NMDA Enhances and Glutamate Attenuates Synchrony of Spontaneous Phase-Locked Locus Coeruleus Network Rhythm in Newborn Rat Brain Slices
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Solutions
2.2. Drug Application
2.3. Electrophysiological Recording
2.4. Data Analysis
3. Results
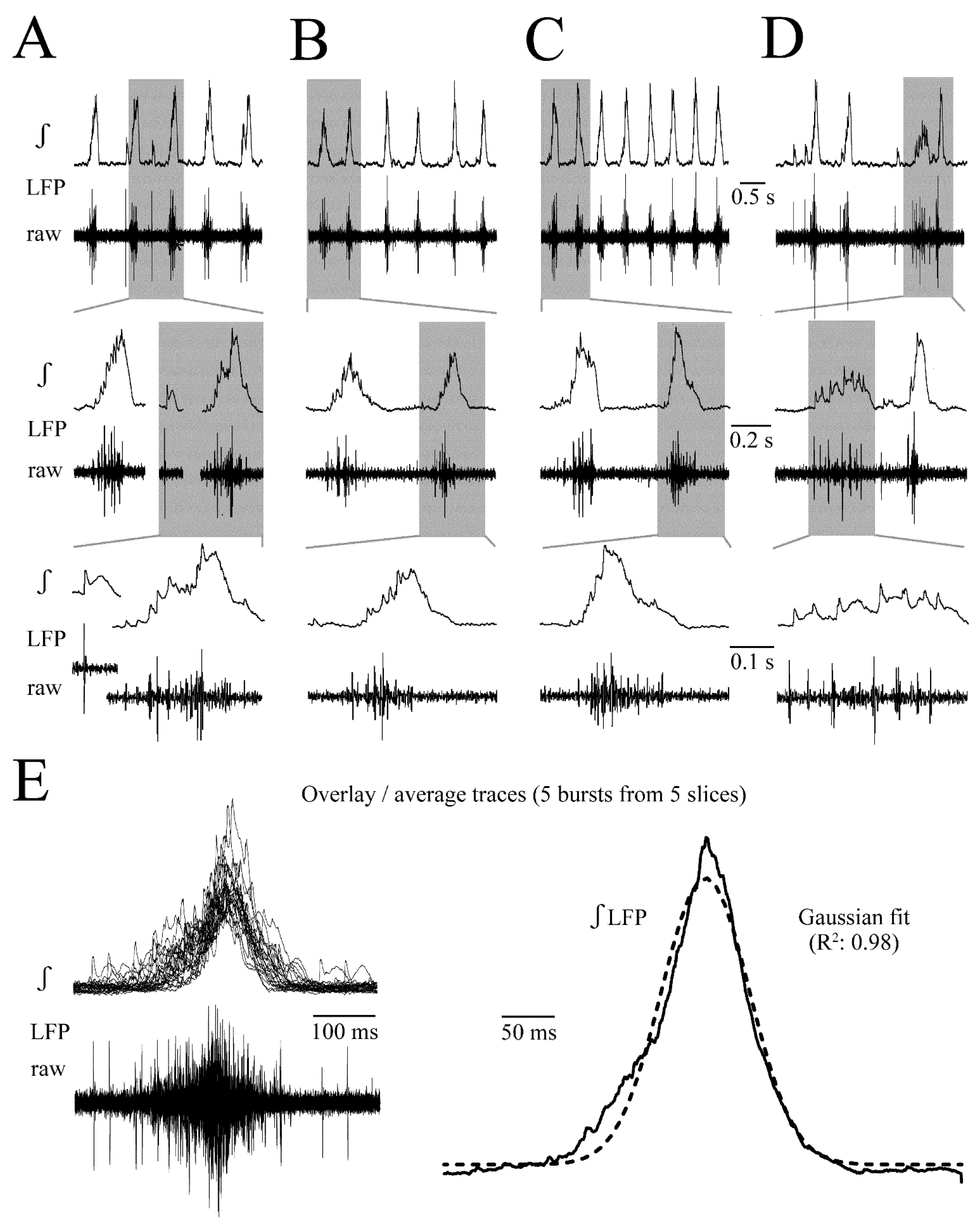
3.1. LFP Shape and Signal Analysis
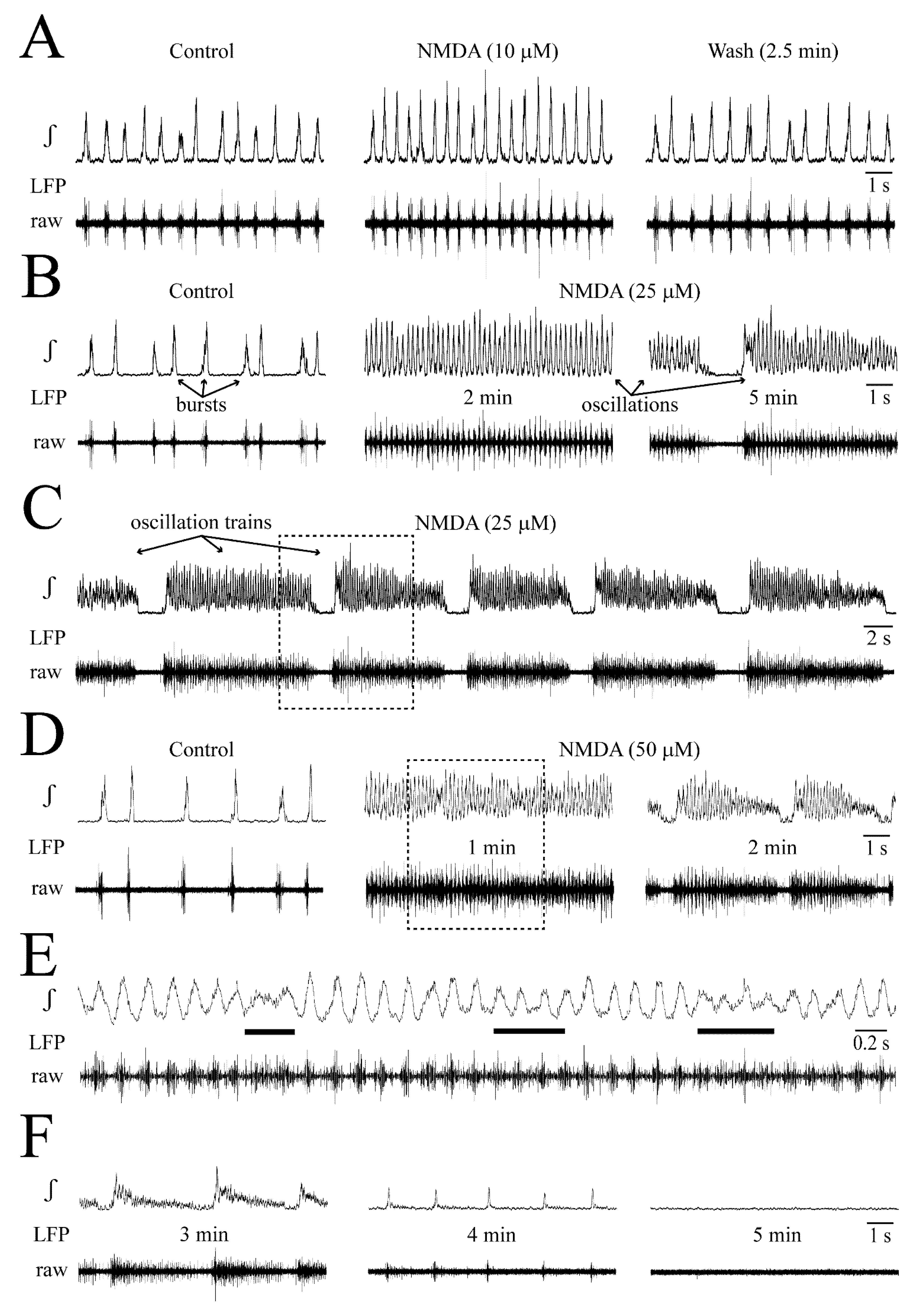
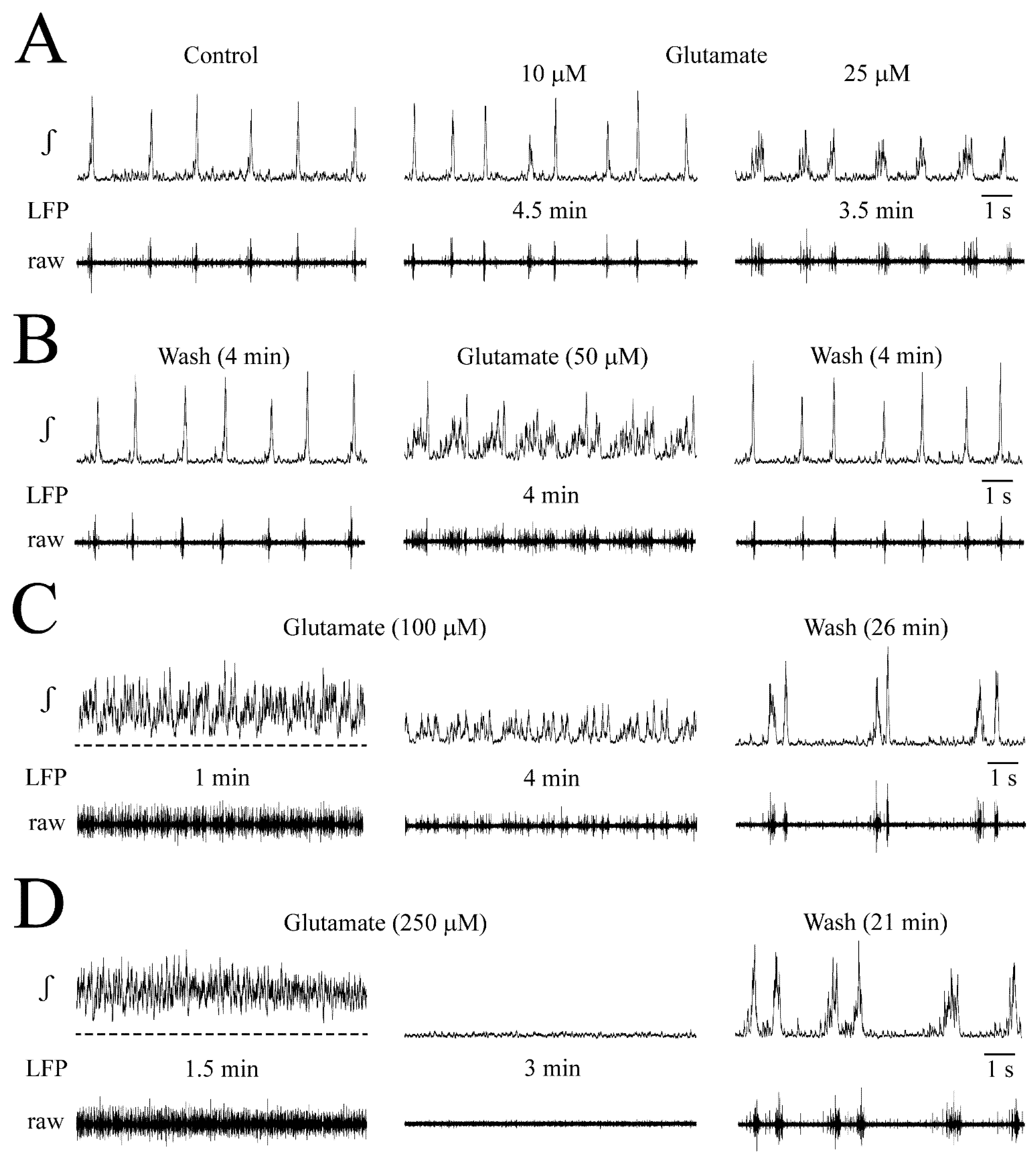
3.2. NMDA- and Glutamate-Evoked LFP Pattern Transformation
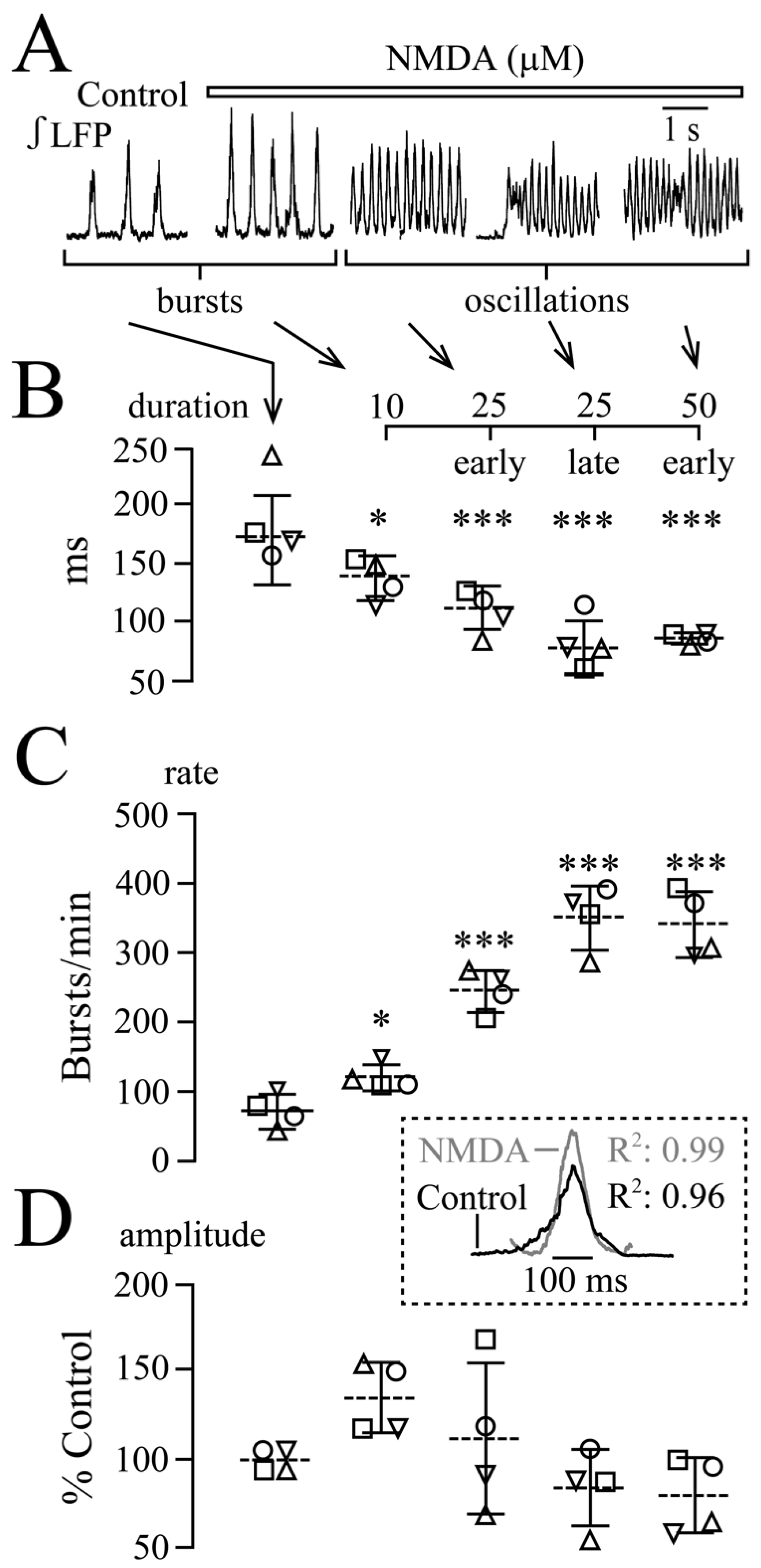
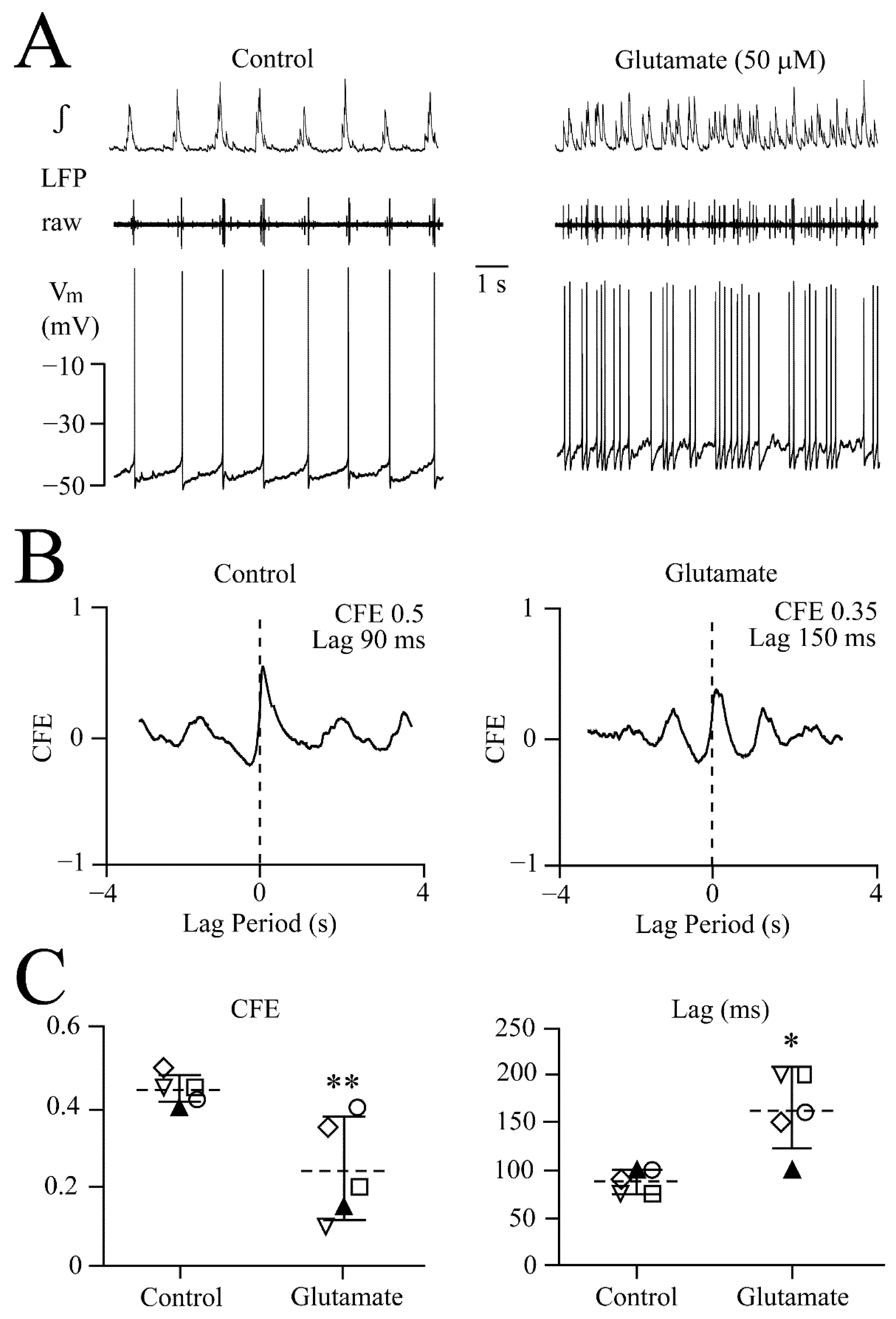
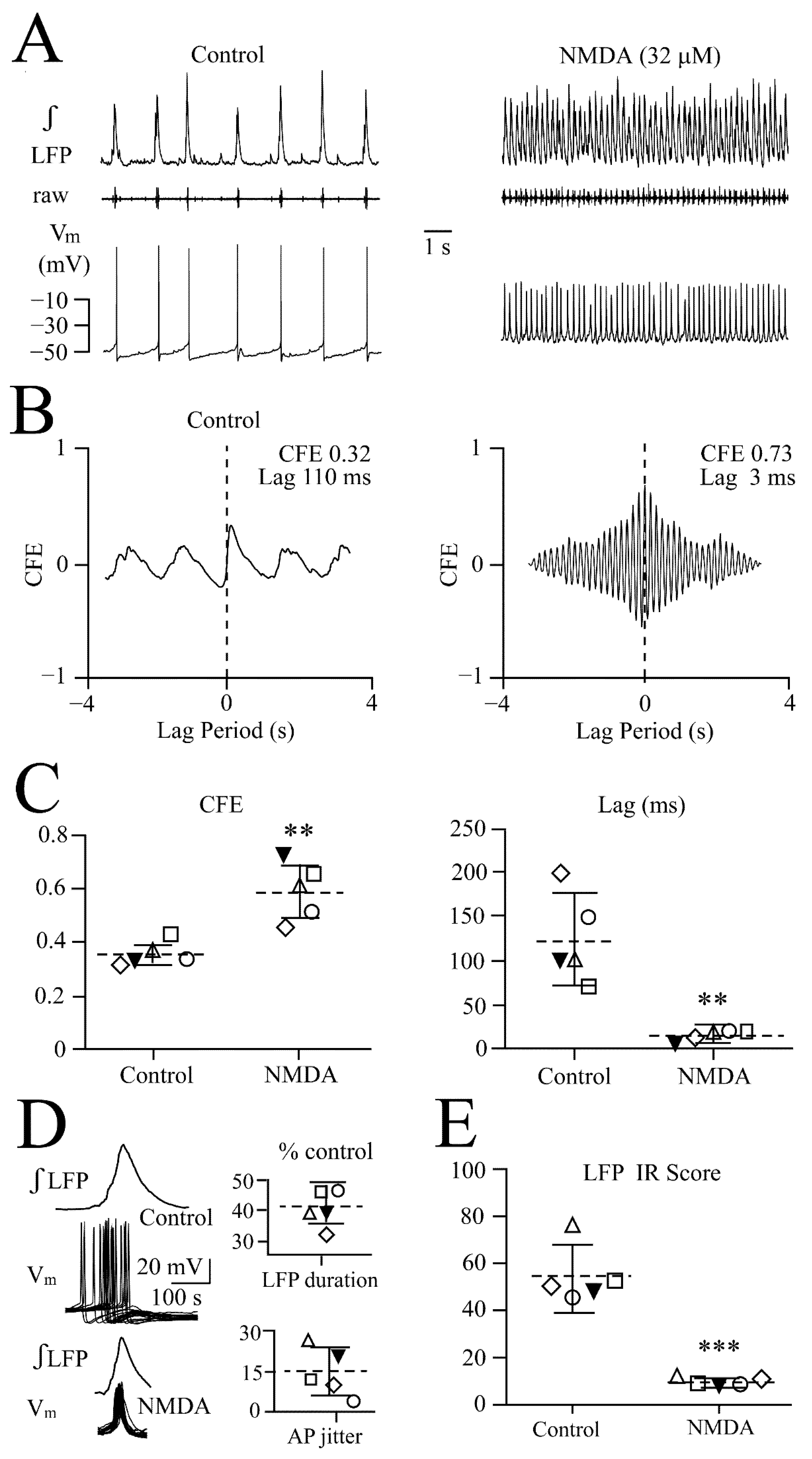
3.3. NMDA and Glutamate Effects on Network Synchrony and Irregularity
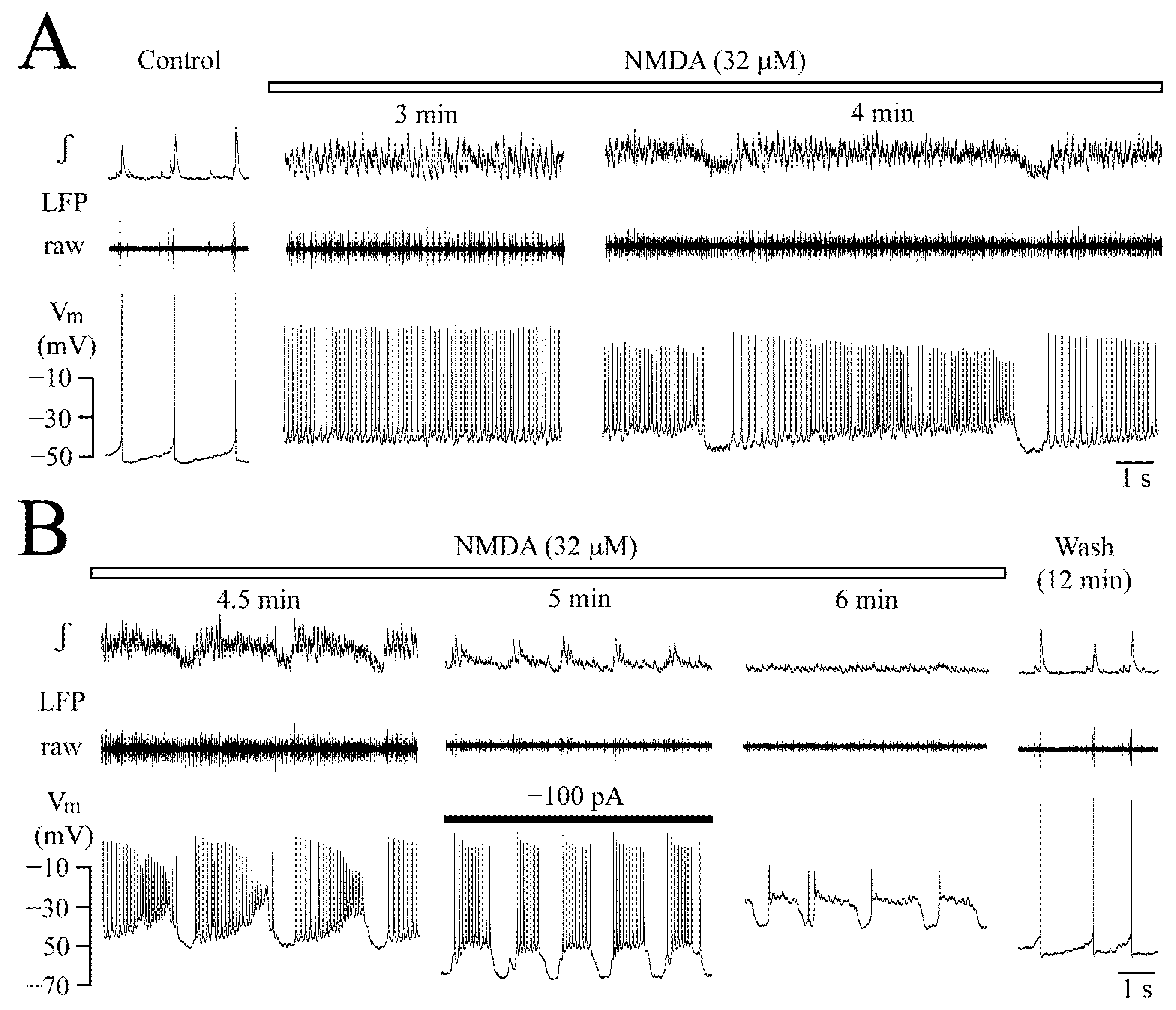
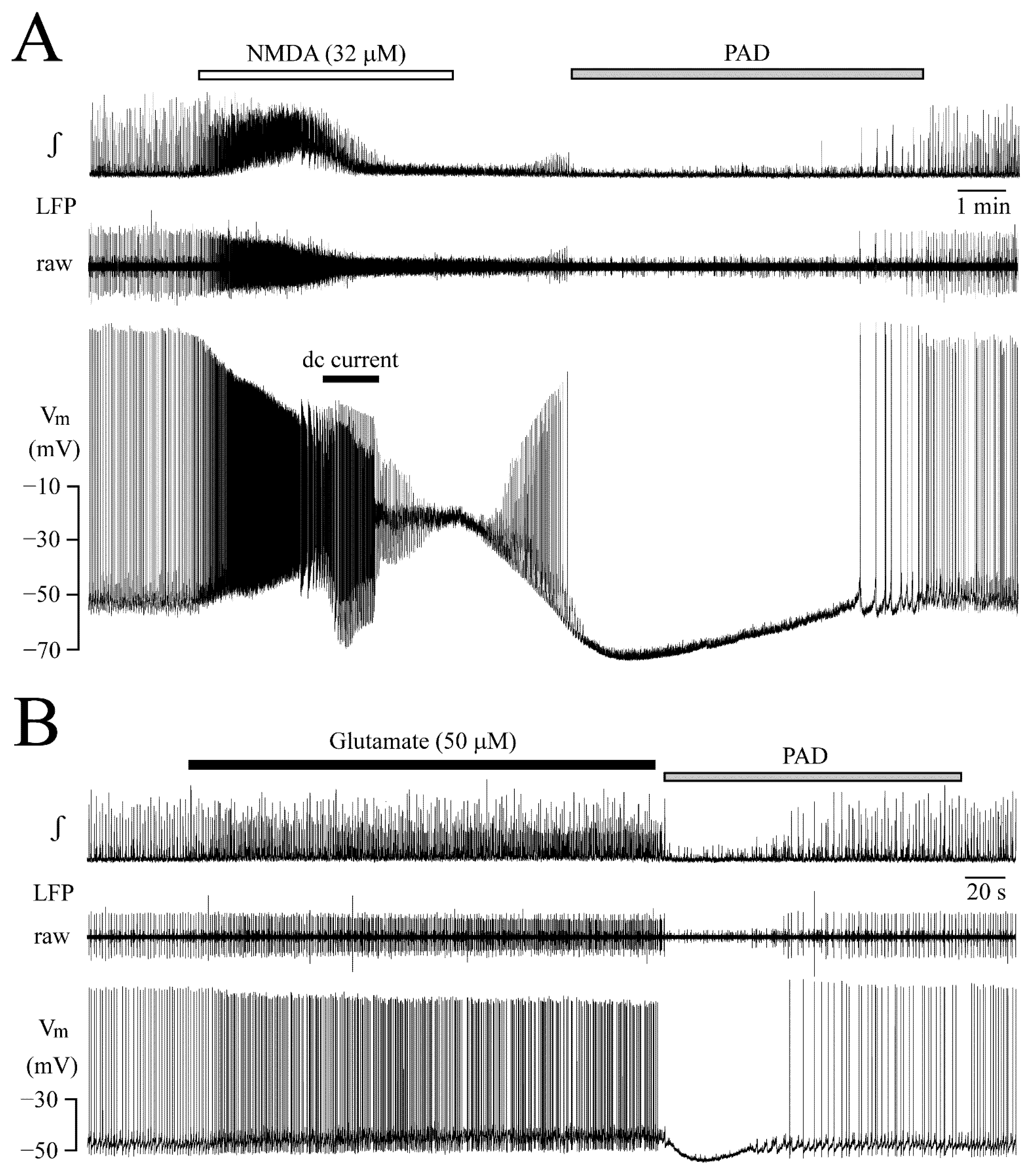
3.4. Vm Changes during NMDA-Evoked Oscillation Trains
3.5. Post-Agonist Depression (PAD)
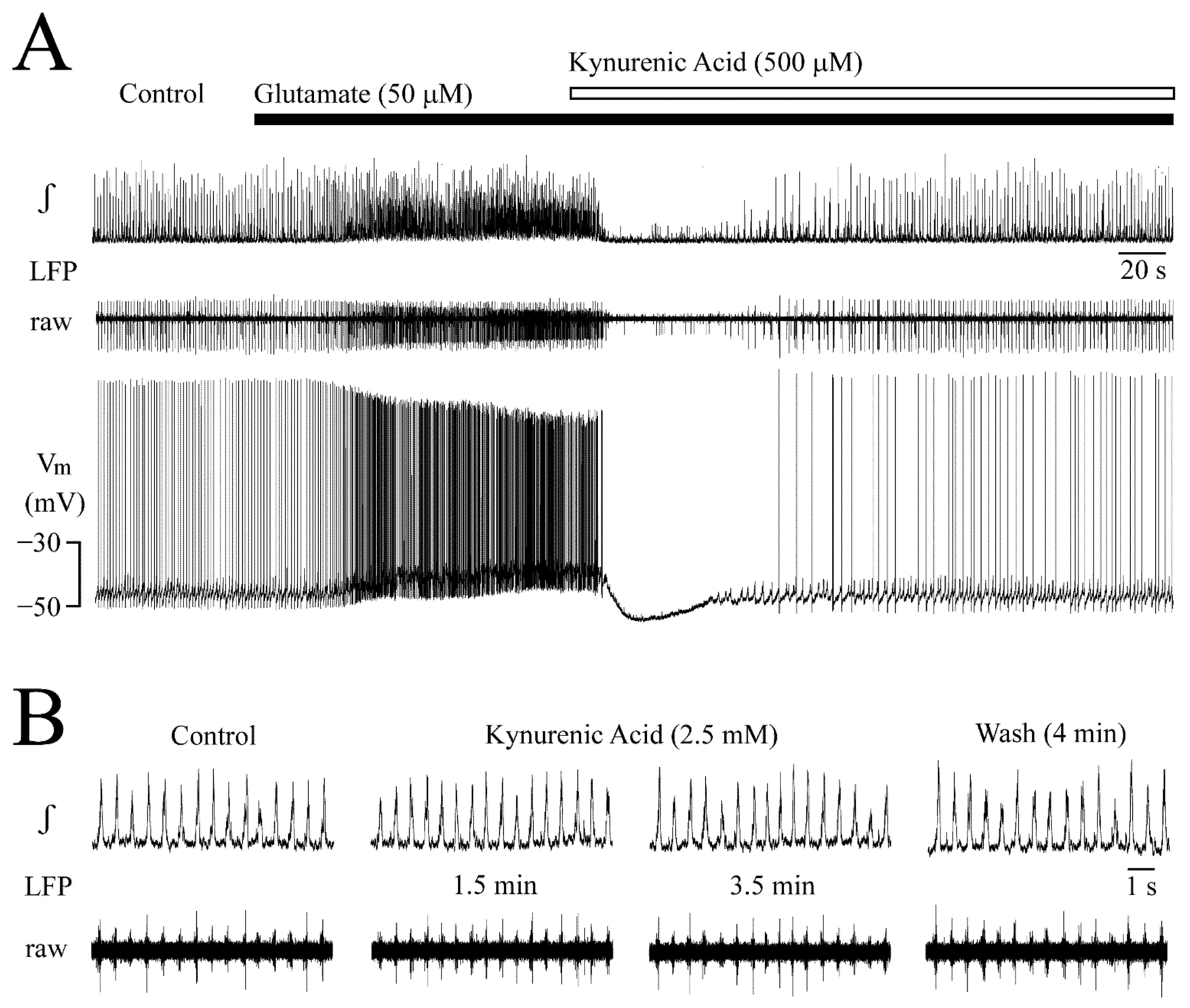
3.6. Receptor Specificity of Glutamate Effects
3.7. Persistence of Single Neuron Spiking during Network Inhibition
4. Discussion
4.1. LFP Recording of LC Discharge Pattern Transformation
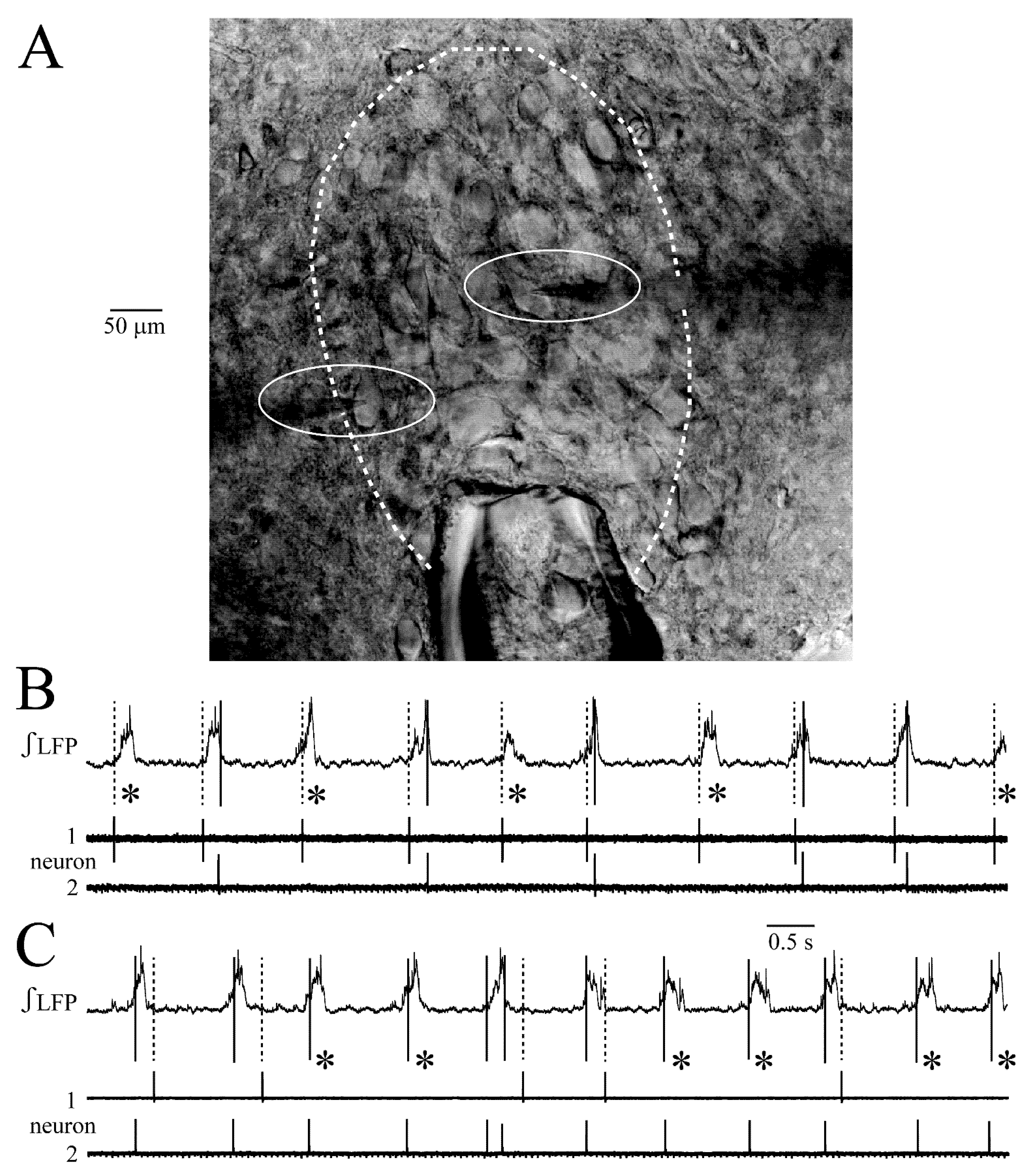
4.2. LC Synchrony Analysis with Combined LFP and Single Neuron Recording
4.3. PAD and Periodic NMDA-Evoked Inhibition
4.4. Potential Role of Modular LC Organization on LFP Pattern Transformation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foote, S.L.; Bloom, F.E.; Aston-Jones, G. Nucleus locus ceruleus: New evidence of anatomical and physiological specificity. Physiol. Rev. 1983, 63, 844–914. [Google Scholar] [CrossRef] [PubMed]
- Berridge, C.W.; Waterhouse, B.D. The locus coeruleus-noradrenergic system: Modulation of behavioral state and state-dependent cognitive processes. Brain Res. Rev. 2003, 42, 33–84. [Google Scholar] [CrossRef]
- Poe, G.R.; Foote, S.; Eschenko, O.; Johansen, J.P.; Bouret, S.; Aston-Jones, G.; Harley, C.W.; Manahan-Vaughan, D.; Weinshenker, D.; Valentino, R.; et al. Locus coeruleus: A new look at the blue spot. Nat. Rev. Neurosci. 2020, 21, 644–659. [Google Scholar] [CrossRef] [PubMed]
- Jodo, E.; Aston-Jones, G. Activation of locus coeruleus by prefrontal cortex is mediated by excitatory amino acid inputs. Brain Res. 1997, 768, 327–332. [Google Scholar] [CrossRef]
