Behavioral Phenotype in Heterozygous DAT Rats: Transgenerational Transmission of Maternal Impact and the Role of Genetic Asset
Abstract
:1. Introduction
- (1)
- As for MIXs, the letter “M” shows the original “maternal” genealogy of the wild allele (coming from the father, and before that from the paternal grandmother);
- (2)
- The letter “U” indicates fetal development in hyperdopaminergic uterus, because the natural dam is KO (here, there is the need of a postnatal adoption as well).
2. Methods
2.1. Exp. 1
2.1.1. Subjects
2.1.2. Procedure
2.2. Exp. 2
2.2.1. Subjects
2.2.2. Procedure
2.2.3. Apparatus
2.3. Analysis
3. Results
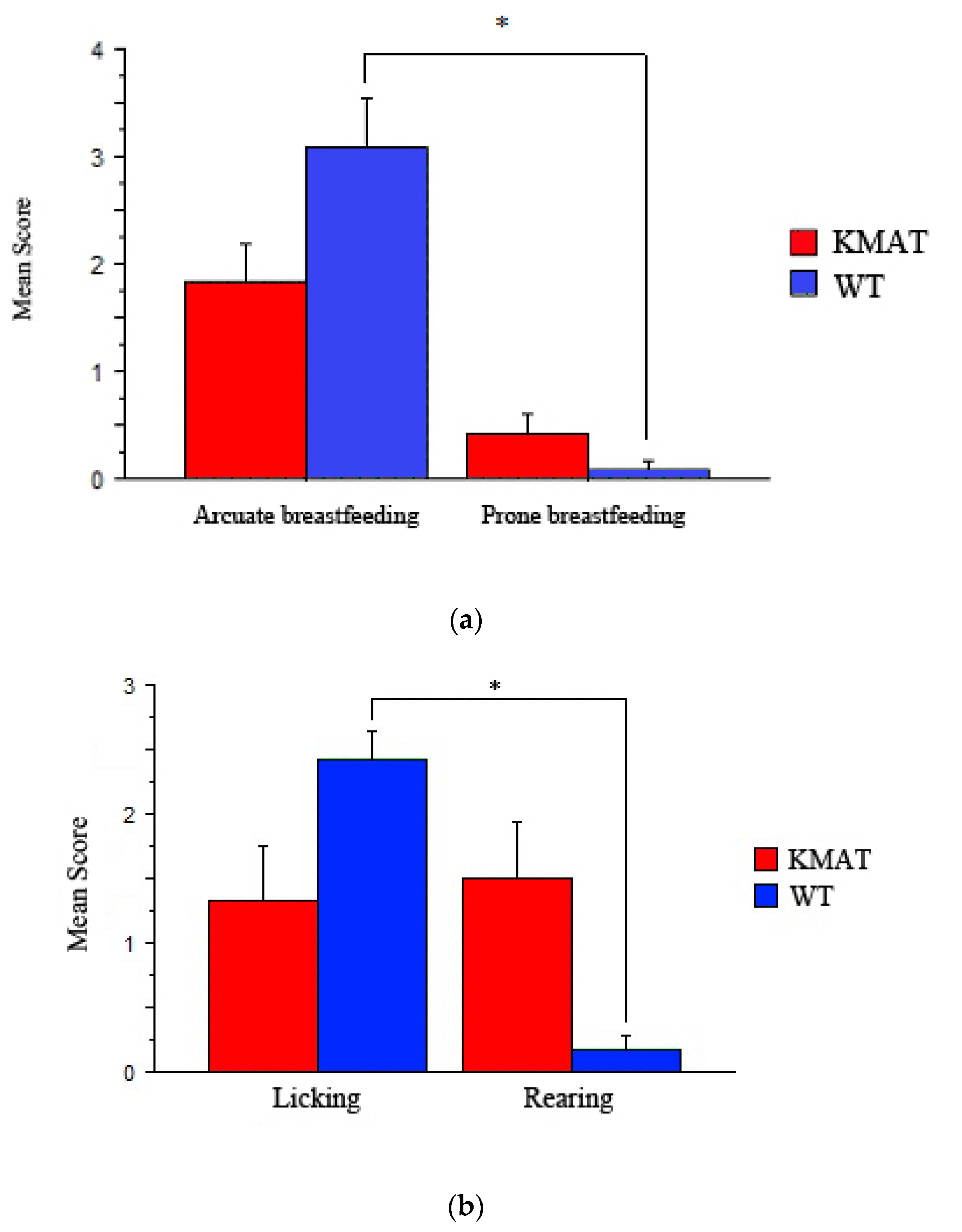
3.1. Maternal Behavior
3.2. Circadian Cycles
4. Discussion
4.1. Evidence of Transgenerational Transmission
4.2. Remarks on Maternal Cares
4.3. Translational Relevance and Limitations
4.4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethical Note
References
- Leo, D.; Sukhanov, I.; Zoratto, F.; Illiano, P.; Caffino, L.; Sanna, F.; Messa, G.; Emanuele, M.; Esposito, A.; Dorofeikova, M.; et al. Pronounced Hyperactivity, Cognitive Dysfunctions, and BDNF Dysregulation in Dopamine Transporter Knock-out Rats. J. Neurosci. 2018, 38, 1959–1972. [Google Scholar] [CrossRef] [Green Version]
- Liberati, A.S.; Calcaprina, B.; Adriani, W. Keeping Track of the Genealogy of Heterozygotes Using Epigenetic Reference Codes and Breeding Tables. Front. Behav. Neurosci. 2022, 15, 781235. [Google Scholar] [CrossRef] [PubMed]
- Adinolfi, A.; Zelli, S.; Leo, D.; Carbone, C.; Mus, L.; Illiano, P.; Alleva, E.; Gainetdinov, R.; Adriani, W. Behavioral characterization of DAT-KO rats and evidence of asocial-like phenotypes in DAT-HET rats: The potential involvement of norepinephrine system. Behav. Brain Res. 2019, 359, 516–527. [Google Scholar] [CrossRef]
- Cinque, S.; Zoratto, F.; Poleggi, A.; Leo, D.; Cerniglia, L.; Cimino, S.; Tambelli, R.; Alleva, E.; Gainetdinov, R.; Laviola, G.; et al. Behavioral Phenotyping of Dopamine Transporter Knockout Rats: Compulsive Traits, Motor Stereotypies, and Anhedonia. Front. Psychiatry 2018, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Oggiano, M.; Buccheri, C.; Alleva, E.; Adriani, W. Dopaminergic modulation of the circadian activity and sociability: Dissecting parental inheritance versus maternal styles as determinants of epigenetic influence. Behav. Brain Res. 2021, 417, 113623. [Google Scholar] [CrossRef] [PubMed]
- Carbone, C.; Brancato, A.; Adinolfi, A.; Russo, S.L.M.L.; Alleva, E.; Cannizzaro, C.; Adriani, W. Motor Transitions’ Peculiarity of Heterozygous DAT Rats When Offspring of an Unconventional KOxWT Mating. Neuroscience 2020, 433, 108–120. [Google Scholar] [CrossRef]
