Anatomically Standardized Detection of MRI Atrophy Patterns in Early-Stage Alzheimer’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Neuropsychological Tests
2.3. MRI Acquisition
2.4. Data Preprocessing
2.5. Statistical Analysis
3. Results
3.1. Principal Component Analysis
3.2. Morphological AD Subtypes Identified by Cluster Analysis
3.3. Demographic and Clinical Characteristics among the 4 AD Subtypes
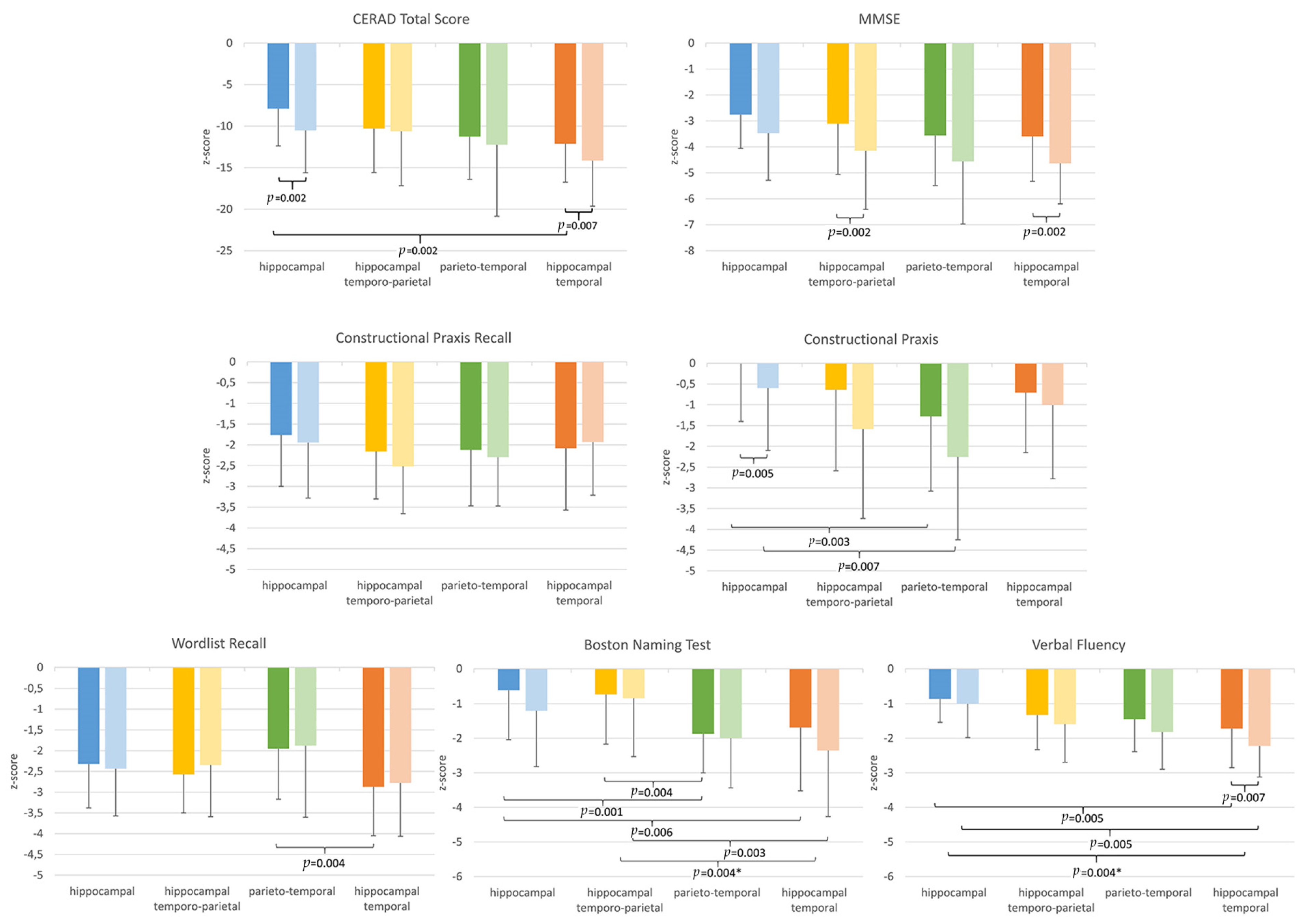
3.4. Cognitive Characteristics among the Four AD Dementia Subtypes
3.4.1. Cross-Sectional Group Comparisons
3.4.2. Longitudinal Group Comparisons
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Braak, H.; Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar] [CrossRef]
- Murray, M.E.; Graff-Radford, N.R.; Ross, O.A.; Petersen, R.C.; Duara, R.; Dickson, D.W. Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: A retrospective study. Lancet Neurol. 2011, 10, 785–796. [Google Scholar] [CrossRef] [Green Version]
- Jack, C.R., Jr.; Dickson, D.W.; Parisi, J.E.; Xu, Y.C.; Cha, R.H.; O’Brien, P.C.; Edland, S.D.; Smith, G.E.; Boeve, B.F.; Tangalos, E.G.; et al. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology 2002, 58, 750–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitwell, J.L.; Josephs, K.A.; Murray, M.E.; Kantarci, K.; Przybelski, S.A.; Weigand, S.D.; Vemuri, P.; Senjem, M.L.; Parisi, J.E.; Knopman, D.S.; et al. MRI correlates of neurofibrillary tangle pathology at autopsy: A voxel-based morphometry study. Neurology 2008, 71, 743–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitwell, J.L.; Dickson, D.W.; Murray, M.E.; Weigand, S.D.; Tosakulwong, N.; Senjem, M.L.; Knopman, D.S.; Boeve, B.F.; Parisi, J.E.; Petersen, R.C.; et al. Neuroimaging correlates of pathologically defined subtypes of Alzheimer’s disease: A case-control study. Lancet Neurol. 2012, 11, 868–877. [Google Scholar] [CrossRef] [Green Version]
- Dong, A.; Toledo, J.B.; Honnorat, N.; Doshi, J.; Varol, E.; Sotiras, A.; Wolk, D.; Trojanowski, J.Q.; Davatzikos, C. Heterogeneity of neuroanatomical patterns in prodromal Alzheimer’s disease: Links to cognition, progression and biomarkers. Brain 2016, 140, 735–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varol, E.; Sotiras, A.; Davatzikos, C. Alzheimer’s Disease Neuroimaging Initiative. HYDRA: Revealing heterogeneity of imaging and genetic patterns through a multiple max-margin discriminative analysis framework. Neuroimage 2017, 145, 346–364. [Google Scholar] [CrossRef] [Green Version]