- Singewald, N.; Philippu, A. Release of neurotransmitters in the locus coeruleus. Prog. Neurobiol. 1998, 56, 237–267. [Google Scholar] [CrossRef]
- Aston-Jones, G.; Cohen, J.D. Adaptive gain and the role of the locus coeruleus-norepinephrine system in optimal performance. J. Comp. Neurol. 1998, 493, 99–110. [Google Scholar] [CrossRef]
- Schwarz, L.A.; Luo, L. Organization of the locus coeruleus-norepinephrine system. Curr. Biol. 2015, 25, R1051–R1056. [Google Scholar] [CrossRef]
- Chandley, M.J.; Ordway, G.A. Noradrenergic Dysfunction in Depression and Suicide. In The Neurobiological Basis of Suicide; Dwivedi, Y., Ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis: New York, NY, USA, 2012; Chapter 3. [Google Scholar]
- Chandley, M.J.; Szebeni, A.; Szebeni, K.; Crawford, J.D.; Stockmeier, C.A.; Turecki, G.; Kostrzewa, R.M.; Ordway, G.A. Elevated gene expressio.n of glutamate receptors in noradrenergic neurons from the locus coeruleus in major depression. Int. J. Neuropsychopharmacol. 2014, 17, 1569–1578. [Google Scholar] [CrossRef]
- Price, R.B.; Nock, M.K.; Charney, D.S.; Mathew, S.J. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol. Psychiatry 2009, 66, 522–526. [Google Scholar] [CrossRef]
- DiazGranados, N.; Ibrahim, L.A.; Brutsche, N.E.; Ameli, R.; Henter, I.D.; Luckenbaugh, D.A.; Machado-Vieira, R.; Zarate, C.A., Jr. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J. Clin. Psychiatry 2010, 71, 1605–1611. [Google Scholar] [CrossRef]
- Zhu, H.; Zhou, W. Excitatory amino acid receptors are involved in morphine-induced synchronous oscillatory discharges in the locus coeruleus of rats. Eur. J. Pharmacol. 2005, 528, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Herkenham, M.; Nauta, W.J. Efferent connections of the habenular nuclei in the rat. J. Comp. Neurol. 1979, 187, 19–47. [Google Scholar] [CrossRef] [PubMed]
- Aston-Jones, G.; Ennis, M.; Pieribone, V.A.; Nickell, W.T.; Shipley, M.T. The brain nucleus locus coeruleus: Restricted afferent control of a broad efferent network. Science 1986, 234, 734–737. [Google Scholar] [CrossRef] [PubMed]
- Olpe, H.R.; Steinmann, M.; Brugger, F.; Pozza, M. Excitatory amino acid receptors in rat locus coeruleus. Naunyn Schmiedebergs Arch. Pharmacol. 1989, 339, 312–314. [Google Scholar] [CrossRef]
- Kogan, J.H.; Aghajanian, G.K. Long-term glutamate desensitization in locus coeruleus neurons and its role in opiate withdrawal. Brain Res. 1995, 689, 111–121. [Google Scholar] [CrossRef]
- Zamalloa, T.; Bailey, C.P.; Pineda, J. Glutamate-induced post-activation inhibition of locus coeruleus neurons is mediated by AMPA/kainate receptors and sodium-dependent potassium currents. Br. J. Pharmacol. 2009, 156, 649–661. [Google Scholar] [CrossRef]
- Ballanyi, K.; Ruangkittisakul, A. Structure-function analysis of rhythmogenic inspiratory pre-Bötzinger complex networks in “calibrated” newborn rat brainstem slices. Respir. Physiol. Neurobiol. 2009, 168, 158–178. [Google Scholar] [CrossRef]
- Buzsáki, G.; Anastassiou, C.A.; Koch, C. The origin of extracellular fields and currents-EEG, ECoG, LFP and spikes. Nat. Rev. Neurosci. 2012, 13, 407–420. [Google Scholar] [CrossRef]
- Einevoll, G.T.; Kayser, C.; Logothetis, N.K.; Panzeri, S. Modelling and analysis of local field potentials for studying the function of cortical circuits. Nat. Rev. Neurosci. 2013, 14, 770–785. [Google Scholar] [CrossRef]
- Totah, N.K.; Neves, R.M.; Panzeri, S.; Logothetis, N.K.; Eschenko, O. The Locus Coeruleus Is a Complex and Differentiated Neuromodulatory System. Neuron 2018, 99, 1055–1068. [Google Scholar] [CrossRef]
- Kantor, C.; Panaitescu, B.; Kuribayashi, J.; Ruangkittisakul, A.; Jovanovic, I.; Leung, V.; Lee, T.F.; MacTavish, D.; Jhamandas, J.H.; Cheung, P.Y.; et al. Spontaneous neural network oscillations in hippocampus, cortex, and locus coeruleus of newborn rat and piglet brain slices. In Isolated Central Nervous System Circuits; Neuromethods; Ballanyi, K., Ed.; Humana Press: Totowa, NJ, USA, 2012; Volume 73, pp. 315–356. ISBN 978-1-62703-019-9. [Google Scholar]
- Rancic, V.; Rawal, B.; Panaitescu, B.; Ruangkittisakul, A.; Ballanyi, K. Suction electrode recording in locus coeruleus of newborn rat brain slices reveals network bursting comprising summated non-synchronous spiking. Neurosci. Lett. 2018, 671, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Neuman, R.S.; Cherubini, E.; Ben-Ari, Y. Endogenous and network bursts induced by N-methyl-D-aspartate and magnesium free medium in the CA3 region of the hippocampal slice. Neuroscience 1998, 28, 393–399. [Google Scholar] [CrossRef]
- Zhu, Z.T.; Munhall, A.; Shen, K.Z.; Johnson, S.W. Calcium-dependent subthreshold oscillations determine bursting activity induced by N-methyl-D-aspartate in rat subthalamic neurons in vitro. Eur. J. Neurosci. 2004, 19, 1296–1304. [Google Scholar] [CrossRef]
- Sharifullina, E.; Ostroumov, K.; Grandolfo, M.; Nistri, A. N-methyl-D-aspartate triggers neonatal rat hypoglossal motoneurons in vitro to express rhythmic bursting with unusual Mg2+ sensitivity. Neuroscience 2008, 154, 804–820. [Google Scholar] [CrossRef] [PubMed]
- Mrejeru, A.; Wei, A.; Ramirez, J.M. Calcium-activated non-selective cation currents are involved in generation of tonic and bursting activity in dopamine neurons of the substantia nigra pars compacta. J. Physiol. 2011, 589, 2497–2514. [Google Scholar] [CrossRef] [PubMed]
- Ishimatsu, M.; Williams, J.T. Synchronous activity in locus coeruleus results from dendritic interactions in pericoerulear regions. J. Neurosci. 1996, 16, 5196–5204. [Google Scholar] [CrossRef]
- Pearlstein, E.; Ben Mabrouk, F.; Pflieger, J.F.; Vinay, L. Serotonin refines the locomotor-related alternations in the in vitro neonatal rat spinal cord. Eur. J. Neurosci. 2005, 21, 1338–1346. [Google Scholar] [CrossRef]
- Taccola, G.; Olivieri, D.; D’Angelo, G.; Blackburn, P.; Secchia, L.; Ballanyi, K. A1 adenosine receptor modulation of chemically and electrically evoked lumbar locomotor network activity in isolated newborn rat spinal cords. Neuroscience 2012, 222, 191–204. [Google Scholar] [CrossRef]
- Christie, M.J.; Williams, J.T.; North, R.A. Electrical coupling synchronizes subthreshold activity in locus coeruleus neurons in vitro from neonatal rats. J. Neurosci. 1989, 9, 3584–3589. [Google Scholar] [CrossRef]
- Waselenchuk, Q.; Ballanyi, K. Autocrine Neuromodulation and Network Activity Patterns in the Locus Coeruleus of Newborn Rat Slices. Brain Sci. 2022, 12, 437. [Google Scholar] [CrossRef]
- Fietkiewicz, C.; Loparo, K.A.; Wilson, C.G. Drive latencies in hypoglossal motoneurons indicate developmental change in the brainstem respiratory network. J. Neural. Eng. 2011, 8, 065011. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.J., 3rd; Dashevskiy, T.; Khuu, M.A.; Ramirez, J.M. Chronic Intermittent Hypoxia Differentially Impacts Different States of Inspiratory Activity at the Level of the preBötzinger Complex. Front. Physiol. 2017, 8, 571. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.B.; Wollmuth, L.P.; Bowie, D.; Furukawa, H.; Menniti, F.S.; Sobolevsky, A.I.; Swanson, G.T.; Swanger, S.A.; Greger, I.H.; Nakagawa, T.; et al. Structure, Function, and Pharmacology of Glutamate Receptor Ion Channels. Pharmacol. Rev. 2021, 73, 298–487. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, F.; Bockaert, J.; Collingridge, G.L.; Conn, P.J.; Ferraguti, F.; Schoepp, D.D.; Wroblewski, J.T.; Pin, J.P. Metabotropic glutamate receptors: From the workbench to the bedside. Neuropharmacology 2011, 60, 1017–1041. [Google Scholar] [CrossRef] [PubMed]
- Rawal, B.; Rancic, V.; Ballanyi, K. TARP mediation of accelerated and more regular locus coeruleus network bursting in neonatal rat brain slices. Neuropharmacology 2019, 148, 169–177. [Google Scholar] [CrossRef]
- Feng, Y.Z.; Zhang, T.; Rockhold, R.W.; Ho, I.K. Increased locus coeruleus glutamate levels are associated with naloxone-precipitated withdrawal from butorphanol in the rat. Neurochem. Res. 1995, 20, 745–751. [Google Scholar] [CrossRef]
- Pál, B. Involvement of extrasynaptic glutamate in physiological and pathophysiological changes of neuronal excitability. Cell. Mol. Life Sci. 2018, 75, 2917–2949. [Google Scholar] [CrossRef]
- Williams, J.T.; Bobker, D.H.; Harris, G.C. Synaptic potentials in locus coeruleus neurons in brain slices. Prog. Brain Res. 1991, 88, 167–172. [Google Scholar]
- Olpe, H.R.; Steinmann, M.W.; Hall, R.G.; Brugger, F.; Pozza, M.F. GABAA and GABAB receptors in locus coeruleus: Effects of blockers. Eur. J. Pharmacol. 1988, 149, 183–185. [Google Scholar] [CrossRef]
- Alvarez, V.A.; Chow, C.C.; van Bockstaele, E.J.; Williams, J.T. Frequency-dependent synchrony in locus ceruleus: Role of electrotonic coupling. Proc. Natl. Acad. Sci. USA 2002, 99, 4032–4036. [Google Scholar] [CrossRef]
- Williams, J.T.; Marshall, K.C. Membrane properties and adrenergic responses in locus coeruleus neurons of young rats. J. Neurosci. 1987, 7, 3687–3694. [Google Scholar] [CrossRef] [PubMed]
- Christie, M.J. Generators of synchronous activity of the locus coeruleus during development. Semin. Cell. Dev. Biol. 1997, 8, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Heister, D.S.; Hayar, A.; Charlesworth, A.; Yates, C.; Zhou, Y.H.; Garcia-Rill, E. Evidence for electrical coupling in the subcoeruleus (SubC) nucleus. J. Neurophysiol. 2007, 97, 3142–3147. [Google Scholar] [CrossRef] [PubMed]
- Rash, J.E.; Olson, C.O.; Davidson, K.G.V.; Yasumura, T.; Kamasawa, N.; Nagy, J.I. Identification of connexin36 in gap junctions between neurons in rodent locus coeruleus. Neuroscience 2007, 147, 938–956. [Google Scholar] [CrossRef][Green Version]
- Turecek, J.; Yuen, G.S.; Han, V.Z.; Zeng, X.H.; Bayer, K.U.; Welsh, J.P. NMDA receptor activation strengthens weak electrical coupling in mammalian brain. Neuron 2014, 81, 1375–1388. [Google Scholar] [CrossRef][Green Version]
- Cedarbaum, J.M.; Aghajanian, G.K. Noradrenergic neurons of the locus coeruleus: Inhibition by epinephrine and activation by the alpha-antagonist piperoxane. Brain Res. 1976, 112, 413–419. [Google Scholar] [CrossRef]
- Foote, S.L.; Aston-Jones, G.; Bloom, F.E. Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc. Natl. Acad. Sci. USA 1980, 77, 3033–3037. [Google Scholar] [CrossRef]
- Metzger, F.; Kulik, A.; Sendtner, M.; Ballanyi, K. Contribution of Ca2+-permeable AMPA/KA receptors to glutamate-induced Ca2+ rise in embryonic lumbar mtoneurons in situ. J. Neurophysiol. 2000, 83, 50–59. [Google Scholar] [CrossRef][Green Version]
- Aghajanian, G.K.; Vandermaelen, C.P.; Andrade, R. Intracellular studies on the role of calcium in regulating the activity and reactivity of locus coeruleus neurons in vivo. Brain Res. 1983, 273, 237–243. [Google Scholar] [CrossRef]
- Andrade, R.; Aghajanian, G.K. Locus coeruleus activity in vitro: Intrinsic regulation by a calcium-dependent potassium conductance but not alpha 2-adrenoceptors. J. Neurosci. 1984, 4, 161–170. [Google Scholar] [CrossRef]
- Matschke, L.A.; Rinné, S.; Snutch, T.P.; Oertel, W.H.; Dolga, A.M.; Decher, N. Calcium-activated SK potassium channels are key modulators of the pacemaker frequency in locus coeruleus neurons. Mol. Cell. Neurosci. 2018, 88, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Matschke, L.A.; Bertoune, M.; Roeper, J.; Snutch, T.P.; Oertel, W.H.; Rinné, S.; Decher, N. A concerted action of L- and T-type Ca2+ channels regulates locus coeruleus pacemaking. Mol. Cell. Neurosci. 2015, 68, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hickey, L.; Perrins, R.; Werlen, E.; Patel, A.A.; Hirschberg, S.; Jones, M.W.; Salinas, S.; Kremer, E.J.; Pickering, A.E. Retrograde optogenetic characterization of the pontospinal module of the locus coeruleus with a canine adenoviral vector. Brain Res. 2016, 1641, 274–290. [Google Scholar] [CrossRef]
- Jin, X.; Li, S.; Bondy, B.; Zhong, W.; Oginsky, M.F.; Wu, Y.; Johnson, C.M.; Zhang, S.; Cui, N.; Jiang, C. Identification of a group of GABAergic neurons in the dorsomedial area of the locus coeruleus. PLoS ONE 2016, 11, e0146470. [Google Scholar] [CrossRef] [PubMed]
- Uematsu, A.; Tan, B.Z.; Ycu, E.A.; Cuevas, J.S.; Koivumaa, J.; Junyent, F.; Kremer, E.J.; Witten, I.B.; Deisseroth, K.; Johansen, J.P. Modular organization of the brainstem noradrenaline system coordinates opposing learning states. Nat. Neurosci. 2017, 20, 1602–1611. [Google Scholar] [CrossRef]
- Tang, F.; Lane, S.; Korsak, A.; Paton, J.F.; Gourine, A.V.; Kasparov, S.; Teschemacher, A.G. Lactate-mediated glia-neuronal signalling in the mammalian brain. Nat. Commun. 2014, 5, 3284. [Google Scholar] [CrossRef]
- Marzo, A.; Totah, N.K.; Neves, R.M.; Logothetis, N.K.; Eschenko, O. Unilateral electrical stimulation of rat locus coeruleus elicits bilateral response of norepinephrine neurons and sustained activation of medial prefrontal cortex. J. Neurophysiol. 2014, 111, 2570–2588. [Google Scholar] [CrossRef]
- Safaai, H.; Neves, R.; Eschenko, O.; Logothetis, N.K.; Panzeri, S. Modeling the effect of locus coeruleus firing on cortical state dynamics and single-trial sensory processing. Proc. Natl. Acad. Sci. USA 2015, 112, 12834–12839. [Google Scholar] [CrossRef]
- Eschenko, O.; Magri, C.; Panzeri, S.; Sara, S.J. Noradrenergic Neurons of the locus coeruleus are phase locked to cortical up-down states during sleep. Cereb. Cortex 2012, 22, 426–435. [Google Scholar] [CrossRef]
- Totah, N.K.; Logothetis, N.K.; Eschenko, O. Synchronous spiking associated with prefrontal high γ oscillations evokes a 5-Hz rhythmic modulation of spiking in locus coeruleus. J. Neurophysiol. 2021, 125, 1191–1201. [Google Scholar] [CrossRef]
- Noei, S.; Zouridis, I.S.; Logothetis, N.K.; Panzeri, S.; Totah, N.K. Distinct ensembles in the noradrenergic locus coeruleus are associated with diverse cortical states. Proc. Natl. Acad. Sci. USA 2022, 119, e2116507119. [Google Scholar] [CrossRef] [PubMed]
- Brofiga, M.; Pisano, M.; Raiteri, R.; Massobrio, P. On the Road to the Brain-on-a-Chip: A Review on Strategies, Methods, and Applications. J. Neural Eng. 2021, 18, 041005. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rawal, B.; Rancic, V.; Ballanyi, K. NMDA Enhances and Glutamate Attenuates Synchrony of Spontaneous Phase-Locked Locus Coeruleus Network Rhythm in Newborn Rat Brain Slices. Brain Sci. 2022, 12, 651. https://doi.org/10.3390/brainsci12050651
Rawal B, Rancic V, Ballanyi K. NMDA Enhances and Glutamate Attenuates Synchrony of Spontaneous Phase-Locked Locus Coeruleus Network Rhythm in Newborn Rat Brain Slices. Brain Sciences. 2022; 12(5):651. https://doi.org/10.3390/brainsci12050651
Chicago/Turabian StyleRawal, Bijal, Vladimir Rancic, and Klaus Ballanyi. 2022. "NMDA Enhances and Glutamate Attenuates Synchrony of Spontaneous Phase-Locked Locus Coeruleus Network Rhythm in Newborn Rat Brain Slices" Brain Sciences 12, no. 5: 651. https://doi.org/10.3390/brainsci12050651
APA StyleRawal, B., Rancic, V., & Ballanyi, K. (2022). NMDA Enhances and Glutamate Attenuates Synchrony of Spontaneous Phase-Locked Locus Coeruleus Network Rhythm in Newborn Rat Brain Slices. Brain Sciences, 12(5), 651. https://doi.org/10.3390/brainsci12050651