- Zelli, S.; Brancato, A.; Mattioli, F.; Pepe, M.; Alleva, E.; Carbone, C.; Cannizzaro, C.; Adriani, W. A new “sudden fright paradigm” to explore the role of (epi)genetic modulations of the DAT gene in fear-induced avoidance behavior. Genes Brain Behav. 2021, 20, e12709. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, R.M.; Fontham, E.H.; Lehmeyer, J.E. Prolactin and Growth Hormone Production as Influenced by Catecholamines and Agents that Affect Brain Catecholamines. Neuroendocrinology 1970, 6, 283–294. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, R.M.; Fontham, H.; Pace, R.C. Influence of Norepinephrine and Catecholamine-Depleting Agents on the Synthesis and Release of Prolactin and Growth Hormone 1. Endocrinology 1969, 85, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Caron, M.G.; Beaulieu, M.; Raymond, V.; Gagne, B.; Drouin, J.; Lefkowitz, R.J.; Labrie, F. Dopaminergic receptors in the anterior pituitary gland. Correlation of [3H] dihydroergocryptine binding with the dopaminergic control of prolactin release. J. Biol. Chem. 1978, 253, 2244–2253. [Google Scholar] [CrossRef]
- Fitzgerald, P.; Dinan, T.G. Prolactin and dopamine: What is the connection? A Review Article. J. Psychopharmacol. 2008, 22, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Raymond, V.; Beaulieu, M.; Labrie, F.; Boissier, J. Potent Antidopaminergic Activity of Estradiol at the Pituitary Level on Prolactin Release. Science 1978, 200, 1173–1175. [Google Scholar] [CrossRef] [PubMed]
- Pepe, M.; Calcaprina, B.; Vaquer, F.; Laviola, G.; Adriani, W. DAT-truncated epigenetics: Heterozigosity of the grand-mother rat temperates the vulnerable phenotype in second-generation offspring. Int. J. Dev. Neurosci. 2022. [Google Scholar] [CrossRef] [PubMed]
- Bonatz, A.E.; Steiner, H.; Huston, J.P. Video image analysis of behavior by microcomputer: Categorization of turning and locomotion after 6-OHDA injection into the substantia nigra. J. Neurosci. Methods 1987, 22, 13–26. [Google Scholar] [CrossRef]
- Pasquali, V.; Scannapieco, E.; Renzi, P. Validation of a microwave radar system for the monitoring of locomotor activity in mice. J. Circadian Rhythm. 2006, 4, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, L.J.; Tuttle, A.H.; Mogil, J.S. The Interaction between Pain and Social Behavior in Humans and Rodents. Behav. Neurobiol. Alcohol Addict. 2014, 20, 233–250. [Google Scholar] [CrossRef]
- Masuo, Y.; Matsumoto, Y.; Morita, S.; Noguchi, J. A novel method for counting spontaneous motor activity in the rat. Brain Res. Protoc. 1997, 1, 321–326. [Google Scholar] [CrossRef]
- Anway, M.D.; Cupp, A.S.; Uzumcu, M.; Skinner, M.K. Epigenetic Transgenerational Actions of Endocrine Disruptors and Male Fertility. Science 2005, 308, 1466–1469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, B.G.; Ressler, K. Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat. Neurosci. 2014, 17, 89–96. [Google Scholar] [CrossRef]
- Stranges, S.; Tigbe, W.; Gómez-Olivé, F.X.; Thorogood, M.; Kandala, N.-B. Sleep Problems: An Emerging Global Epidemic? Findings from the INDEPTH WHO-SAGE Study among More Than 40,000 Older Adults from 8 Countries across Africa and Asia. Sleep 2012, 35, 1173–1181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregg, C.; Zhang, J.; Butler, J.E.; Haig, D.; Dulac, C. Sex-Specific Parent-of-Origin Allelic Expression in the Mouse Brain. Science 2010, 329, 682–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregg, C.; Zhang, J.; Weissbourd, B.; Luo, S.; Schroth, G.P.; Haig, D.; Dulac, C. High-Resolution Analysis of Parent-of-Origin Allelic Expression in the Mouse Brain. Science 2010, 329, 643–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perera, F.; Herbstman, J. Prenatal environmental exposures, epigenetics, and disease. Reprod. Toxicol. 2011, 31, 363–373. [Google Scholar] [CrossRef] [Green Version]
- Hackett, J.A.; Sengupta, R.; Zylicz, J.J.; Murakami, K.; Lee, C.; Down, T.A.; Surani, M.A. Germline DNA Demethylation Dynamics and Imprint Erasure Through 5-Hydroxymethylcytosine. Science 2013, 339, 448–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ly, L.; Chan, D.; Trasler, J.M. Developmental windows of susceptibility for epigenetic inheritance through the male germline. Semin. Cell Dev. Biol. 2015, 43, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Duffy, K.A.; Bale, T.L.; Epperson, C.N. Germ Cell Drivers: Transmission of Preconception Stress across Generations. Front. Hum. Neurosci. 2021, 15, 642762. [Google Scholar] [CrossRef] [PubMed]
- Belleannee, C.; Calvo, É.; Caballero, J.; Sullivan, R. Epididymosomes Convey Different Repertoires of MicroRNAs throughout the Bovine Epididymis1. Biol. Reprod. 2013, 89, 30. [Google Scholar] [CrossRef]
- Nixon, B.; Stanger, S.J.; Mihalas, B.P.; Reilly, J.N.; Anderson, A.L.; Tyagi, S.; Holt, J.E.; McLaughlin, E.A. The MicroRNA Signature of Mouse Spermatozoa Is Substantially Modified During Epididymal Maturation1. Biol. Reprod. 2015, 93, 91. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.; Conine, C.C.; Shea, J.M.; Boskovic, A.; Derr, A.G.; Bing, X.Y.; Belleannee, C.; Kucukural, A.; Serra, R.W.; Sun, F.; et al. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science 2016, 351, 391–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, C.; Feng, J.; Sun, H.; Shao, N.-Y.; Mazei-Robison, M.; Damez-Werno, D.; Scobie, K.N.; Bagot, R.C.; Labonté, B.; Ribeiro, E.; et al. β-catenin mediates stress resilience through Dicer1/microRNA regulation. Nature 2014, 516, 51–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodgers, A.B.; Morgan, C.P.; Leu, N.A.; Bale, T.L. Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc. Natl. Acad. Sci. USA 2015, 112, 13699–13704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caldji, C.; Tannenbaum, B.; Sharma, S.; Francis, D.; Plotsky, P.M.; Meaney, M.J. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc. Natl. Acad. Sci. USA 1998, 95, 5335–5340. [Google Scholar] [CrossRef] [Green Version]
- Meaney, M.J. Maternal Care, Gene Expression, and the Transmission of Individual Differences in Stress Reactivity across Generations. Annu. Rev. Neurosci. 2001, 24, 1161–1192. [Google Scholar] [CrossRef]
- Vallee, M.; Mayo, W.; Dellu, F.; Le Moal, M.; Simon, H.; Maccari, S. Prenatal Stress Induces High Anxiety and Postnatal Handling Induces Low Anxiety in Adult Offspring: Correlation with Stress-Induced Corticosterone Secretion. J. Neurosci. 1997, 17, 2626–2636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weaver, I.C.G.; Szyf, M.; Meaney, M.J. From maternal care to gene expression: DNA methylation and the maternal programming of stress responses. Endocr. Res. 2002, 28, 699. [Google Scholar] [CrossRef] [PubMed]
- Weaver, I.C.; Cervoni, N.; Champagne, F.A.; D’Alessio, A.C.; Sharma, S.; Seckl, J.R.; Dymov, S.; Szyf, M.; Meaney, M.J. Epi-genetic programming by maternal behavior. Nat. Neurosci. 2004, 7, 847–854. [Google Scholar] [CrossRef]
- Beery, A.K.; McEwen, L.M.; MacIsaac, J.L.; Francis, D.D.; Kobor, M. Natural variation in maternal care and cross-tissue patterns of oxytocin receptor gene methylation in rats. Horm. Behav. 2016, 77, 42–52. [Google Scholar] [CrossRef] [Green Version]
- van der Doelen, R.H.A.; Arnoldussen, I.A.; Ghareh, H.; van Och, L.; Homberg, J.R.; Kozicz, T. Early life adversity and serotonin transporter gene variation interact to affect DNA methylation of the corticotropin-releasing factor gene promoter region in the adult rat brain. Dev. Psychopathol. 2015, 27, 123–135. [Google Scholar] [CrossRef]
- Agrawal, A.A. Phenotypic Plasticity in the Interactions and Evolution of Species. Science 2001, 294, 321–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossiter, M.C. Maternal Effects as Adaptations; Fox, T.A., Mousseau, C.W., Eds.; Oxford University Press: London, UK, 1999. [Google Scholar]
- Gudsnuk, K.M.; Champagne, F.A. Epigenetic Effects of Early Developmental Experiences. Clin. Perinatol. 2011, 38, 703–717. [Google Scholar] [CrossRef]
- Champagne, F.A. Epigenetic mechanisms and the transgenerational effects of maternal care. Front. Neuroendocrinol. 2008, 29, 386–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franklin, T.; Russig, H.; Weiss, I.C.; Gräff, J.; Linder, N.; Michalon, A.; Vizi, S.; Mansuy, I.M. Epigenetic Transmission of the Impact of Early Stress Across Generations. Biol. Psychiatry 2010, 68, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Francis, D.D.; Meaney, M.J. Maternal care and the development of stress responses. Curr. Opin. Neurobiol. 1999, 9, 128–134. [Google Scholar] [CrossRef]
- Bennett, A.J.; Lesch, K.-P.; Heils, A.; Long, J.C.; Lorenz, J.G.; Shoaf, S.E.; Champoux, M.; Suomi, S.J.; Linnoila, M.V.; Higley, J.D. Early experience and serotonin transporter gene variation interact to influence primate CNS function. Mol. Psychiatry 2002, 7, 118–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Champagne, F.; Francis, D.D.; Mar, A.; Meaney, M.J. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol. Behav. 2003, 79, 359–371. [Google Scholar] [CrossRef]
- Maestripieri, D.; Higley, J.D.; Lindell, S.G.; Newman, T.K.; McCormack, K.M.; Sanchez, M.M. Early maternal rejection affects the development of monoaminergic systems and adult abusive parenting in rhesus macaques (Macaca mulatta). Behav. Neurosci. 2006, 120, 1017–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, A.; Lovic, V.; Ward, G.R.; Wainwright, P.E.; Fleming, A.S. Intergenerational effects of complete maternal deprivation and replacement stimulation on maternal behaviour and emotionality in female rats. Dev. Psychobiol. 2000, 38, 11–32. [Google Scholar] [CrossRef]
- Fleming, A.S.; Kraemer, G.W.; Gonzalez, A.; Lovic, V.; Rees, S.; Melo, A. Mothering begets mothering: The transmission of behavior and its neurobiology across generations. Pharmacol. Biochem. Behav. 2002, 73, 61–75. [Google Scholar] [CrossRef]
- Maestripieri, D.; Lindell, S.G.; Ayala, A.; