Dopamine Function and Hypothalamic-Pituitary-Thyroid Axis Activity in Major Depressed Patients with Suicidal Behavior
Abstract
:1. Introduction
2. Materials and Methods
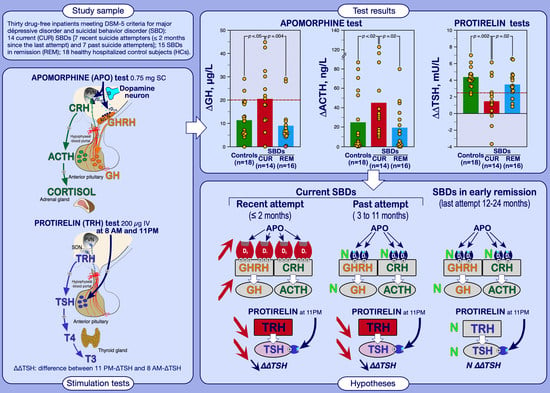
2.1. Subjects
2.2. Procedures
2.3. Assays
2.4. Data Analysis
2.5. Statistical Analyses
3. Results
3.1. Comparison between Healthy Control Subjects and Depressed Patients with Suicidal Behavior Subgrouped according to DSM-5 Specifiers (i.e., Current or in Early Remission)
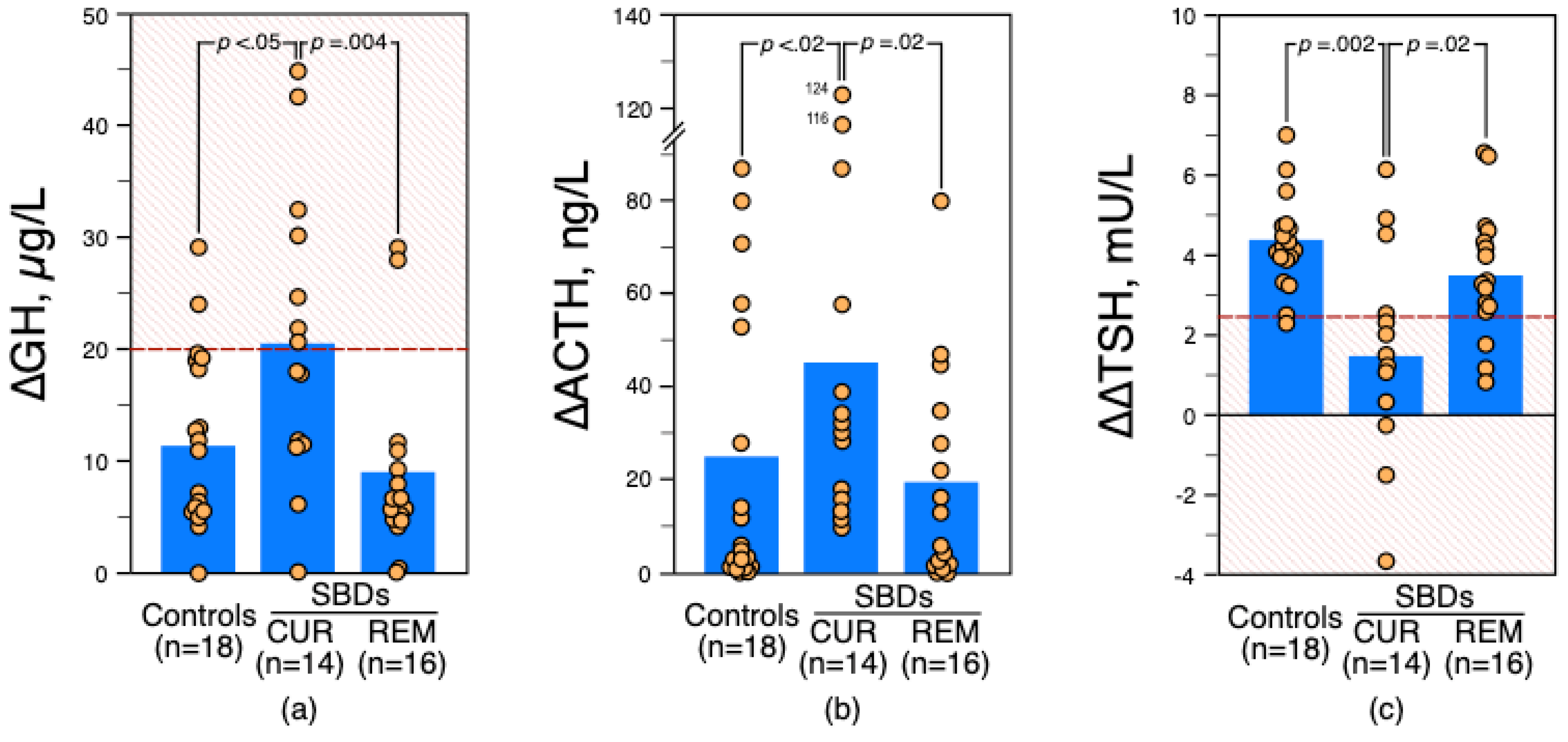
3.1.1. Apomorphine Test Results
3.1.2. TRH 8 AM and 11 PM Test Results
3.1.3. Dexamethasone Suppression Test
3.2. Cross Correlations between Hormonal Responses to Apomorphine and TRH Tests in Patients and Controls
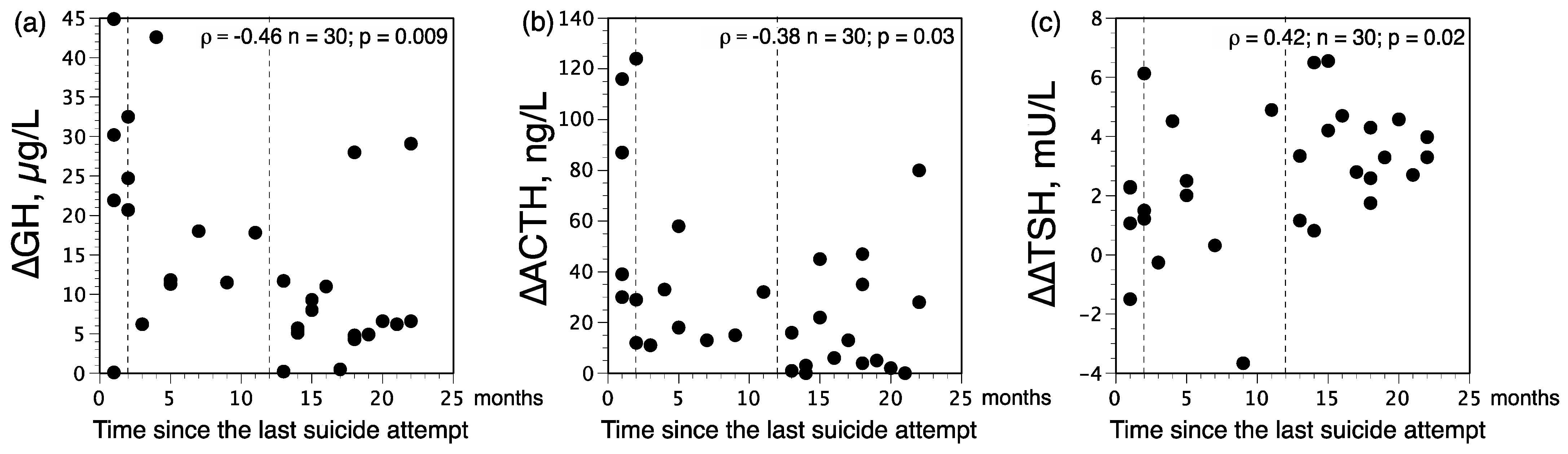
3.3. Distribution of ∆GH and ∆∆TSH Status among Patients according to the Time Elapsed since the Last Suicide Attempt
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Oquendo, M.A.; Sullivan, G.M.; Sudol, K.; Baca-Garcia, E.; Stanley, B.H.; Sublette, M.E.; Mann, J.J. Toward a biosignature for suicide. Am. J. Psychiatry 2014, 171, 1259–1277. [Google Scholar] [CrossRef] [PubMed]
- Sudol, K.; Mann, J.J. Biomarkers of suicide attempt behavior: Towards a biological model of risk. Curr. Psychiatry Rep. 2017, 19, 31. [Google Scholar] [CrossRef] [PubMed]
- Loosen, P.T.; Prange, A.J., Jr. Serum thyrotropin response to thyrotropin-releasing hormone in psychiatric patients: A review. Am. J. Psychiatry 1982, 139, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Duval, F.; Mokrani, M.C.; Erb, A.; Gonzalez Lopera, F.G.; Calleja, C.; Paris, V. Relationship between chronobiological thyrotropin and prolactin responses to protirelin (TRH) and suicidal behavior in depressed patients. Psychoneuroendocrinology 2017, 85, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Duval, F.; Mokrani, M.C.; Erb, A.; Danila, V.; Gonzalez Lopera, F.; Foucher, J.; Jeanjean, L. Thyroid activity and dopamine function in depression. Psychoneuroendocrinology 2021, 128, 105219. [Google Scholar] [CrossRef]
- Pitchot, W.; Hansenne, M.; Gonzalez Moreno, A.; Pinto, E.; Reggers, J.; Fuchs, S.; Pirard, S.; Ansseau, M. Reduced dopamine function in depressed patients is related to suicidal behavior but not its lethality. Psychoneuroendocrinology 2001, 26, 689–696. [Google Scholar] [CrossRef]
- Fitzgerald, M.L.; Kassir, S.A.; Underwood, M.D.; Bakalian, M.J.; Mann, J.J.; Arango, V. Dysregulation of striatal dopamine receptor binding in suicide. Neuropsychopharmacology 2017, 42, 974–982. [Google Scholar] [CrossRef] [Green Version]
- Hoertel, N.; Cipel, H.; Blanco, C.; Oquendo, M.A.; Ellul, P.; Leaune, E.; Limosin, F.; Peyre, H.; Costemale-Lacoste, J.F. Cerebrospinal fluid levels of monoamines among suicide attempters: A systematic review and random-effects meta-analysis. J. Psychiatr. Res. 2021, 136, 224–235. [Google Scholar] [CrossRef]
- Ohmori, T.; Arora, R.C.; Meltzer, H.Y. Serotonergic measures in suicide brain: The concentration of 5-HIAA, HVA, and tryptophan in frontal cortex of suicide victims. Biol. Psychiatry 1992, 32, 57–71. [Google Scholar] [CrossRef]
- Bowden, C.; Theodorou, A.E.; Cheetham, S.C.; Lowther, S.; Katona, C.L.; Crompton, M.R.; Horton, R.W. Dopamine D1 and D2 receptor binding sites in brain samples from depressed suicides and controls. Brain Res. 1997, 752, 227–233. [Google Scholar] [CrossRef]
- Laasonen-Balk, T.; Kuikka, J.; Viinamäki, H.; Husso-Saastamoinen, M.; Lehtonen, J.; Tiihonen, J. Striatal dopamine transporter density in major depression. Psychopharmacology 1999, 144, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Pitchot, W.; Hansenne, M.; Moreno, A.G.; Ansseau, M. Suicidal behavior and growth hormone response to apomorphine test. Biol. Psychiatry 1992, 31, 1213–1219. [Google Scholar] [CrossRef]
- Allard, P.; Norlén, M. Caudate nucleus dopamine D (2) receptors in depressed suicide victims. Neuropsychobiology 2001, 44, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Duval, F.; Mokrani, M.C.; Monreal, J.; Weiss, T.; Fattah, S.; Hamel, B.; Macher, J.P. Interaction between the serotonergic system and HPA and HPT axes in patients with major depression: Implications for pathogenesis of suicidal behavior. Dialogues Clin. Neurosci. 2002, 4, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Pettorruso, M.; d’Andrea, G.; Martinotti, G.; Cocciolillo, F.; Miuli, A.; Di Muzio, I.; Collevecchio, R.; Verrastro, V.; De-Giorgio, F.; Janiri, L.; et al. Hopelessness, Dissociative Symptoms, and Suicide Risk in Major Depressive Disorder: Clinical and Biological Correlates. Brain Sci. 2020, 10, 519. [Google Scholar] [CrossRef]
- Pizzagalli, D.A.; Berretta, S.; Wooten, D.; Goer, F.; Pilobello, K.T.; Kumar, P.; Murray, L.; Beltzer, M.; Boyer-Boiteau, A.; Alpert, N.; et al. Assessment of striatal dopamine transporter binding in individuals with major depressive disorder: In vivo positron emission tomography and postmortem evidence. JAMA Psychiatry 2019, 76, 854–861. [Google Scholar] [CrossRef]
- Duval, F.; Mokrani, M.C. Thyroid Axis Activity in Depression. Ann. Thyroid Res. 2018, 4, 166–171. [Google Scholar]
- Duval, F.; Mokrani, M.C.; Gonzalez Lopera, F.G.; Diep, T.S.; Rabia, H.; Fattah, S. Thyroid axis activity and suicidal behavior in depressed patients. Psychoneuroendocrinology 2010, 35, 1045–1054. [Google Scholar] [CrossRef]
- Duval, F.; Macher, J.P.; Mokrani, M.C. Difference between evening and morning thyrotropin response to protirelin in major depressive episode. Arch. Gen. Psychiatry 1990, 47, 443–448. [Google Scholar] [CrossRef]
- Mokrani, M.C.; Duval, F.; Erb, A.; Gonzalez Lopera, F.; Danila, V. Are the thyroid and adrenal system alterations linked in depression? Psychoneuroendocrinology 2020, 122, 104831. [Google Scholar] [CrossRef]
- Peng, R.; Dai, W.; Li, Y. Low serum free thyroxine level is correlated with lipid profile in depressive patients with suicide attempt. Psychiatry Res. 2018, 266, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Butkute-Sliuoziene, K.; Berentaite, B.; Steibliene, V. Thyroid Axis Functioning in patients with high suicide risk. Ann. Thyroid Res. 2018, 4, 141–145. [Google Scholar]
- Alkemade, A.; Unmehopa, U.A.; Wiersinga, W.M.; Swaab, D.F.; Fliers, E. Glucocorticoids decrease thyrotropin-releasing hormone messenger ribonucleic acid expression in the paraventricular nucleus of the human hypothalamus. J. Clin. Endocrinol. Metab. 2005, 90, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Gary, K.A.; Sevarino, K.A.; Yarbrough, G.G.; Prange, A.J., Jr.; Winokur, A. The thyrotropin-releasing hormone (TRH) hypothesis of homeostatic regulation: Implications for TRH-based therapeutics. J. Pharmacol Exp. Ther. 2003, 305, 410–416. [Google Scholar] [CrossRef] [Green Version]
- Duval, F.; Mokrani, M.C.; Bailey, P.; Correa, H.; Diep, T.S.; Crocq, M.A.; Macher, J.P. Thyroid axis activity and serotonin function in major depressive episode. Psychoneuroendocrinology 1999, 24, 695–712. [Google Scholar] [CrossRef]
- Corrêa, H.; Duval, F.; Mokrani, M.C.; Bailey, P.; Trémeau, F.; Staner, L.; Diep, T.S.; Hodé, Y.; Crocq, M.A.; Macher, J.P. Prolactin response to d-fenfluramine and suicidal behavior in depressed patients. Psychiatry Res. 2000, 93, 189–199. [Google Scholar] [CrossRef]
- Przegaliński, E.; Jaworska, L.; Budziszewska, B. The role of dopamine receptors in the release of thyrotropin-releasing hormone from the rat striatum and nucleus accumbens: An in vitro study. Neuropeptides 1993, 25, 277–282. [Google Scholar] [CrossRef]
- Krulich, L.; Giachetti, A.; Marchlewska-Koj, A.; Hefco, E.; Jameson, H.E. On the role of the central noradrenergic and dopaminergic systems in the regulation of TSH secretion in the rat. Endocrinology 1977, 100, 496–505. [Google Scholar] [CrossRef]
- Scanlon, M.F.; Weightman, D.R.; Shale, D.J.; Mora, B.; Heath, M.; Snow, M.H.; Lewis, M.; Hall, R. Dopamine is a physiological regulator of thyrotrophin (TSH) secretion in normal man. Clin. Endocrinol. 1979, 10, 7–15. [Google Scholar] [CrossRef]
- Kalivas, P.W.; Stanley, D.; Prange, A.J., Jr. Interaction between thyrotropin-releasing hormone and the mesolimbic dopamine system. Neuropharmacology 1987, 26, 33–38. [Google Scholar] [CrossRef]
- Kreutz, M.R.; Acworth, N.; Lehnert, H.; Wurtman, R.J. Systemic administration of thyrotropin-releasing hormone enhances striatal dopamine release in vivo. Brain Res. 1990, 536, 347–352. [Google Scholar] [CrossRef]
- Puga, L.; Alcántara-Alonso, V.; Coffeen, U.; Jaimes, O.; de Gortari, P. TRH injected into the nucleus accumbens shell releases dopamine and reduces feeding motivation in rats. Behav. Brain Res. 2016, 306, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Lal, S. Apomorphine in the evaluation of dopaminergic function in man. Prog. Neuro Psychopharmacol. Biol. Psychiatry 1988, 12, 117–164. [Google Scholar] [CrossRef]
- Mokrani, M.C.; Duval, F.; Crocq, M.A.; Bailey, P.E.; Macher, J.P. Multihormonal responses to apomorphine in mental illness. Psychoneuroendocrinology 1995, 20, 365–375. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5), 5th ed.; Psychiatric Publishing: Washington, DC, USA, 2013; pp. 160–168. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5), 5th ed.; Psychiatric Publishing: Washington, DC, USA, 2013; pp. 801–803. [Google Scholar]
- Hamilton, M.A. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endicott, J.; Andreasen, N.; Spitzer, R.L. Family History—Research Diagnostic Criteria; Research Assessment and Training Unit; New York State Psychiatric Institute: New York, NY, USA, 1978. [Google Scholar]
- Carroll, B.J.; Feinberg, M.; Greden, J.F.; Tarika, J.; Albala, A.A.; Hasket, R.F.; James, N.M.; Kronfol, Z.; Lohr, N.; Steiner, M.; et al. A specific laboratory test for the diagnosis of melancholia: Standardisation, validation, and clinical utility. Arch. Gen. Psychiatry 1981, 38, 15–22. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.r-project.org/ (accessed on 18 May 2021).
- Metz, C.E. Basic principles of ROC analysis. Semin. Nucl. Med. 1978, 8, 283–298. [Google Scholar] [CrossRef]
- Zimmerman, M.; Martinez, J.H.; Young, D.; Chelminski, I.; Dalrymple, K. Severity classification on the Hamilton Depression Rating Scale. J. Affect. Disord. 2013, 150, 384–388. [Google Scholar] [CrossRef]
- Duval, F.; Mokrani, M.C.; Monreal-Ortiz, J.A.; Fattah, S.; Champeval, C.; Schulz, P.; Macher, J.P. Cortisol hypersecretion in unipolar major depression with melancholic and psychotic features: Dopaminergic, noradrenergic and thyroid correlates. Psychoneuroendocrinology 2006, 31, 876–888. [Google Scholar] [CrossRef]
- Jezova, D.; Vigas, M. Apomorphine injection stimulates beta-endorphin, adrenocorticotropin, and cortisol release in healthy man. Psychoneuroendocrinology 1988, 13, 479–485. [Google Scholar] [CrossRef]
- Wood, D.F.; Johnston, J.M.; Johnston, D.G. Dopamine. the dopamine D2 receptor and pituitary tumour. Clin. Endocrinol. 1991, 35, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Pivonello, R.; Waaijers, M.; Kros, J.M.; Pivonello, C.; de Angelis, C.; Cozzolino, A.; Colao, A.; Lamberts, S.W.J.; Hofland, L.J. Dopamine D2 receptor expression in the corticotroph cells of the human normal pituitary gland. Endocrine 2017, 57, 314–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Creese, I.; Burt, D.R.; Snyder, S.H. Dopamine receptor binding enhancement accompanies lesion-induced behavioral supersensitivity. Science 1977, 197, 596–598. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K.; Marshall, A.M.; Varmuza, S.L. Supersensitivity in rat caudate nucleus: Effects of 6-hydroxydopamine on the time course of dopamine receptor and cyclic AMP changes. Brain Res. 1980, 200, 47–57. [Google Scholar] [CrossRef]
- Savitz, J.B.; Drevets, W.C. Neuroreceptor imaging in depression. Neurobiol. Dis. 2013, 52, 49–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grace, A.A. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat. Rev. Neurosci. 2016, 17, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Kvernmo, T.; Hartter, S.; Burger, E. A review of the receptor-binding and pharmacokinetic properties of dopamine agonists. Clin. Ther. 2006, 28, 1065–1078. [Google Scholar]
- Duval, F. Endocrinologie et psychiatrie. In Encyclopédie Médico Chirurgicale Psychiatrie; 37-640-A-10; Elsevier: Paris, France, 2016; 20. [Google Scholar]
- Patel, P.A.; Patel, V.R. Formulation and evaluation of anti-suicidal nasal spray of Thyrotropin releasing hormone. Pharmaceut Anal. Acta 2014, 5, 3. [Google Scholar] [CrossRef]
- Wilkinson, S.T.; Ballard, E.D.; Bloch, M.H.; Mathew, S.J.; Murrough, J.W.; Feder, A.; Sos, P.; Wang, G.; Zarate, C.A., Jr.; Sanacora, G. The Effect of a Single Dose of Intravenous Ketamine on Suicidal Ideation: A Systematic Review and Individual Participant Data Meta-Analysis. Am. J. Psychiatry 2018, 175, 150–158. [Google Scholar] [CrossRef]
- Pekary, A.E.; Sattin, A.; Lloyd, R.L. Ketamine modulates TRH and TRH-like peptide turnover in brain and peripheral tissues of male rats. Peptides 2015, 69, 66–76. [Google Scholar] [CrossRef]
- Kokkinou, M.; AH Ashok, A.H.; Howes, O.D. The effects of ketamine on dopaminergic function: Meta-analysis and review of the implications for neuropsychiatric disorders. Mol. Psychiatry 2018, 23, 59–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Argyropoulos, S.V.; Nutt, D.J. Anhedonia revisited: Is there a role for dopamine-targeting drugs for depression? Psychopharmacol 2013, 27, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Torrisi, S.A.; Leggio, G.M.; Drago, F.; Salomone, S. Therapeutic challenges of post-traumatic stress disorder: Focus on the dopaminergic system. Front. Pharmacol. 2019, 10, 404. [Google Scholar] [CrossRef] [PubMed]
| Control Subjects (n = 18) | SBD-REM (n = 16) | SBD-CUR (n = 14) | |
|---|---|---|---|
| Age, years a | 37.8 ± 7.8 | 36.9 ± 11.4 | 33.0 ± 6.9 |
| Gender | 9 M/9 F | 5 M/11 F | 7 M/7 F |
| HAM-D | … | 26.4 ± 4.5 | 28.0 ± 4.3 |
| Melancholic features (n) | … | 4 | 4 |
| TSA, months | … | 17.2 ± 3.1 | 3.9 ± 3.2 ††† |
| NSA | … | 1.4 ± 0.6 | 1.3 ± 0.6 |
| 8 AM and 11 PM TRH tests | |||
| 8 AM-FT4BL, pmol/L | 13.6 ± 1.5 | 14.5 ± 3.0 | 12.3 ± 2.1 * |
| 8 AM-FT3BL, pmol/L | 5.1 ± 0.4 | 5.5 ± 0.7 | 5.3 ± 0.8 |
| 8 AM-TSHBL, mU/L | 1.23 ± 0.44 | 1.42 ± 0.69 | 1.10 ± 0.38 |
| 8 AM-∆TSH, mU/L | 9.32 ± 4.41 | 8.83 ± 3.61 | 7.30 ± 3.60 |
| 11 PM-TSHBL, mU/L | 1.33 ± 0.75 | 1.12 ± 0.52 | 0.75 ± 0.29 * |
| 11 PM-∆TSH, mU/L | 13.66 ± 4.94 | 12.35 ± 3.67 | 9.00 ± 3.93 **† |
| ∆∆TSH, mU/L | 4.31 ± 1.16 | 3.54 ± 1.63 | 1.67 ± 2.55 ***† |
| Apomorphine test | |||
| ACTHBL (ng/L) | 23.0 ± 17.1 | 20.0 ± 8.6 | 30.9 ± 19.9 |
| ∆ACTH (ng/L) | 24.8 ± 30.1 | 19.2 ± 22.7 | 44.1 ± 38.2 *† |
| CORBL (nmol/L) | 282 ± 110 | 321 ±114 | 251 ± 77 |
| ∆COR (nmol/L) | 176 ± 181 | 111 ± 131 | 167 ± 124 |
| GHBL (µg/L) | 0.6 ± 0.3 | 0.7 ± 0.3 | 0.7 ± 0.4 |
| ∆GH (µg/L) | 12.0 ± 7.4 | 8.9 ± 8.3 | 21.0 ± 13.0 *††† |
| Maximum post-DST | |||
| COR, nmol/L | 44 ± 56 | 85 ± 103 | 66 ± 62 |
| Regression Coefficients (Standard Errors); p | Odds Ratios | 95% CI Low-High | Overall Model Fit χ2; df; p | |
|---|---|---|---|---|
| ∆ACTH | 0.03 (0.02); 0.065 | 1.03 | 0.99–1.06 | 4.92; 1; 0.027 |
| ∆COR | 0.004 (0.003); 0.23 | 1.00 | 0.99–1.01 | 1.53; 1; 0.22 |