- Poulakis, K.; Pereira, J.B.; Mecocci, P.; Vellas, B.; Tsolaki, M.; Kłoszewska, I.; Soininen, H.; Lovestone, S.; Simmons, A.; Wahlund, L.O.; et al. Heterogeneous patterns of brain atrophy in Alzheimer’s disease. Neurobiol. Aging 2018, 65, 98–108. [Google Scholar] [CrossRef] [Green Version]
- Friston, K.J.; Ashburner, J.; Frith, C.D.; Poline, J.B.; Heather, J.D.; Frackowiak, R.S.J. Spatial registration and normalization of images. Hum. Brain Map. 1995, 3, 165–189. [Google Scholar] [CrossRef]
- Fischl, B.; Dale, A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. USA 2000, 97, 11050–11055. [Google Scholar] [CrossRef] [Green Version]
- Dahnke, R.; Yotter, R.A.; Gaser, C. Cortical thickness and central surface estimation. Neuroimage 2013, 65, 336–348. [Google Scholar] [CrossRef]
- Noh, Y.; Jeon, S.; Lee, J.M.; Seo, S.W.; Kim, G.H.; Cho, H.; Ye, B.S.; Yoon, C.W.; Kim, H.J.; Chin, J.; et al. Anatomical heterogeneity of Alzheimer disease: Based on cortical thickness on MRIs. Neurology 2014, 83, 1936–1944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Mormino, E.C.; Sun, N.; Sperling, R.A.; Sabuncu, M.R.; Yeo, B.T. Alzheimer’s Disease Neuroimaging Initiative. Bayesian model reveals latent atrophy factors with dissociable cognitive trajectories in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2016, 113, E6535–E6544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, J.; Kim, C.M.; Jeon, S.; Lee, J.M.; Hong, Y.J.; Roh, J.H.; Lee, J.H.; Koh, J.Y.; Na, D.L. Alzheimer’s Disease Neuroimaging Initiative. Prediction of Alzheimer’s disease pathophysiology based on cortical thickness patterns. Alzheimers Dement. 2016, 2, 58–67. [Google Scholar]
