum albumin (mean ± SD) | 35.63 ± 6.84 | 33.97 ± 6.38 | 0.072 |
| Plasma HIV RNA (Log copies/mL), median (IQR) | 5.86 (5.50–6.27) | 5.69 (5.19–6.19) | 0.158 |
| CSF HIV RNA (log copies/mL), median (IQR) | 4.78 (3.67–5.55) | 3.98 (3.10–4.65) | <0.001 |
| CSF/plasma HIV RNA ratio, (mean ± SD) | 0.82 ± 0.22 | 0.71 ± 0.19 | <0.001 |
| CSF protein (mg/L), median (IQR) | 594.86 (362.96–978.64) | 359.01 (310.93–451.22) | <0.001 |
| CSF chloride (mmol/L), mean ± SD | 121.0 ± 8.75 | 124.42 ± 4.36 | 0.001 |
| CSF glucose, (mmol/L), median (IQR) | 3.19 (2.44–3.60) | 3.66 (3.23–4.33) | <0.001 |
| CSF white blood cells (cell per µL), median (IQR) | 5 (0–84) | 1 (0–4) | <0.001 |
| Variables | CNS Co-Infection (N = 98) | TBM (N = 30) | CM (N = 18) | TM (N = 11) | VE (N = 14) | NS (N = 6) | MI (N = 19) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |
| CSF/plasma HRR | ||||||||||||||
| <1.00 | ||||||||||||||
| ≥1.00 | 5.19 (2.02–13.33) | 0.001 | 6.50 (2.08–20.25) | 0.001 | 7.58 (2.10–27.32) | 0.002 | 3.37 (0.59–19.21) | 0.171 | 4.13 (0.90–18.92) | 0.067 | 3.03 (0.30–30.27) | 0.344 | 4.04 (1.02–16.04) | 0.047 |
| CSF/plasma HRR | 13.78 (3.27–58.13) | <0.001 | 8.92 (1.11–71.19) | 0.039 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Age | 0.98 (0.96–1.00) | 0.153 | 0.98 (0.96–1.01) | 0.348 | 0.98 (0.95–1.02) | 0.478 | 0.99 (0.95–1.03) | 0.785 | 0.99 (0.96–1.03) | 0.855 | 0.94 (0.88–1.00) | 0.073 | 0.99 (0.95–1.02) | 0.532 |
| Male | 0.98 (0.50–1.93) | 0.969 | 1.24 (0.48–3.16) | 0.654 | 1.31 (0.42–4.08) | 0.640 | 1.27 (0.31–5.23) | 0.733 | 0.93 (0.23–3.63) | 0.917 | NA | NA | 0.63 (0.17–2.39) | 0.507 |
| CD 4 count (log 10 cell per µL) | 0.71 (0.39–1.28) | 0.262 | 0.79 (0.35–1.77) | 0.572 | 0.47 (0.17–1.36) | 0.165 | 0.76 (0.21–2.78) | 0.683 | 1.21 (0.39–3.76) | 0.738 | 1.89 (0.32–10.85) | 0.475 | 0.44 (0.15–1.28) | 0.133 |
| Ratio of CD4/CD8 | 0.25 (0.03–1.79) | 0.170 | 0.30 (0.01–5.32) | 0.418 | 0.00 (0.00–0.98) | 0.050 | 0.03 (0.00–9.13) | 0.234 | 1.12 (0.03–35.72) | 0.947 | NA | NA | 0.89 (0.03–20.61) | 0.946 |
| Serum albumin | 1.03 (0.99–1.08) | 0.087 | 1.02 (0.95–1.08) | 0.550 | 1.04 (0.97–1.11) | 0.183 | 1.04 (0.94–1.15) | 0.389 | 1.04 (0.96–1.14) | 0.281 | 1.06 (0.93–1.21) | 0.324 | 1.04 (0.96–1.13) | 0.304 |
| Plasma HIV RNA (Log copies/mL) | 1.25 (0.90–1.73) | 0.167 | 1.25 (0.78–1.99) | 0.346 | 1.79 (0.88–3.64) | 0.103 | 1.225 (0.63–2.39) | 0.551 | 0.87 (0.48–1.58) | 0.666 | 0.65 (0.30–1.42) | 0.284 | 1.89 (0.93–3.84) | 0.076 |
