Flavonoid Derivatives as Potential Cholinesterase Inhibitors in Scopolamine-Induced Amnesic Mice: An In Vitro, In Vivo and Integrated Computational Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Equipment
2.2. In-Vitro Anticholinesterase Activity
2.3. Receptor Preparation
2.4. Re-Docking Setup and Ligand Preparation
2.5. Docking of Acetyl and Butyryl Cholinesterase Inhibitors
2.6. Dynamics Understanding and Binding Free Energies of Systems
2.7. Animals
2.8. Acute Toxicity Study
2.9. Experimental Design for Anti-Amnesic Activity in Scopolamine-Induced Amnesic Model
2.10. Assessment of Cognitive Function
2.10.1. Y-Maze Paradigm
2.10.2. NOD Task
2.11. Measurement of Antioxidant Enzyme Activities and Oxidative Stress Markers
2.12. Statistical Analysis
3. Results
3.1. In Vitro Anticholinesterase Activity
3.2. Validation of Docking Protocol
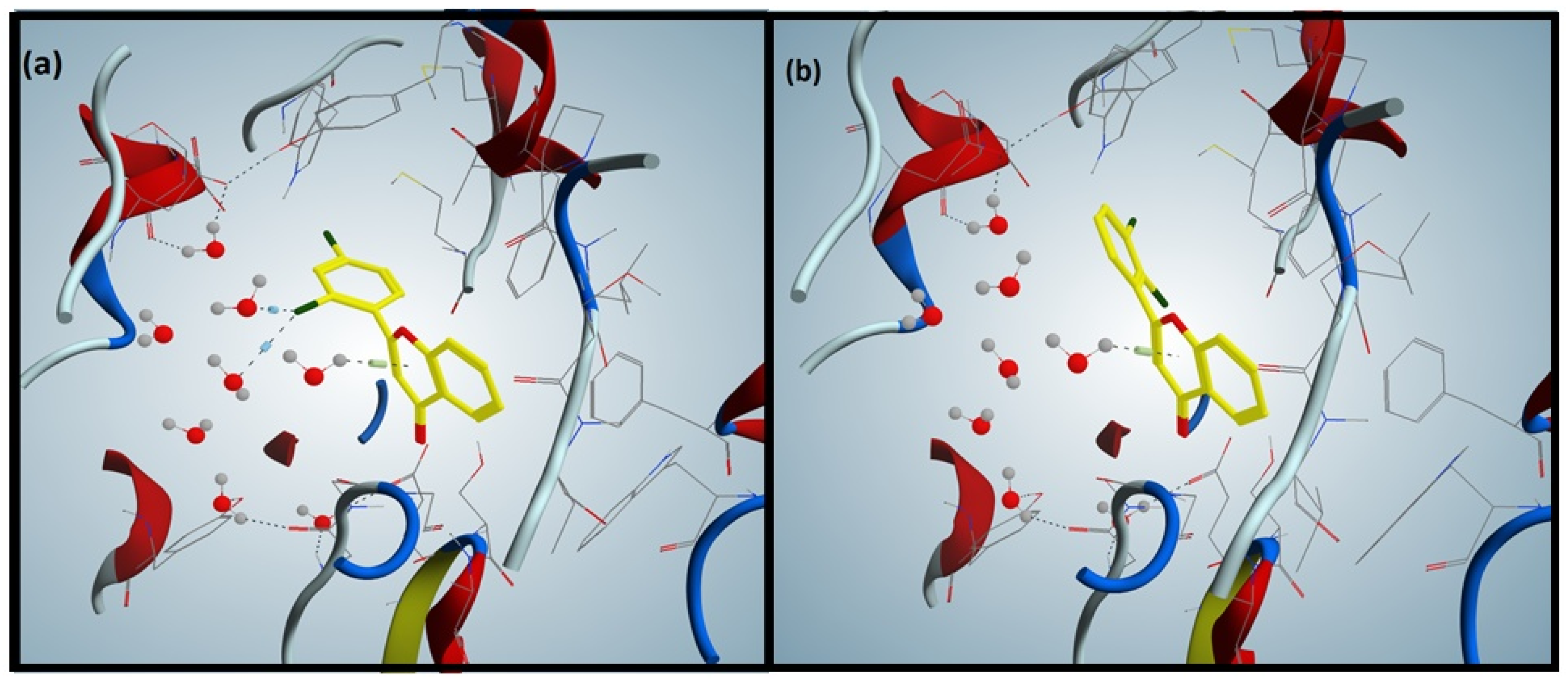
3.3. Docking of Flavones against Acetylcholinesterase
3.4. Docking of Flavones against Butyrylcholinesterase
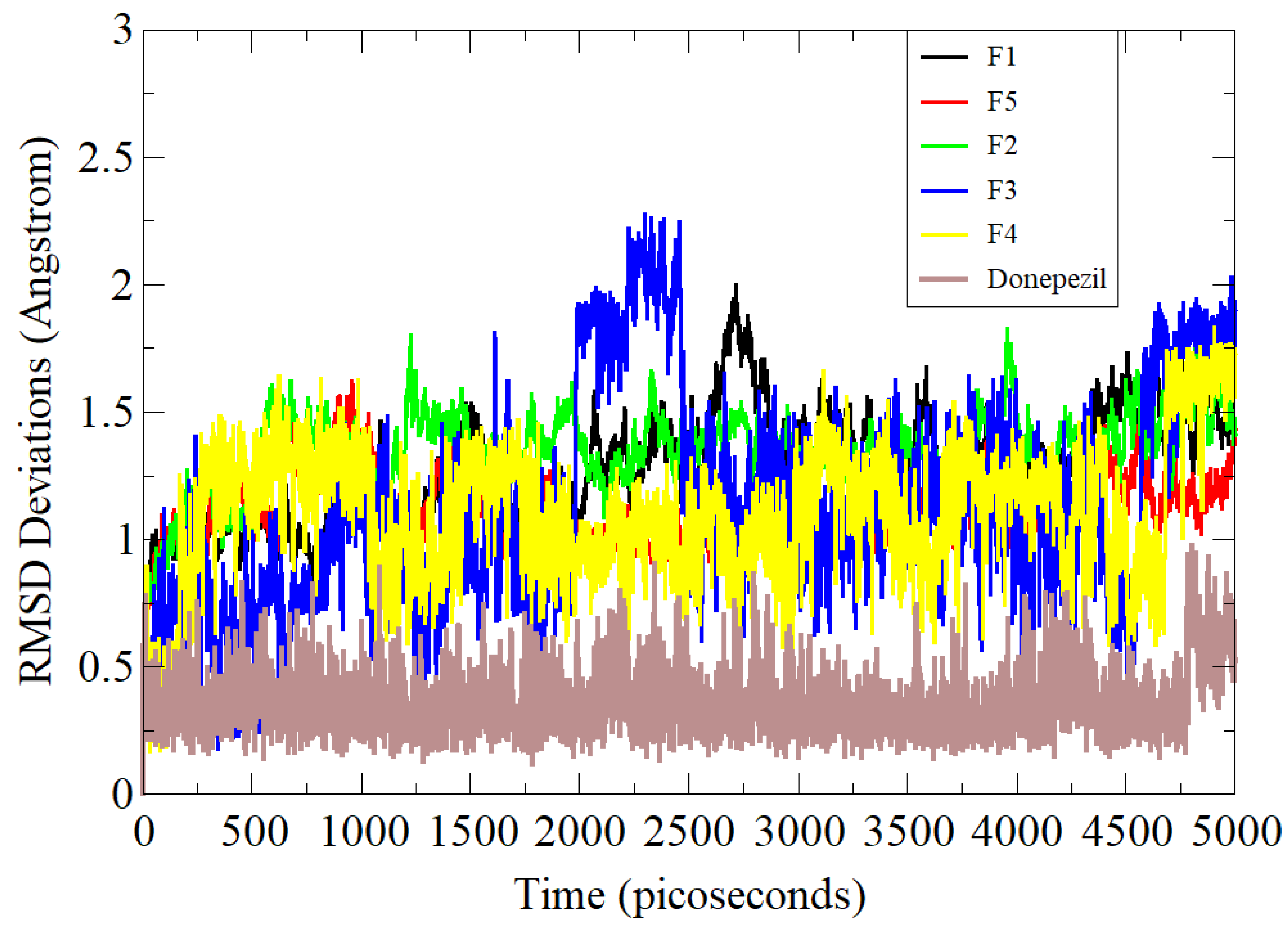
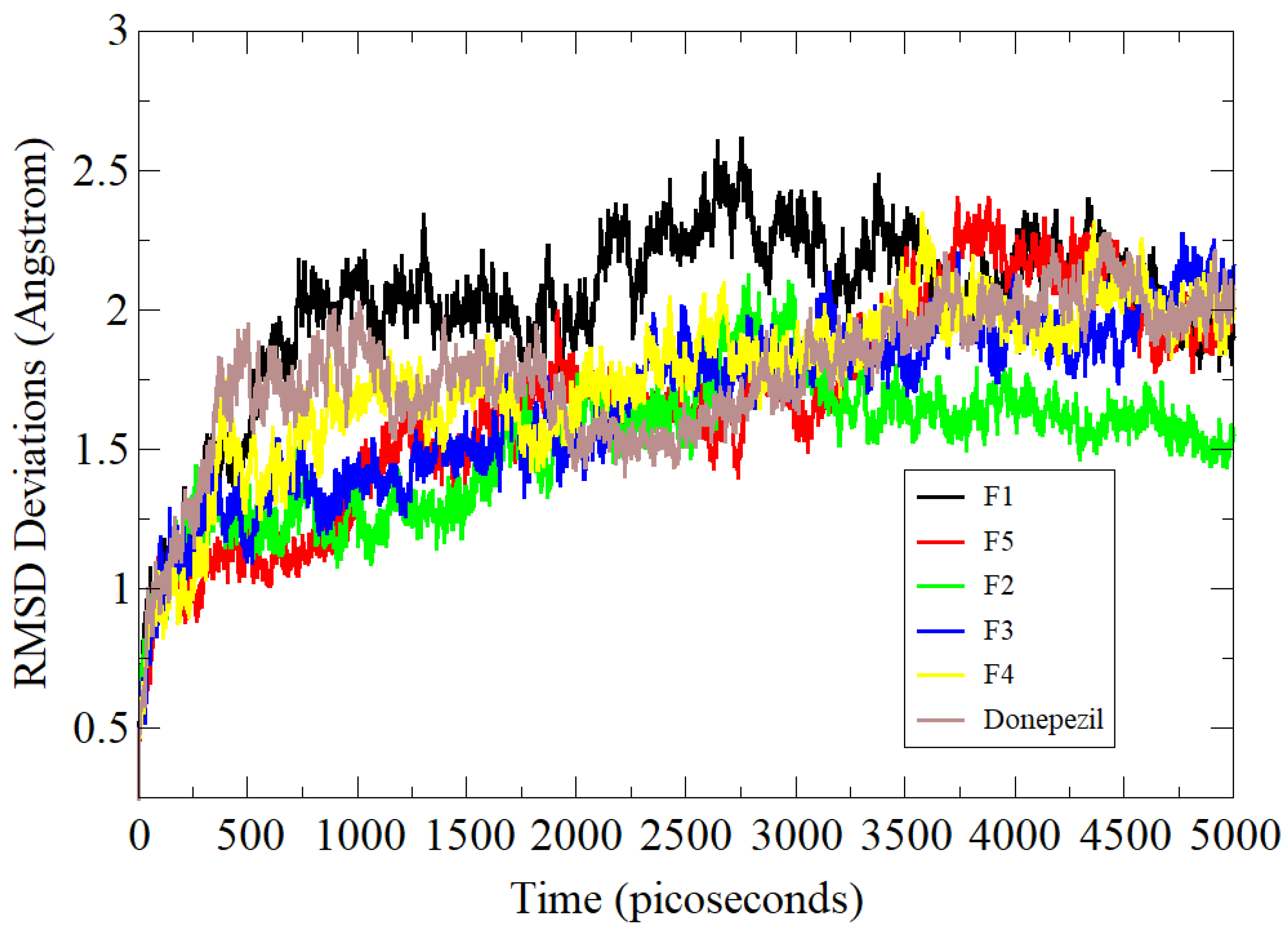
3.5. Molecular Dynamics Simulation Assay
3.6. Estimation of Binding Free Energies
3.7. Acute Toxicity
3.8. Evaluation of Learning Behaviors
3.9. Y-Maze Spontaneous Alternation
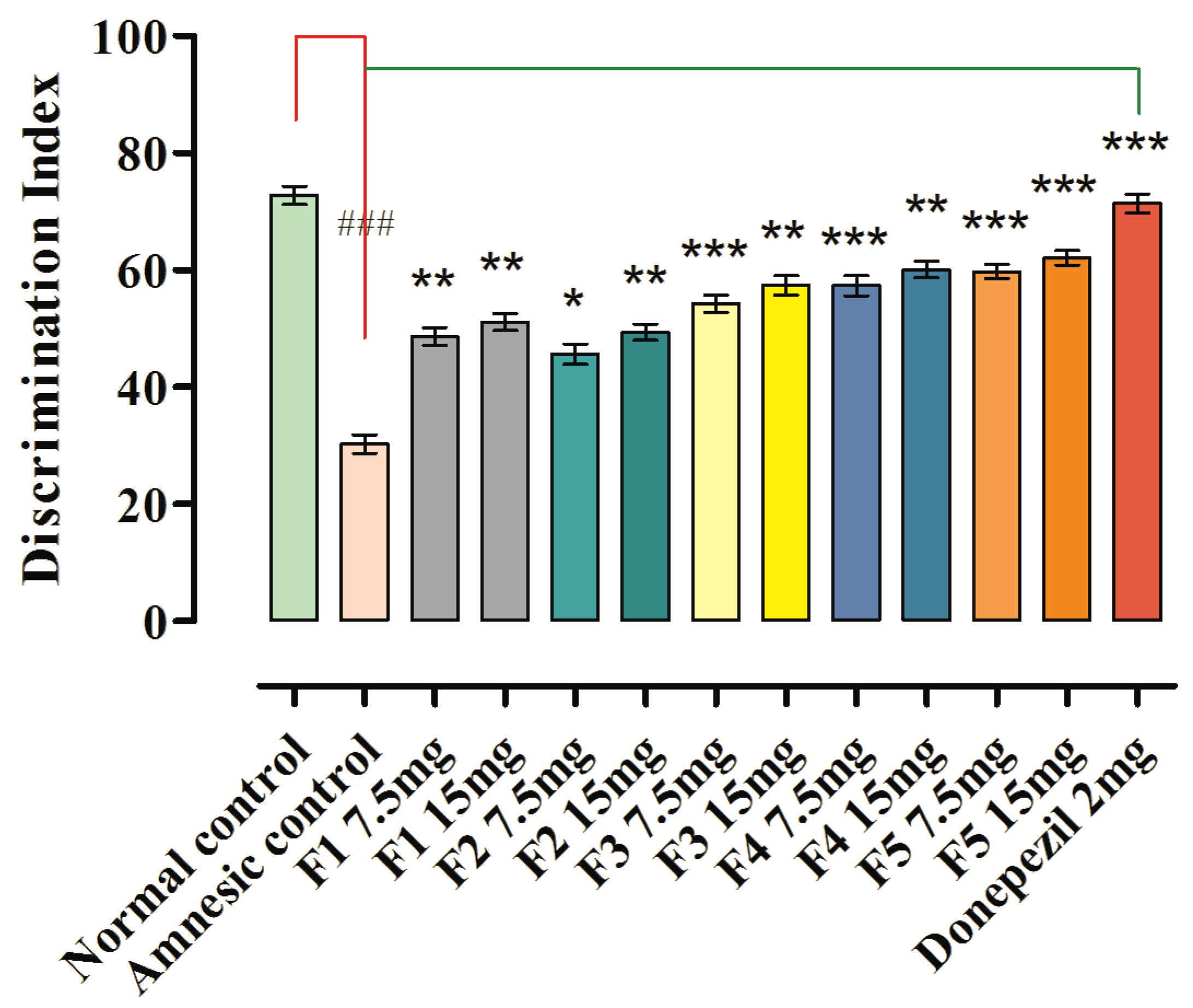
3.10. Novel Object Discrimination Task
3.11. Assessment of Antioxidant Enzyme Activities on Oxidative Stress
3.12. Effect on AChE and ACh Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Bartus, R.T.; Dean, R.L.; Pontecorvo, M.J.; Flicker, C. The Cholinergic Hypothesis: A Historical Overview, Current Perspective, and Future Directions. Ann. N. Y. Acad. Sci. 1985, 444, 332–358. [Google Scholar] [CrossRef] [PubMed]
- Bartus, R.T.; Dean, R.L.; Beer, B.; Lippa, A.S. The cholinergic hypothesis of geriatric memory dysfunction. Science 1982, 217, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Klinkenberg, I.; Blokland, A. The validity of scopolamine as a pharmacological model for cognitive impairment: A review of animal behavioral studies. Neurosci. Biobehav. Rev. 2010, 34, 1307–1350. [Google Scholar] [CrossRef] [PubMed]
- DeKosky, D.S.T.; Harbaugh, R.E.; Schmitt, F.A.; Bakay, R.A.E.; Chui, H.C.; Knopman, D.S.; Reeder, T.M.; Shetter, A.G.; Senter, H.J.; Markesbery, W.R.; et al. Cortical biopsy in Alzheimer’s disease: Diagnostic accuracy and neurochemical, neuropathological, and cognitive correlations. Ann. Neurol. 1992, 32, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Sims, N.R.; Bowen, D.M.; Allen, S.J.; Smith, C.C.T.; Neary, D.; Thomas, D.J.; Davison, A.N. Presynaptic Cholinergic Dysfunction in Patients with Dementia. J. Neurochem. 1983, 40, 503–509. [Google Scholar] [CrossRef]
- Greig, N.H.; Utsuki, T.; Ingram, D.K.; Wang, Y.; Pepeu, G.; Scali, C.; Yu, Q.-S.; Mamczarz, J.; Holloway, H.W.; Giordano, T.; et al. Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer β-amyloid peptide in rodent. Proc. Natl. Acad. Sci. USA 2005, 102, 17213–17218. [Google Scholar] [CrossRef] [Green Version]
- Owokotomo, I.A.; Ekundayo, O.; Abayomi, T.G.; Chukwuka, A.V. In-vitro anti-cholinesterase activity of essential oil from four tropical medicinal plants. Toxicol. Rep. 2015, 2, 850–857. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, A.; Boga, M.; Topçu, G. Novel terpenoids with potential anti-alzheimer activity from Nepeta obtusicrena. Rec. Nat. Prod. 2016, 10, 530. [Google Scholar]
- Shoaib, M.; Shah, A.; Wadood, S.; Ali, N.; Shah, I.; Naveed Umar, M.; Ayaz, M.; Tahir, M.N.; Akhtar, S. In vitro enzyme inhibition potentials and antioxidant activity of synthetic flavone derivatives. J. Chem. 2015, 2015, 516878. [Google Scholar] [CrossRef] [Green Version]
- Shoaib, M.; Ali Shah, S.W.; Shah, S.; Khan, N.; Naeem Ahmed, M. Synthesis, characterization and antinociceptive activities of Novel 2-(2, 4-dichlorophenyl)-4H-chromen-4-one. Pak. J. Pharm. Sci. 2017, 30, 2085–2089. [Google Scholar]
- Shoaib, M.; Shah, S.; Ali, N.; Shah, I.; Umar, M.; Shafiullah; Tahir, M.; Ghias, M. Synthetic flavone derivatives. An antibacterial evaluation and structure-activity relationship study. Bulg. Chem. Commun. 2016, 48, 414–421. [Google Scholar]