- Park, J.Y.; Na, H.K.; Kim, S.; Kim, H.; Kim, H.J.; Seo, S.W.; Na, D.L.; Han, C.E.; Seong, J.K. Alzheimer’s Disease Neuroimaging Initiative. Robust identification of Alzheimer’s disease subtypes based on cortical atrophy patterns. Sci. Rep. 2017, 7, 43270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ten Kate, M.; Barkhof, F.; Boccardi, M.; Visser, P.J.; Jack, C.R., Jr.; Lovblad, K.O.; Frisoni, G.B.; Scheltens, P. Geneva Task Force for the Roadmap of Alzheimer’s Biomarkers. Clinical validity of medial temporal atrophy as a biomarker for Alzheimer’s disease in the context of a structured 5-phase development framework. Neurobiol. Aging 2017, 52, 167–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischl, B.; Salat, D.H.; Busa, E.; Albert, M.; Dieterich, M.; Haselgrove, C.; van der Kouwe, A.; Killiany, R.; Kennedy, D.; Klaveness, S.; et al. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron 2002, 33, 341–355. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, C.G.; Gunter, J.L.; Wiste, H.J.; Przybelski, S.A.; Weigand, S.D.; Ward, C.P.; Senjem, M.L.; Vemuri, P.; Murray, M.E.; Dickson, D.W.; et al. Alzheimer’s Disease Neuroimaging Initiative. A large-scale comparison of cortical thickness and volume methods for measuring Alzheimer’s disease severity. Neuroimage Clin. 2016, 11, 802–812. [Google Scholar] [CrossRef] [Green Version]
- Desikan, R.S.; Cabral, H.J.; Hess, C.P.; Dillon, W.P.; Glastonbury, C.M.; Weiner, M.W.; Schmansky, N.J.; Greve, D.N.; Salat, D.H.; Buckner, R.L.; et al. Alzheimer’s Disease Neuroimaging Initiative. Automated MRI measures identify individuals with mild cognitive impairment and Alzheimer’s disease. Brain 2009, 132, 2048–2057. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, J.E.; Augustinack, J.C.; Nguyen, K.; Player, C.M.; Player, A.; Wright, M.; Roy, N.; Frosch, M.P.; McKee, A.C.; Wald, L.L.; et al. Alzheimer’s Disease Neuroimaging Initiative. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: Application to adaptive segmentation of in vivo MRI. Neuroimage 2015, 115, 117–137. [Google Scholar] [CrossRef]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, J.C. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology 1993, 43, 2412–2414. [Google Scholar] [CrossRef] [PubMed]
- Fazekas, F.; Chawluk, J.B.; Alavi, A.; Hurtig, H.I.; Zimmerman, R.A. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am. J. Roentgenol. 1987, 149, 351–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, R.; Fazekas, F.; Kapeller, P.; Schmidt, H.; Hartung, H.P. MRI white matter hyperintensities: Three-year follow-up of the Austrian Stroke Prevention Study. Neurology 1999, 53, 132–139. [Google Scholar] [CrossRef]
- Berres, M.; Monsch, A.U.; Bernasconi, F.; Thalmann, B.; Stähelin, H.B. Normal ranges of neuropsychological tests for the diagnosis of Alzheimer’s disease. Stud. Health. Technol. Inform. 2000, 77, 195–199. [Google Scholar]
- Kaplan, E.; Goodglass, H.; Weintraub, S. Boston Naming Test, 2nd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001. [Google Scholar]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. Mini Mental State: A practical method for grading the cognitive state of patients for the clinicians. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Chandler, M.J.; Lacritz, L.H.; Hynan, L.S.; Barnard, H.D.; Allen, G.; Deschner, M.; Weiner, M.F.; Cullum, C.M. A total score for the CERAD neuropsychological battery. Neurology 2005, 65, 102–106. [Google Scholar] [CrossRef]
- Gelinas, I.; Gauthier, L.; McIntyre, M.; Gauthier, S. Development of a functional measure for persons with Alzheimer’s disease: The Disability Assessment for Dementia. Am. J. Occup. Ther. 1999, 53, 471–481. [Google Scholar] [CrossRef] [Green Version]
- Yesavage, J.A.; Brink, T.L.; Rose, T.L.; Lum, O.; Huang, V.; Adey, M.; Leirer, V.O. Development and validation of a geriatric depression screening scale: A preliminary report. J. Psychiatr. Res. 1982, 17, 37–49. [Google Scholar] [CrossRef]
- Sled, J.G.; Zijdenbos, A.P.; Evans, A.C. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans. Med. Imaging 1998, 17, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Fischl, B.; Liu, A.; Dale, A.M. Automated manifold surgery: Constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans. Med. Imaging 2001, 20, 70–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segonne, F.; Pacheco, J.; Fischl, B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans. Med. Imaging 2007, 26, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Dale, A.M.; Fischl, B.; Sereno, M.I. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage 1999, 9, 179–194. [Google Scholar] [CrossRef]
- Buckner, R.L.; Head, D.; Parker, J.; Fotenos, A.F.; Marcus, D.; Morris, J.C.; Snyder, A.Z. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: Reliability and validation against manual measurement of total intracranial volume. Neuroimage 2004, 23, 724–738. [Google Scholar] [CrossRef] [PubMed]
- Voevodskaya, O.; Simmons, A.; Nordenskjöld, R.; Kullberg, J.; Ahlström, H.; Lind, L.; Wahlund, L.O.; Larsson, E.M.; Westman, E. Alzheimer’s Disease Neuroimaging Initiative. The effects of intracranial volume adjustment approaches on multiple regional MRI volumes in healthy aging and Alzheimer’s disease. Front. Aging Neurosci. 2014, 6, 264. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef]
- Whitwell, J.L.; Przybelski, S.A.; Weigand, S.D.; Ivnik, R.J.; Vemuri, P.; Gunter, J.L.; Josephs, K.A. Distinct anatomical subtypes of the behavioural variant of frontotemporal dementia: A cluster analysis study. Brain 2009, 132, 2932–2946. [Google Scholar] [CrossRef] [Green Version]
- Byun, M.S.; Kim, S.E.; Park, J.; Yi, D.; Choe, Y.M.; Sohn, B.K. Heterogeneity of regional brain atrophy patterns associated with distinct progression rates in Alzheimer’s disease. PLoS ONE 2015, 10, e0142756. [Google Scholar]
- Apostolova, L.G.; Thompson, P.M.; Green, A.E.; Hwang, K.S.; Zoumalan, C.; Jack, C.R., Jr.; Harvey, D.J.; Petersen, R.C.; Thal, L.J.; Aisen, P.S.; et al. 3D comparison of low intermediate, and advanced hippocampal atrophy in MCI. Hum. Brain Map. 2010, 31, 786–797. [Google Scholar] [CrossRef] [Green Version]
- Wisse, L.E.; Biessels, G.J.; Heringa, S.M.; Kuijf, H.J.; Koek, D.H.; Luijten, P.R.; Geerlings, M.I. Utrecht Vascular Cognitive Impairment (VCI) Study Group. Hippocampal subfield volumes at 7T in early Alzheimer’s disease and normalaging. Neurobiol. Aging 2014, 35, 2039–2045. [Google Scholar] [CrossRef]
- Maruszak, A.; Thuret, S. Why looking at the whole hippocampus is not enough-a critical role for anteroposterior axis, subfield and activation analyses to enhance predictive value of hippocampal changes for Alzheimer’s disease diagnosis. Front. Cell. Neurosci. 2014, 8, 95. [Google Scholar] [CrossRef] [Green Version]
- Mueller, S.G.; Yushkevich, P.A.; Das, S.; Wang, L.; Van Leemput, K.; Iglesias, J.E.; Alpert, K.; Mezher, A.; Ng, P.; Paz, K.; et al. Alzheimer’s Disease Neuroimaging Initiative. Systematic comparison of different techniques to measure hippocampal subfield volumes in ADNI2. Neuroimage Clin. 2018, 17, 1006–1018. [Google Scholar] [CrossRef]
- Baron, J.C.; Chételat, G.; Desgranges, B.; Perchey, G.; Landeau, B.; de la Sayette, V.; Eustache, F. In vivo mapping of gray matter loss with voxel-based morphometry in mild Alzheimer’s disease. Neuroimage 2001, 14, 298–309. [Google Scholar] [CrossRef]
- Scheltens, P.; Blennow, K.; Breteler, M.M.; de Strooper, B.; Frisoni, G.B.; Salloway, S.; Van der Flier, W.M. Alzheimer’s disease. Lancet 2016, 388, 505–517. [Google Scholar] [CrossRef]
- Vanhoutte, M.; Semah, F.; Rollin Sillaire, A.; Jaillard, A.; Petyt, G.; Kuchcinski, G.; Maureille, A.; Delbeuck, X.; Fahmi, R.; Pasquier, F.; et al. (18)F-FDG PET hypometabolism patterns reflect clinical heterogeneity in sporadic forms of early-onset Alzheimer’s disease. Neurobiol. Aging 2017, 59, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Lam, B.; Masellis, M.; Freedman, M.; Stuss, D.T.; Black, S.E. Clinical, imaging, and pathological heterogeneity of the Alzheimer’s disease syndrome. Alzheimers Res. Ther. 2013, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Jovicich, J.; Salat, D.; van der Kouwe, A.; Quinn, B.; Czanner, S.; Busa, E.; Pacheco, J.; Albert, M.; Killiany, R.; et al. Reliability of MRI-derived measurements of human cerebral cortical thickness: The effects of field strength, scanner upgrade and manufacturer. Neuroimage 2006, 32, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Whelan, C.D.; Hibar, D.P.; van Velzen, L.S.; Zannas, A.S.; Carrillo-Roa, T.; McMahon, K.; Prasad, G.; Kelly, S.; Faskowitz, J.; deZubiracay, G.; et al. Heritability and reliability of automatically segmented human hippocampal formation subregions. Neuroimage 2016, 128, 125–137. [Google Scholar] [CrossRef] [Green Version]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
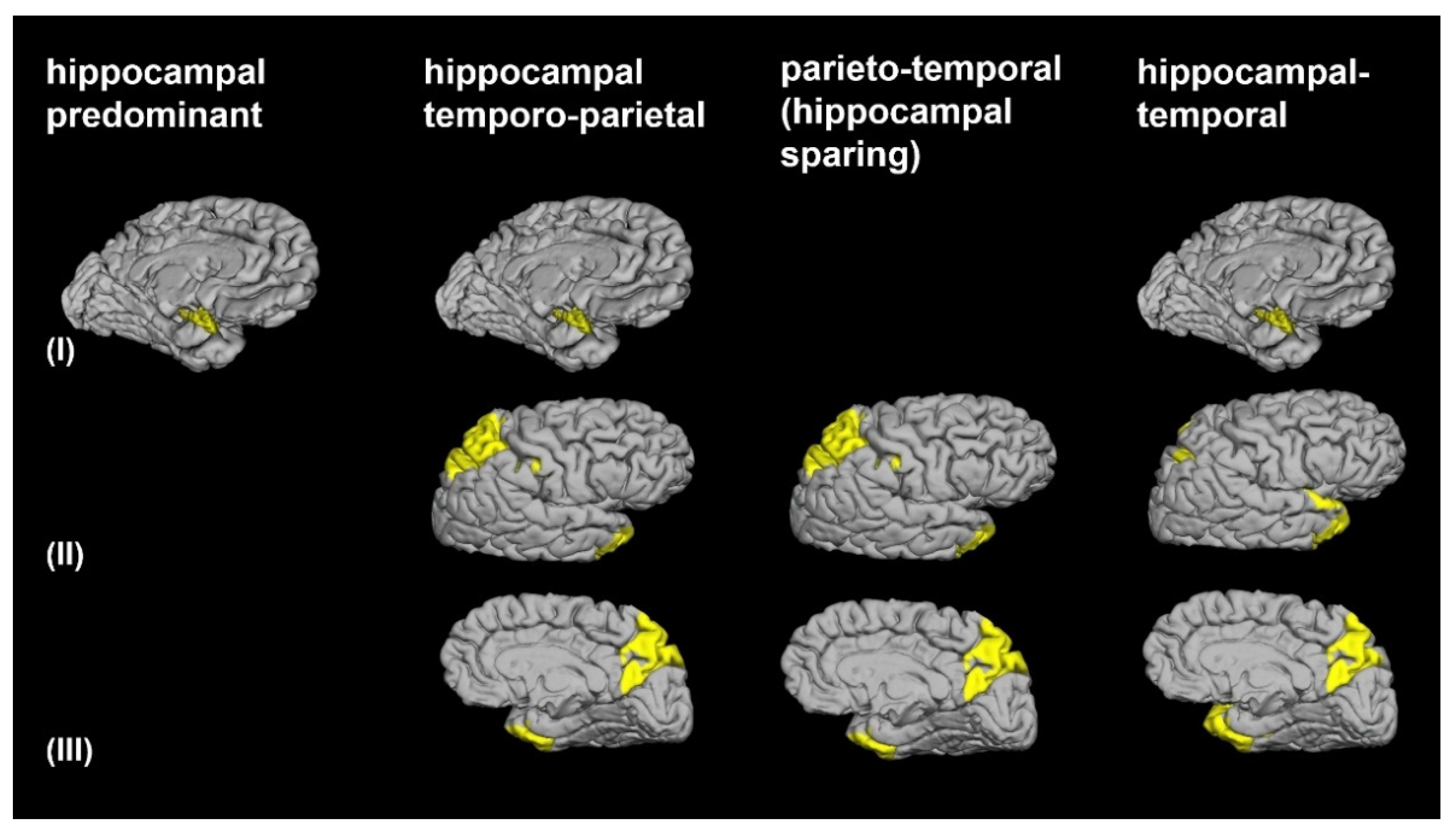
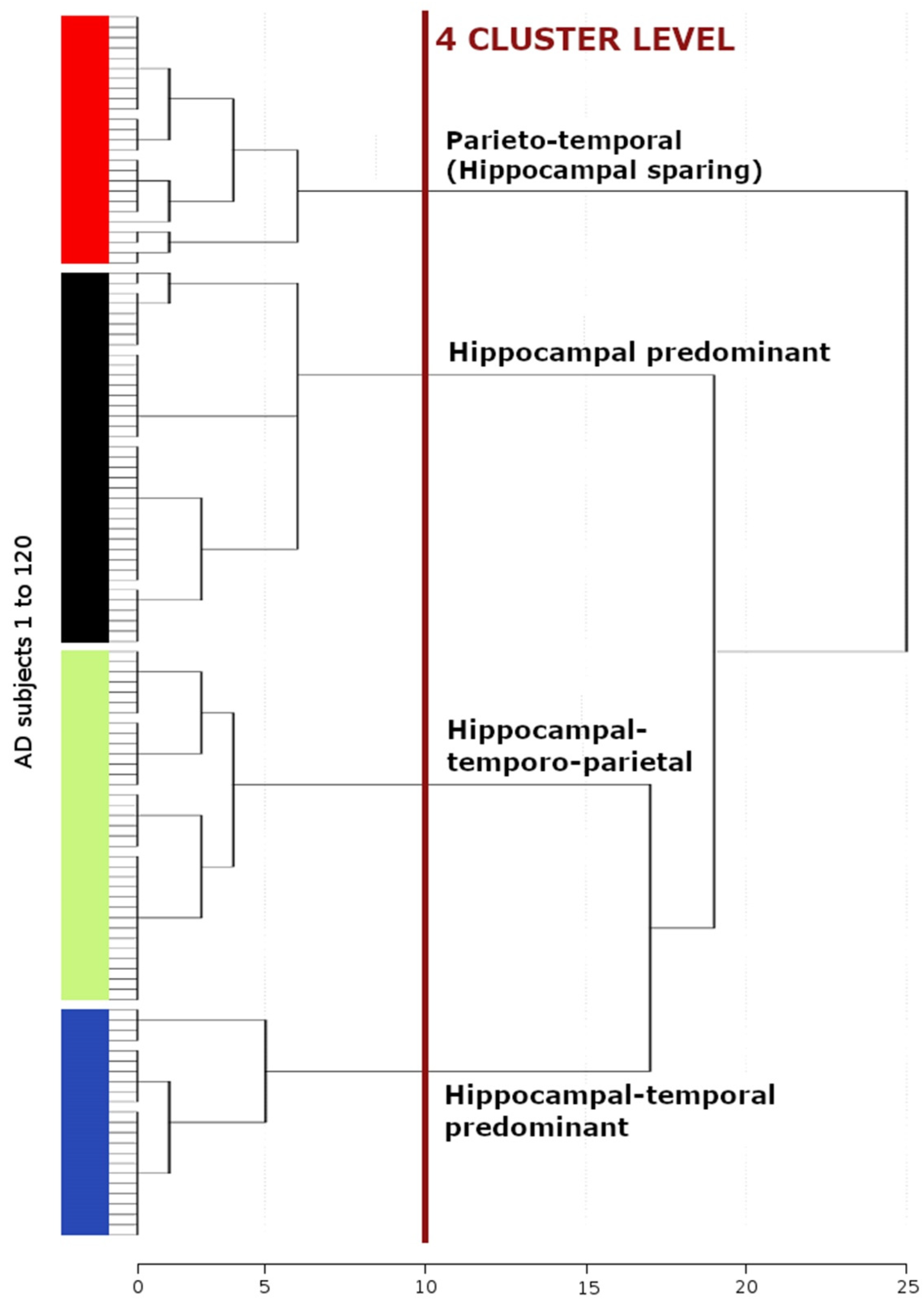
| Hippocampal Predominant (n = 37) | Hippocampal-Temporo-Parietal (n = 35) | Parieto-Temporal (n = 25) | Hippocampal-Temporal Predominant (n = 23) | |
|---|---|---|---|---|
| Principal component I | ||||
| Subiculum | −2.55 (1.32) | −3.50 (0.86) | −1.32 (0.87) | −3.41 (0.54) |