| ∆GH | 0.11 (0.05); 0.017 | 1.12 | 1.02–1.22 | 8.63; 1; 0.003 |
| Normal/High 1 | 1.94 (0.92); 0.035 | 7.00 | 1.14–44.97 | 5.19; 1; 0.023 |
| FT4BL | −0.32 (0.16); 0.048 | 0.72 | 0.52–0.99 | 4.83; 1; 0.028 |
| 8 AM-∆TSH | −0.13 (0.11); 0.25 | 0.88 | 0.71–1.09 | 1.41; 1; 0.23 |
| 11 PM-∆TSH | −024 (0.11); 0.033 | 0.79 | 0.63–0.98 | 5.55; 1; 0.019 |
| ∆∆TSH | −0.46 (0.22); 0.04 | 0.63 | 0.40–0.98 | 5.77; 1; 0.016 |
| Normal/Low 2 | 2.77 (0.91); 0.0025 | 15.89 | 2.65–95.21 | 11.46; 1; 0.0007 |
| 11PM-∆TSH | ∆∆TSH | ∆ACTH | ∆COR | ∆GH | COR Post-DST | |
|---|---|---|---|---|---|---|
| 8 AM-∆TSH | ||||||
| Controls | 0.93 *** | 0.29 | 0.10 | 0.22 | −0.04 | −0.34 |
| SBDs-CUR | 0.83 *** | 0.07 | 0.35 | 0.03 | 0.34 | 0.16 |
| SBDs-REM | 0.90 *** | 0.14 | −0.14 | 0.14 | −0.15 | −0.39 |
| 11 PM-∆TSH | ||||||
| Controls | 0.53 * | 0.02 | 0.14 | −0.09 | −0.09 | |
| SBDs-CUR | 0.54 * | 0.30 | 0.17 | 0.41 | −0.11 | |
| SBDs-REM | 0.22 | −0.24 | 0.03 | −0.07 | −0.34 | |
| ∆∆TSH | ||||||
| Controls | 0.09 | 0.11 | −0.05 | −0.10 | ||
| SBDs-CUR | −0.07 | −0.01 | 0.28 | −0.07 | ||
| SBDs-REM | 0.01 | 0.07 | 0.34 | 0.01 | ||
| ∆ACTH | ||||||
| Controls | 0.72 *** | 0.46 * | −0.14 | |||
| SBDs-CUR | 0.48 | 0.51 | 0.09 | |||
| SBDs-REM | 0.69 ** | 0.45 | −0.10 | |||
| ∆COR | ||||||
| Controls | 0.41 | 0.12 | ||||
| SBDs-CUR | 0.35 | 0.04 | ||||
| SBDs-REM | 0.42 | −0.11 | ||||
| ∆GH | ||||||
| Controls | −0.32 | |||||
| SBDs-CUR | −0.07 | |||||
| SBDs-REM | −0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duval, F.; Mokrani, M.-C.; Danila, V.; Erb, A.; Gonzalez Lopera, F.; Tomsa, M. Dopamine Function and Hypothalamic-Pituitary-Thyroid Axis Activity in Major Depressed Patients with Suicidal Behavior. Brain Sci. 2022, 12, 621. https://doi.org/10.3390/brainsci12050621
Duval F, Mokrani M-C, Danila V, Erb A, Gonzalez Lopera F, Tomsa M. Dopamine Function and Hypothalamic-Pituitary-Thyroid Axis Activity in Major Depressed Patients with Suicidal Behavior. Brain Sciences. 2022; 12(5):621. https://doi.org/10.3390/brainsci12050621
Chicago/Turabian StyleDuval, Fabrice, Marie-Claude Mokrani, Vlad Danila, Alexis Erb, Felix Gonzalez Lopera, and Mihaela Tomsa. 2022. "Dopamine Function and Hypothalamic-Pituitary-Thyroid Axis Activity in Major Depressed Patients with Suicidal Behavior" Brain Sciences 12, no. 5: 621. https://doi.org/10.3390/brainsci12050621
APA StyleDuval, F., Mokrani, M.-C., Danila, V., Erb, A., Gonzalez Lopera, F., & Tomsa, M. (2022). Dopamine Function and Hypothalamic-Pituitary-Thyroid Axis Activity in Major Depressed Patients with Suicidal Behavior. Brain Sciences, 12(5), 621. https://doi.org/10.3390/brainsci12050621