- Shoaib, M.; Shah, I.; Ali, N.; Shah, S.W.A. In vitro acetylcholinesterase and butyrylcholinesterase inhibitory potentials of essential oil of Artemisia macrocephala. Bangladesh J. Pharmacol. 2015, 10, 87–91. [Google Scholar] [CrossRef] [Green Version]
- Zaheer-ul-haq; Wellenzohn, B.; Liedl, K.R.; Rode, B.M. Molecular Docking Studies of Natural Cholinesterase-Inhibiting Steroidal Alkaloids from Sarcococca s aligna. J. Med. Chem. 2003, 46, 5087–5090. [Google Scholar] [CrossRef] [PubMed]
- Case, D.; Cerutti, D.; Cheateham, T.; Darden, T.; Duke, R.; Giese, T.; Gohlke, H.; Goetz, A.; Greene, D.; Homeyer, N.; et al. AMBER16 Package; University of California: San Francisco, CA, USA, 2016; Available online: https://ambermd.org (accessed on 20 April 2022).
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef] [PubMed]
- Kräutler, V.; Van Gunsteren, W.F.; Hünenberger, P.H. A fast SHAKE algorithm to solve distance constraint equations for small molecules in molecular dynamics simulations. J. Comput. Chem. 2001, 22, 501–508. [Google Scholar] [CrossRef]
- Miller, B.R., III; McGee, T.D., Jr.; Swails, J.M.; Homeyer, N.; Gohlke, H.; Roitberg, A.E. MMPBSA. py: An efficient program for end-state free energy calculations. J. Chem. Theory Comput. 2012, 8, 3314–3321. [Google Scholar] [CrossRef]
- Lorke, D. A new approach to practical acute toxicity testing. Arch. Toxicol. 1983, 54, 275–287. [Google Scholar] [CrossRef]
- Hussain, H.; Ahmad, S.; Shah, S.W.A.; Ghias, M.; Ullah, A.; Rahman, S.U.; Kamal, Z.; Khan, F.A.; Khan, N.M.; Muhammad, J. Neuroprotective Potential of Synthetic Mono-Carbonyl Curcumin Analogs Assessed by Molecular Docking Studies. Molecules 2021, 26, 7168. [Google Scholar] [CrossRef]
- Choi, J.-H.; Lee, E.-B.; Jang, H.-H.; Cha, Y.-S.; Park, Y.-S.; Lee, S.-H. Allium Hookeri Extracts Improve Scopolamine-Induced Cognitive Impairment via Activation of the Cholinergic System and Anti-neuroinflammation in Mice. Nutrients 2021, 13, 2890. [Google Scholar] [CrossRef]
- Mushtaq, A.; Anwar, R.; Ahmad, M. Lavandula stoechas (L.) a Very Potent Antioxidant Attenuates Dementia in Scopolamine Induced Memory Deficit Mice. Front. Pharmacol. 2018, 9, 1375. [Google Scholar] [CrossRef] [PubMed]
- Karplus, M.; McCammon, J.A. Molecular dynamics simulations of biomolecules. Nat. Struct. Biol. 2002, 9, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Maiorov, V.N.; Crippen, G.M. Significance of root-mean-square deviation in comparing three-dimensional structures of globular proteins. J. Mol. Biol. 1994, 235, 625–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genheden, S.; Ryde, U. The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin. Drug Discov. 2015, 10, 449–461. [Google Scholar] [CrossRef]
- Schroeter, H.; Spencer, J.P.; Rice-Evans, C.; Williams, R.J. Flavonoids protect neurons from oxidized low-density-lipoprotein-induced apoptosis involving c-Jun N-terminal kinase (JNK), c-Jun and caspase-3. Biochem. J. 2001, 358, 547–557. [Google Scholar] [CrossRef]
- Lou, H.; Fan, P.; Perez, R.G.; Lou, H. Neuroprotective effects of linarin through activation of the PI3K/Akt pathway in amyloid-β-induced neuronal cell death. Bioorganic Med. Chem. 2011, 19, 4021–4027. [Google Scholar] [CrossRef]
- Li, R.-S.; Wang, X.-B.; Hu, X.-J.; Kong, L.-Y. Design, synthesis and evaluation of flavonoid derivatives as potential multifunctional acetylcholinesterase inhibitors against Alzheimer’s disease. Bioorganic Med. Chem. Lett. 2013, 23, 2636–2641. [Google Scholar] [CrossRef]
- Kim, H.; Park, B.-S.; Lee, K.-G.; Choi, C.Y.; Jang, S.S.; Kim, Y.-H.; Lee, S.-E. Effects of naturally occurring compounds on fibril formation and oxidative stress of β-amyloid. J. Agric. Food Chem. 2005, 53, 8537–8541. [Google Scholar] [CrossRef]
- Sugihara, N.; Arakawa, T.; Ohnishi, M.; Furuno, K. Anti-and pro-oxidative effects of flavonoids on metal-induced lipid hydroperoxide-dependent lipid peroxidation in cultured hepatocytes loaded with α-linolenic acid. Free. Radic. Biol. Med. 1999, 27, 1313–1323. [Google Scholar] [CrossRef]
