Eye Tracking in Patients with Parkinson’s Disease Treated with Nabilone–Results of a Phase II, Placebo-Controlled, Double-Blind, Parallel-Group Pilot Study
Abstract
:1. Introduction
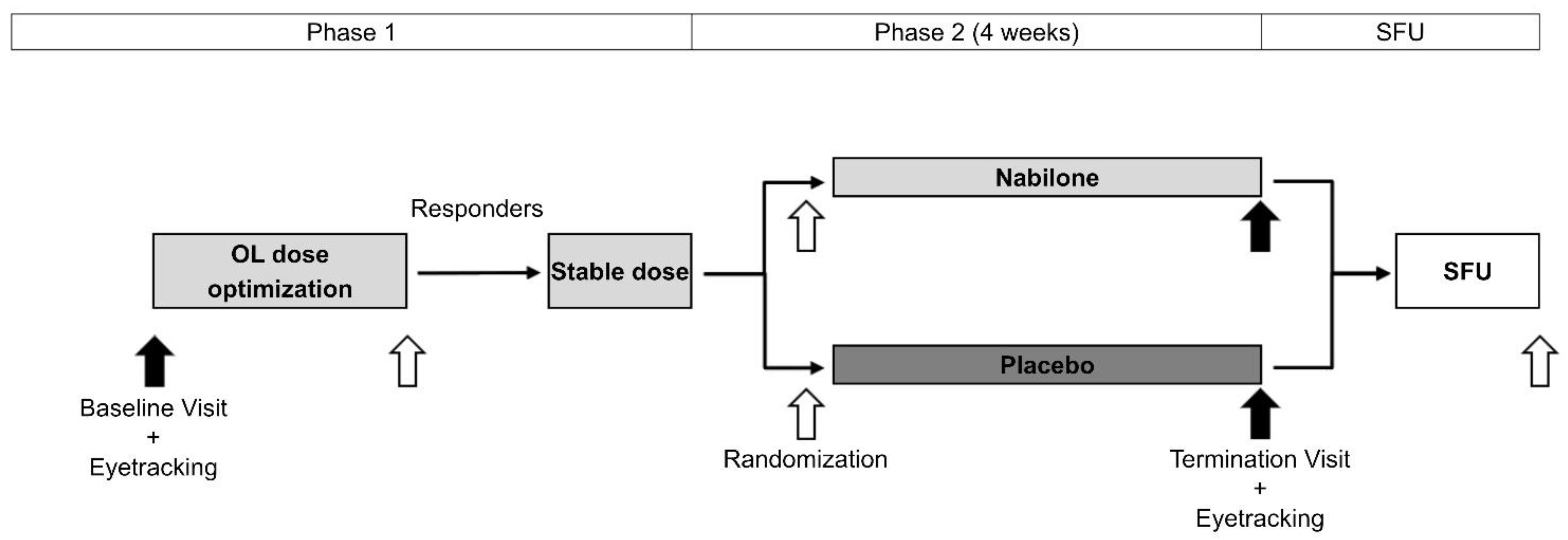
2. Materials and Methods
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Peball, M.; Werkmann, M.; Ellmerer, P.; Stolz, R.; Valent, D.; Knaus, H.G.; Ulmer, H.; Djamshidian, A.; Poewe, W.; Seppi, K. Nabilone for non-motor symptoms of Parkinson’s disease: A randomized placebo-controlled, double-blind, parallel-group, enriched enrolment randomized withdrawal study (The NMS-Nab Study). J. Neural Transm. 2019, 126, 1061–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buhmann, C.; Mainka, T.; Ebersbach, G.; Gandor, F. Evidence for the use of cannabinoids in Parkinson’s disease. J. Neural Transm. 2019, 126, 913–924. [Google Scholar] [CrossRef] [PubMed]
- Peball, M.; Krismer, F.; Knaus, H.G.; Djamshidian, A.; Werkmann, M.; Carbone, F.; Ellmerer, P.; Heim, B.; Marini, K.; Valent, D.; et al. Non-Motor Symptoms in Parkinson’s Disease are Reduced by Nabilone. Ann. Neurol. 2020, 88, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Ware, M.A.; St Arnaud-Trempe, E. The abuse potential of the synthetic cannabinoid nabilone. Addiction 2010, 105, 494–503. [Google Scholar] [CrossRef]
- Murray, R.M.; Englund, A.; Abi-Dargham, A.; Lewis, D.A.; Di Forti, M.; Davies, C.; Sherif, M.; McGuire, P.; D’Souza, D.C. Cannabis-associated psychosis: Neural substrate and clinical impact. Neuropharmacology 2017, 124, 89–104. [Google Scholar] [CrossRef]
- Bueno, A.P.A.; Sato, J.R.; Hornberger, M. Eye tracking—The overlooked method to measure cognition in neurodegeneration? Neuropsychologia 2019, 133, 107191. [Google Scholar] [CrossRef]
- Ellmerer, P.; Heim, B.; Stefani, A.; Peball, M.; Werkmann, M.; Holzknecht, E.; Bergmann, M.; Brandauer, E.; Sojer, M.; Zamarian, L.; et al. Augmentation in restless legs syndrome: An eye tracking study on emotion processing. Ann. Clin. Transl. Neurol. 2020, 7, 1620–1627. [Google Scholar] [CrossRef]
- Briand, K.A.; Strallow, D.; Hening, W.; Poizner, H.; Sereno, A.B. Control of voluntary and reflexive saccades in Parkinson’s disease. Exp. Brain Res. 1999, 129, 38–48. [Google Scholar] [CrossRef]
- Carbone, F.; Ellmerer, P.; Ritter, M.; Spielberger, S.; Mahlknecht, P.; Hametner, E.; Hussl, A.; Hotter, A.; Granata, R.; Seppi, K.; et al. Impaired Inhibitory Control of Saccadic Eye Movements in Cervical Dystonia: An Eye-Tracking Study. Mov. Disord. 2021, 36, 1246–1250. [Google Scholar] [CrossRef]