| CA1-2 transition zones | −2.40 (1.02) | −3.03 (0.83) | −0.96 (0.88) | −2.94 (0.65) |
| CA3 | −2.24 (0.79) | −2.66 (0.63) | −0.35 (1.19) | −2.81 (0.82) |
| CA4/dentate gyrus | −2.71 (0.94) | −3.23 (0.76) | −0.72 (1.02) | −3.29 (0.84) |
| whole hippocampus | −2.80 (1.26) | −3.58 (0.83) | −1.21 (1.89) | −3.53 (0.60) |
| Principal component II | ||||
| superior parietal lobule | 0.36 (1.36) | −2.06 (0.81) | −1.50 (1.67) | −1.22 (1.21) |
| Precuneus | 0.26 (1.31) | −1.72 (1.08) | −1.59 (1.50) | −1.81 (0.98) |
| cingulate sulcus marginal branch | 1.09 (1.38) | −1.01 (1.21) | −1.44 (1.06) | −0.82 (1.36) |
| intraparietal sulcus and transverse parietal sulci | 0.06 (1.18) | −1.85 (0.87) | −2.15 (1.39) | −1.75 (0.96) |
| postcentral sulcus | 0.56 (1.22) | −1.66 (0.82) | −1.55 (1.12) | −1.31 (0.80) |
| Principal component III | ||||
| superior temporal gyrus planum polare | −0.66 (1.55) | −1.22 (1.29) | −1.22 (1.35) | −3.79 (1.16) |
| temporal pole | −0.78 (1.57) | −2.12 (1.64) | −1.91 (1.76) | −4.37 (1.58) |
| inferior segment of the circular sulcus of the insula | −0.42 (1.38) | −0.34 (1.26) | −0.97 (1.42) | −2.86 (0.78) |
| anterior transverse collateral sulcus | −0.71 (1.36) | −1.05 (1.42) | −1.33 (1.62) | −3.03 (1.03) |
| At Baseline | Hippocampal Predominant | Hippocampal-Temporo-Parietal | Parieto-Temporal | Hippocampal-Temporal | Alzheimer’s Dementia | Control Group |
| Sample size, n (%) | 37 (38.8) | 35 (29.2) | 25 (20.8) | 23 (19.2) | 120 | 348 |
| Age at MRI, years, mean (± 1 SD) | 74.13 (4.92) | 73.66 (4.8) | 71.81 (5.38) | 74.8 (5.44) | 73.52 (5.43) | 72.52 (6.47) |
| Sex, females, n (%) | 25 (67.57) | 20 (57.14) | 17 (68) | 15 (65.22) | 77 (64.17) | 210 (60.44) |
| Disease duration, years, median (IQR) | 1.85 (2.35) | 2.06 (2.65) | 1.55 (2.32) | 3.1 (2.41) | 2.18 (2.55) | - |
| CDR, median (IQR) | 0.5 (0.5) | 0.5 (0.5) | 0.5 (0.5) | 0.5 (0.5) | 0.5 (0.5) | - |
| GDS, median (IQR) | 1 (2) | 2 (2) | 2 (2) | 1 (2) | 1.5 (2) | - |
| MMSE, mean (± 1 SD) | 23.46 (3.17) | 22.71 (4.59) | 21.52 (4.16) | 21.91 (4.40) | 22.27 (4.3) | - |
| NPI, median (IQR) | 9 (17) | 4 (10) | 4 (7) | 2 (7) | 4 (9) | - |
| DAD median %, (IQR) | 85 (27.5) | 92.5 (22.5) | 84 (38) | 87.5 (27.5) | 88.49 (25) | - |
| At One-Year Follow-Up | Hippocampal Predominant | Hippocampal-Temporo-Parietal | Parieto-Temporal | Hippocampal-Temporal | Alzheimer’s Dementia | Control Group |
| Sample size, n (%) | 24 (21.6) | 30 (27) | 16 (14.4) | 20 (18) | 90 | - |
| Age at MRI, years, mean (± 1 SD) | 74.73 (4.9) | 74.21 (6.81) | 74.2 (6.27) | 75.84 (3.57) | 74.46 (5.38) | - |
| Disease duration, years, median (IQR) | 2.82 (2.98) | 3.16 (2.6) | 3.42 (1.84) | 4.16 (3.3) | 3.1 (2.48) | - |
| CDR, median (IQR) | 1 (0.5) | 1 (0.5) | 1 (0.5) | 1 (0.5) | 1 (0.5) | - |
| GDS, median (IQR) | 1 (2.75) | 2 (3) | 2 (2.5) | 1 (3) | 2 (2) | - |
| MMSE, mean (± 1 SD) | 22.04 (3.86) | 20.20 (6.00) | 18.75 (5.47) | 19.95 (4.01) | 20.39 (4.9) | - |
| NPI, median (IQR) | 9.5 (14.25) | 8 (15.5) | 5 (9.75) | 2.5 (8.5) | 6 (10.5) | - |
| DAD median %, (IQR) | 65 (41.88) | 67.5 (25.8) | 70.83 (41.8) | 75 (40) | 71.25 (39.66) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lenhart, L.; Seiler, S.; Pirpamer, L.; Goebel, G.; Potrusil, T.; Wagner, M.; Dal Bianco, P.; Ransmayr, G.; Schmidt, R.; Benke, T.; et al. Anatomically Standardized Detection of MRI Atrophy Patterns in Early-Stage Alzheimer’s Disease. Brain Sci. 2021, 11, 1491. https://doi.org/10.3390/brainsci11111491
Lenhart L, Seiler S, Pirpamer L, Goebel G, Potrusil T, Wagner M, Dal Bianco P, Ransmayr G, Schmidt R, Benke T, et al. Anatomically Standardized Detection of MRI Atrophy Patterns in Early-Stage Alzheimer’s Disease. Brain Sciences. 2021; 11(11):1491. https://doi.org/10.3390/brainsci11111491
Chicago/Turabian StyleLenhart, Lukas, Stephan Seiler, Lukas Pirpamer, Georg Goebel, Thomas Potrusil, Michaela Wagner, Peter Dal Bianco, Gerhard Ransmayr, Reinhold Schmidt, Thomas Benke, and et al. 2021. "Anatomically Standardized Detection of MRI Atrophy Patterns in Early-Stage Alzheimer’s Disease" Brain Sciences 11, no. 11: 1491. https://doi.org/10.3390/brainsci11111491
APA StyleLenhart, L., Seiler, S., Pirpamer, L., Goebel, G., Potrusil, T., Wagner, M., Dal Bianco, P., Ransmayr, G., Schmidt, R., Benke, T., & Scherfler, C. (2021). Anatomically Standardized Detection of MRI Atrophy Patterns in Early-Stage Alzheimer’s Disease. Brain Sciences, 11(11), 1491. https://doi.org/10.3390/brainsci11111491