| CSF HIV RNA concentration (Log copies/mL) | 1.71 (1.32–2.20) | <0.001 | 1.59 (1.11–2.29) | 0.011 | 2.92 (1.67–5.10) | <0.001 | 1.55 (0.84–2.87) | 0.158 | 1.34 (0.79–2.26) | 0.266 | 1.34 (0.61–2.92) | 0.457 | 2.27 (1.36–3.79) | 0.002 |
| CSF protein (mg/L) | 1.003 (1.002–1.004) | <0.001 | 1.003 (1.001–1.004) | <0.001 | 1.002 (1.001–1.004) | 0.006 | 1.002 (1.002–1.004) | 0.014 | 1.002 (1.000–1.004) | 0.027 | 1.003 (1.000–1.005) | 0.021 | 1.003 (1.001–1.004) | 0.001 |
| CSF chloride(mmol/L) | 0.92 (0.88–0.97) | <0.001 | 0.91 (0.84–0.97) | 0.011 | 0.86 (0.78–0.96) | 0.009 | 0.96 (0.83–1.10) | 0.574 | 0.93 (0.82–1.05) | 0.271 | 0.96 (0.79–1.17) | 0.744 | 0.86 (0.79–0.94) | 0.001 |
| CSF glucose(mmol/L) | 0.62 (0.47–0.82) | 0.001 | 0.59 (0.38–0.89) | 0.013 | 0.31 (0.16–0.61) | 0.001 | 0.29 (0.10–0.87) | 0.027 | 0.54 (0.25–1.17) | 0.122 | 0.18 (0.04–0.66) | 0.010 | 0.74 (0.45–1.22) | 0.249 |
| CSF white blood cells (cell per µL) | 1.05 (1.01–1.09) | 0.005 | 1.05 (0.99–1.10) | 0.061 | 1.10 (1.03–1.18) | 0.005 | 1.08 (0.99–1.17) | 0.053 | 1.04 (1.00–1.08) | 0.035 | 1.05 (0.99–1.11) | 0.069 | 1.07 (1.00–1.14) | 0.039 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Tao, W.; Yang, H.; Wu, Y.; Yu, Q.; Liu, M. Association of High Ratio of CSF/Plasma HIV-1 RNA with Central Nervous System Co-Infection in HIV-1-Positive Treatment-Naive Patients. Brain Sci. 2022, 12, 791. https://doi.org/10.3390/brainsci12060791
Liu Q, Tao W, Yang H, Wu Y, Yu Q, Liu M. Association of High Ratio of CSF/Plasma HIV-1 RNA with Central Nervous System Co-Infection in HIV-1-Positive Treatment-Naive Patients. Brain Sciences. 2022; 12(6):791. https://doi.org/10.3390/brainsci12060791
Chicago/Turabian StyleLiu, Qian, Wendan Tao, Honghong Yang, Yushan Wu, Qing Yu, and Min Liu. 2022. "Association of High Ratio of CSF/Plasma HIV-1 RNA with Central Nervous System Co-Infection in HIV-1-Positive Treatment-Naive Patients" Brain Sciences 12, no. 6: 791. https://doi.org/10.3390/brainsci12060791
APA StyleLiu, Q., Tao, W., Yang, H., Wu, Y., Yu, Q., & Liu, M. (2022). Association of High Ratio of CSF/Plasma HIV-1 RNA with Central Nervous System Co-Infection in HIV-1-Positive Treatment-Naive Patients. Brain Sciences, 12(6), 791. https://doi.org/10.3390/brainsci12060791