- Ettinger, U.; Kumari, V.; Crawford, T.J.; Davis, R.E.; Sharma, T.; Corr, P.J. Reliability of smooth pursuit, fixation, and saccadic eye movements. Psychophysiology 2003, 40, 620–628. [Google Scholar] [CrossRef]
- Płomecka, M.B.; Barańczuk-Turska, Z.; Pfeiffer, C.; Langer, N. Aging Effects and Test–Retest Reliability of Inhibitory Control for Saccadic Eye Movements. eNeuro 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Coe, B.C.; Munoz, D.P. Mechanisms of saccade suppression revealed in the anti-saccade task. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.J.; Gao, M.; Gao, F.F.; Su, Q.X.; Wu, J. Brain cannabinoid receptor 2: Expression, function and modulation. Acta Pharmacol. Sin. 2017, 38, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Hampson, R.E.; Deadwyler, S.A. Cannabinoids, hippocampal function and memory. Life Sci. 1999, 65, 715–723. [Google Scholar] [CrossRef]
- Espadas, I.; Ortiz, O.; García-Sanz, P.; Sanz-Magro, A.; Alberquilla, S.; Solis, O.; Delgado-García, J.M.; Gruart, A.; Moratalla, R. Dopamine D2R is Required for Hippocampal-Dependent Memory and Plasticity at the CA3-CA1 Synapse. Cereb. Cortex 2020, 31, 2187–2204. [Google Scholar] [CrossRef]
- Liu, X.; Dimidschstein, J.; Fishell, G.; Carter, A.G. Hippocampal inputs engage CCK+ interneurons to mediate endocannabinoid-modulated feed-forward inhibition in the prefrontal cortex. eLife 2020, 9, e55267. [Google Scholar] [CrossRef]
- Fried, P.A.; Watkinson, B.; Gray, R. Neurocognitive consequences of marihuana—A comparison with pre-drug performance. Neurotoxicol. Teratol. 2005, 27, 231–239. [Google Scholar] [CrossRef]
- Kindred, J.H.; Li, K.; Ketelhut, N.B.; Proessl, F.; Fling, B.W.; Honce, J.M.; Shaffer, W.R.; Rudroff, T. Cannabis use in people with Parkinson’s disease and Multiple Sclerosis: A web-based investigation. Complement. Ther. Med. 2017, 33, 99–104. [Google Scholar] [CrossRef]
- Averbeck, B.B.; O’Sullivan, S.S.; Djamshidian, A. Impulsive and compulsive behaviors in Parkinson’s disease. Annu. Rev. Clin. Psychol. 2014, 10, 553–580. [Google Scholar] [CrossRef] [Green Version]
- Picazio, S.; Ponzo, V.; Caltagirone, C.; Brusa, L.; Koch, G. Dysfunctional inhibitory control in Parkinson’s disease patients with levodopa-induced dyskinesias. J. Neurol. 2018, 265, 2088–2096. [Google Scholar] [CrossRef]
- Sieradzan, K.A.; Fox, S.H.; Hill, M.; Dick, J.P.R.; Crossman, A.R.; Brotchie, J.M. Cannabinoids reduce levodopa-induced dyskinesia in Parkinson’s disease: A pilot study. Neurology 2001, 57, 2108–2111. [Google Scholar] [CrossRef] [PubMed]
- Haney, M.; Cooper, Z.D.; Bedi, G.; Vosburg, S.K.; Comer, S.D.; Foltin, R.W. Nabilone decreases marijuana withdrawal and a laboratory measure of marijuana relapse. Neuropsychopharmacology 2013, 38, 1557–1565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, K.P.; Palastro, M.D.; Gruber, S.A.; Fitzmaurice, G.M.; Greenfield, S.F.; Lukas, S.E.; Weiss, R.D. Nabilone pharmacotherapy for cannabis dependence: A randomized, controlled pilot study. Am. J. Addict. 2017, 26, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, N.; Ruthirakuhan, M.; Gallagher, D.; Verhoeff, N.P.L.; Kiss, A.; Black, S.E.; Lanctôt, K.L. Randomized Placebo-Controlled Trial of Nabilone for Agitation in Alzheimer’s Disease. Am. J. Geriatr. Psychiatry 2019, 27, 1161–1173. [Google Scholar] [CrossRef]
- Dyckman, K.A.; McDowell, J.E. Behavioral plasticity of antisaccade performance following daily practice. Exp. Brain Res. 2005, 162, 63–69. [Google Scholar] [CrossRef]
| Nabilone | Placebo | p Value | |
|---|---|---|---|
| n (f) | 14 (6) | 10 (1) | 0.081 d |
| Age (y) | 65.9 ± 7.5 | 62.9 ± 9.3 | 0.376 a |
| Disease duration (y) | 7.0 ± 5.7 | 5.4 ± 2.0 | 0.347 a |
| Education (y) | 12.3 ± 2.6 | 13.3 ± 3.0 | 0.391 b |
| MMSE | 30.0 (29.0–30.0) | 29.5 (29.0–30.0) | 0.503 c |
| MoCA | 28.0 (27.0–29.75) | 28.0 (27.0–29.0) | 0.928 c |
| HADS-A | 6.6 ± 3.6 | 4.3 ± 2.8 | 0.099 a |
| HADS-D | 5.6 ± 3.6 | 4.2 ± 2.7 | 0.296 a |
| MDS-UPDRS I | 13.9 ± 4.4 | 11.2 ± 4.5 | 0.152 a |
| MDS-UPDRS II | 10.9 ± 6.3 | 10.8 ± 4.1 | 0.956 a |
| MDS-UPDRS III | 25.9 ± 12.7 | 30.3 ± 11.4 | 0.395 a |
| MDS-UPDRS IV | 1.0 (0.0–3.0) | 1.5 (0.0–3.8) | 0.900 c |
| Nabilone | Placebo | Main Effect Time | Main Effect Group | Main Effect Time × Group | |||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | p | p | p | |
| PS RT (ms) a | |||||||
| BL | 277 | 110 | 300 | 67 | 0.766 | 0.799 | 0.158 |
| TV | 297 | 81 | 287 | 124 | |||
| PS ER (%) a | |||||||
| BL | 59.5 | 31.8 | 59.8 | 33.6 | 0.484 | 0.893 | 0.292 |
| TV | 49.7 | 28.9 | 58.2 | 35.5 | |||
| AS RT (ms) a | |||||||
| BL | 295 | 110 | 302 | 83 | 0.070 | 0.763 | 0.705 |
| TV | 327 | 82 | 330 | 90 | |||
| AS ER (%) | |||||||
| BL | 58.7 | 27.8 | 46.7 | 37.4 | 0.046 | 0.215 | 0.539 |
| TV | 51.8 | 29.6 | 34.1 | 26.1 | |||
| SS RT (ms) a | |||||||
| BL | 227 | 46 | 246 | 72 | 0.248 | 0.514 | 0.941 |
| TV | 216 | 77 | 231 | 51 | |||
| SS ER (%) | |||||||
| BL | 28.25 | 19.95 | 31.78 | 26.62 | 0.137 | 0.723 | 0.109 |
| TV | 28.73 | 20.85 | 19.28 | 19.15 | |||
| PAS RT (ms) a | |||||||
| BL | 264 | 65 | 237 | 48 | 0.133 | 0.525 | 0.313 |
| TV | 269 | 53 | 271 | 88 | |||
| PAS ER (%) | |||||||
| BL | 25.5 | 15.5 | 25.0 | 20.0 | 0.757 | 0.831 | 0.455 |
| TV | 29.4 | 22.9 | 25.0 | 20.7 | |||
| RPAS RT (ms) a | |||||||
| BL | 231 | 51 | 237 | 43 | 0.188 | 0.912 | 0.748 |
| TV | 243 | 50 | 237 | 51 | |||
| RPAS ER (%) b | |||||||
| BL | 27.7 | 14.2 | 27.5 | 21.7 | 0.735 | 0.85 | 0.16 |
| TV | 29.4 | 14.7 | 21.6 | 25.0 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ellmerer, P.; Peball, M.; Carbone, F.; Ritter, M.; Heim, B.; Marini, K.; Valent, D.; Krismer, F.; Poewe, W.; Djamshidian, A.; et al. Eye Tracking in Patients with Parkinson’s Disease Treated with Nabilone–Results of a Phase II, Placebo-Controlled, Double-Blind, Parallel-Group Pilot Study. Brain Sci. 2022, 12, 661. https://doi.org/10.3390/brainsci12050661
Ellmerer P, Peball M, Carbone F, Ritter M, Heim B, Marini K, Valent D, Krismer F, Poewe W, Djamshidian A, et al. Eye Tracking in Patients with Parkinson’s Disease Treated with Nabilone–Results of a Phase II, Placebo-Controlled, Double-Blind, Parallel-Group Pilot Study. Brain Sciences. 2022; 12(5):661. https://doi.org/10.3390/brainsci12050661
Chicago/Turabian StyleEllmerer, Philipp, Marina Peball, Federico Carbone, Marcel Ritter, Beatrice Heim, Kathrin Marini, Dora Valent, Florian Krismer, Werner Poewe, Atbin Djamshidian, and et al. 2022. "Eye Tracking in Patients with Parkinson’s Disease Treated with Nabilone–Results of a Phase II, Placebo-Controlled, Double-Blind, Parallel-Group Pilot Study" Brain Sciences 12, no. 5: 661. https://doi.org/10.3390/brainsci12050661
APA StyleEllmerer, P., Peball, M., Carbone, F., Ritter, M., Heim, B., Marini, K., Valent, D., Krismer, F., Poewe, W., Djamshidian, A., & Seppi, K. (2022). Eye Tracking in Patients with Parkinson’s Disease Treated with Nabilone–Results of a Phase II, Placebo-Controlled, Double-Blind, Parallel-Group Pilot Study. Brain Sciences, 12(5), 661. https://doi.org/10.3390/brainsci12050661