- Farias, G.; Cornejo, A.; Jimenez, J.; Guzmán, L.; B Maccioni, R. Mechanisms of tau self-aggregation and neurotoxicity. Curr. Alzheimer Res. 2011, 8, 608–614. [Google Scholar] [CrossRef]
- Guzmán-Martinez, L.; Farías, G.A.; Maccioni, R.B. Tau oligomers as potential targets for Alzheimer’s diagnosis and novel drugs. Front. Neurol. 2013, 4, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meraz Rios, M.A.; Toral-Rios, D.; Franco-Bocanegra, D.; Villeda-Hernández, J.; Campos-Peña, V. Inflammatory process in Alzheimer’s Disease. Front. Integr. Neurosci. 2013, 7, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millington, C.; Sonego, S.; Karunaweera, N.; Rangel, A.; Aldrich-Wright, J.R.; Campbell, I.L.; Gyengesi, E.; Münch, G. Chronic neuroinflammation in Alzheimer’s disease: New perspectives on animal models and promising candidate drugs. BioMed Res. Int. 2014, 2014, 309129. [Google Scholar] [CrossRef] [PubMed]
- Sperling, R.; Mormino, E.; Johnson, K. The evolution of preclinical Alzheimer’s disease: Implications for prevention trials. Neuron 2014, 84, 608–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [Green Version]
- Allgaier, M.; Allgaier, C. An update on drug treatment options of Alzheimer’s disease. Front. Biosci. 2014, 19, 1345–1354. [Google Scholar] [CrossRef] [Green Version]
- Schneider, L.S.; Mangialasche, F.; Andreasen, N.; Feldman, H.; Giacobini, E.; Jones, R.; Mantua, V.; Mecocci, P.; Pani, L.; Winblad, B. Clinical trials and late-stage drug development for A lzheimer’s disease: An appraisal from 1984 to 2014. J. Intern. Med. 2014, 275, 251–283. [Google Scholar] [CrossRef]
- Fernández-Bachiller, M.I.; Pérez, C.N.; Monjas, L.; Rademann, J.R.; Rodríguez-Franco, M.I. New Tacrine–4-Oxo-4 H-chromene hybrids as multifunctional agents for the treatment of alzheimer’s disease, with cholinergic, antioxidant, and β-amyloid-reducing properties. J. Med. Chem. 2012, 55, 1303–1317. [Google Scholar] [CrossRef] [Green Version]
- Ng, Y.P.; Or, T.C.T.; Ip, N.Y. Plant alkaloids as drug leads for Alzheimer’s disease. Neurochem. Int. 2015, 89, 260–270. [Google Scholar] [CrossRef]



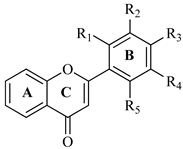 | ||||||
| Flavone | R1 | R2 | R3 | R4 | IC50 (μg/mL) | |
| AChE | BChE | |||||
| F1 | H | H | Cl | H | 165.21 ± 1.53 | 154.71 ± 1.38 |
| F2 | Cl | H | Cl | H | 131.33 ± 1.12 | 105.20 ± 1.43 |
| F3 | Cl | Cl | H | H | 126.29 ± 1.33 | 147.12 ± 1.23 |
| F4 | H | Cl | Cl | H | 112.33 ± 1.16 | 121.77 ± 1.19 |
| F5 | Cl | H | H | Cl | 98.42 ± 0.97 | 109.61 ± 1.11 |
| Donepezil | 4.91 ± 0.51 | 3.98 ± 0.67 | ||||
| Sample Test | AChE IC50 (µg/mL) | Net Binding Energy (Kcal/mol) | BChE IC50 (µg/mL) | Net Binding Energy (Kcal/mol) | ||
|---|---|---|---|---|---|---|
| MMGBSA | MMPBSA | MMGBSA | MMPBSA | |||
| F1 | 165.21 ± 1.53 | −42.31 | −41.15 | 154.71 ± 1.38 | −35.64 | −31.45 |
| F2 | 131.33 ± 1.12 | −45.32 | −43.87 | 105.20 ± 1.43 | −49.74 | −51.42 |
| F3 | 126.29 ± 1.33 | −46.87 | −50.78 | 147.12 ± 1.23 | −43.48 | −38.61 |
| F4 | 112.33 ± 1.16 | −50.99 | −48.65 | 121.77 ± 1.19 | −48.19 | −43.64 |
| F5 | 98.42 ± 0.97 | −49.45 | −53.14 | 109.61 ± 1.11 | −51.88 | −52.78 |
| Donepezil | 4.91 ± 0.51 | −55.91 | −54.51 | 3.98 ± 0.67 | −40.17 | −43.14 |
| Treatment/Dose (mg) | Total Weight (g) | Kidney Weight (g) | Liver Weight (g) | |
|---|---|---|---|---|
| Normal Control | 22.68 ± 1.40 | 0.31 ± 0.13 | 1.22 ± 0.27 | |
| F1 | 600 | 21.82 ± 1.39 | 0.29 ± 0.19 | 1.24 ± 0.21 |
| 1000 | 22.93 ± 1.91 | 0.29 ± 0.18 | 1.23 ± 0.26 | |
| 2000 | 21.79 ± 1.72 | 0.30 ± 0.20 | 1.23 ± 0.24 | |
| 3000 | 22.71 ± 1.79 | 0.31 ± 0.27 | 1.25 ± 0.29 | |
| F5 | 600 | 20.97 ± 1.66 | 0.30 ± 0.21 | 1.24 ± 0.25 |
| 1000 | 21.88 ± 1.89 | 0.31 ± 0.24 | 1.22 ± 0.29 | |
| 2000 | 22.91 ± 1.67 | 0.29 ± 0.20 | 1.26 ± 0.21 | |
| 3000 | 22.65 ± 1.91 | 0.30 ± 0.19 | 1.23 ± 0.25 | |
| Treatment/Dose (mg) | Glucose (mg/dL) | Triglyceride (mg/dL) | AST (U/L) | ALP (U/L) | Creatinine (mg/dL) | Total Cholesterol (mg/dL) | |
|---|---|---|---|---|---|---|---|
| Normal control | 84.72 ± 2.41 | 94.39 ± 2.13 | 107.62 ± 1.87 | 132.98 ± 3.47 | 0.25 ± 0.04 | 99.67 ± 1.79 | |
| F1 | 600 | 85.51 ± 2.33 | 95.20 ± 2.76 | 107.89 ± 2.01 | 132.54 ± 2.78 | 0.36 ± 0.05 | 103.19 ± 2.06 |
| 1000 | 86.98 ± 2.09 | 94.78 ± 1.98 | 108.34 ± 2.66 | 136.33 ± 2.39 | 0.38 ± 0.06 | 102.76 ± 1.87 | |
| 2000 | 86.45 ± 2.27 | 96.22 ± 1.70 | 109.73 ± 1.98 | 135.29 ± 4.17 | 0.34 ± 0.04 | 102.05 ± 2.01 | |
| 3000 | 85.72 ± 1.97 | 97.41 ± 1.83 | 109.90 ± 2.09 | 136.09 ± 3.12 | 0.29 ± 0.03 | 101.28 ± 1.98 | |
| F5 | 600 | 86.98 ± 2.12 | 95.31 ± 2.61 | 108.37 ± 1.97 | 134.41 ± 2.81 | 0.28 ± 0.04 | 102.91 ± 1.93 |
| 1000 | 87.01 ± 2.21 | 96.08 ± 2.01 | 107.41 ± 2.03 | 135.87 ± 2.24 | 0.35 ± 0.05 | 103.02 ± 1.79 | |
| 2000 | 86.89 ± 1.98 | 95.37 ± 1.98 | 108.39 ± 1.89 | 133.91 ± 3.71 | 0.33 ± 0.06 | 102.73 ± 2.10 | |
| 3000 | 85.34 ± 2.01 | 96.50 ± 2.05 | 107.76 ± 1.92 | 134.02 ± 3.28 | 0.30 ± 0.04 | 103.09 ± 1.83 | |
| Treatment/Dose (mg) | Spontaneous Alternation Performance | |
|---|---|---|
| Normal control | 82.90 ± 1.67 | |
| Amnesic control (Scopolamine) | 37.89 ± 1.61 ### | |
| F1 | 7.5 | 49.90 ± 1.33 * |
| 15 | 51.78 ± 1.45 ** | |
| F2 | 7.5 | 47.13 ± 1.65 * |
| 15 | 49.88 ± 1.28 * | |
| F3 | 7.5 | 59.61 ± 1.61 ** |
| 15 | 65.78 ± 1.57 *** | |
| F4 | 7.5 | 57.92 ± 1.41 ** |
| 15 | 66.70 ± 1.63 *** | |
| F5 | 7.5 | 67.94 ± 1.51 *** |
| 15 | 73.11 ± 1.63 *** | |
| Donepezil | 2 | 80.72 ± 1.59 *** |
| Sample Test (mg) | SOD (U/mg of Protein) | CAT (U/mg of Protein) | MDA (nmol/mg Protein) | GSH (μg/mg of Protein) | |
|---|---|---|---|---|---|
| Control | 14.11 ± 1.08 | 32.21 ± 1.37 | 8.99 ± 1.01 | 45.87 ± 1.41 | |
| Amnesic control | 5.18 ± 0.91 ### | 7.08 ± 1.13 ### | 26.60 ± 1.41 ### | 15.06 ± 1.37 ### | |
| F1 | 7.5 | 8.97 ± 1.12 ** | 21.93 ± 1.37 ** | 16.39 ± 1.28 ** | 34.62 ± 1.50 ** |
| 15 | 9.12 ± 1.28 ** | 22.31 ± 1.22 ** | 16.12 ± 1.47 *** | 35.70 ± 1.47 ** | |
| F2 | 7.5 | 9.25 ± 1.36 ** | 22.83 ± 1.19 *** | 15.16 ± 1.21 ** | 34.93 ± 1.22 ** |
| 15 | 9.33 ± 1.45 ** | 23.71 ± 1.45 *** | 14.90 ± 1.33 *** | 35.91 ± 1.39 *** | |
| F3 | 7.5 | 9.19 ± 1.21 ** | 22.97 ± 1.71 ** | 16.37 ± 1.13 ** | 35.61 ± 1.55 ** |
| 15 | 9.41 ± 1.29 *** | 24.50 ± 1.41 *** | 15.94 ± 1.65 ** | 36.98 ± 1.66 *** | |
| F4 | 7.5 | 9.30 ± 1.41 *** | 22.21 ± 1.40 ** | 15.74 ± 1.21 ** | 36.21 ± 1.35 *** |
| 15 | 9.71 ± 1.56 *** | 23.39 ± 1.32 ** | 15.01 ± 1.37 ** | 38.30 ± 1.40 *** | |
| F5 | 7.5 | 9.88 ± 1.49 *** | 24.18 ± 1.41 *** | 14.98 ± 1.22 *** | 37.28 ± 1.60 *** |
| 15 | 10.20 ± 1.62 *** | 24.91 ± 1.29 *** | 14.48 ± 1.38 ** | 39.01 ± 1.39 *** | |
| Donepezil | 2 | 12.95 ± 1.47 *** | 30.88 ± 1.44 *** | 10.95 ± 1.28 *** | 45.09 ± 1.30 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Joufi, F.A.; Shah, S.W.A.; Shoaib, M.; Ghias, M.; Shafiullah; Khalil, A.A.K.; Jamal, S.B.; Shah, S.M.H.; Zahoor, M. Flavonoid Derivatives as Potential Cholinesterase Inhibitors in Scopolamine-Induced Amnesic Mice: An In Vitro, In Vivo and Integrated Computational Approach. Brain Sci. 2022, 12, 731. https://doi.org/10.3390/brainsci12060731
Al-Joufi FA, Shah SWA, Shoaib M, Ghias M, Shafiullah, Khalil AAK, Jamal SB, Shah SMH, Zahoor M. Flavonoid Derivatives as Potential Cholinesterase Inhibitors in Scopolamine-Induced Amnesic Mice: An In Vitro, In Vivo and Integrated Computational Approach. Brain Sciences. 2022; 12(6):731. https://doi.org/10.3390/brainsci12060731
Chicago/Turabian StyleAl-Joufi, Fakhria A., Syed Wadood Ali Shah, Mohammad Shoaib, Mehreen Ghias, Shafiullah, Atif Ali Khan Khalil, Syed Babar Jamal, Syed Muhammad Hassan Shah, and Muhammad Zahoor. 2022. "Flavonoid Derivatives as Potential Cholinesterase Inhibitors in Scopolamine-Induced Amnesic Mice: An In Vitro, In Vivo and Integrated Computational Approach" Brain Sciences 12, no. 6: 731. https://doi.org/10.3390/brainsci12060731
APA StyleAl-Joufi, F. A., Shah, S. W. A., Shoaib, M., Ghias, M., Shafiullah, Khalil, A. A. K., Jamal, S. B., Shah, S. M. H., & Zahoor, M. (2022). Flavonoid Derivatives as Potential Cholinesterase Inhibitors in Scopolamine-Induced Amnesic Mice: An In Vitro, In Vivo and Integrated Computational Approach. Brain Sciences, 12(6), 731. https://doi.org/10.3390/brainsci12060731






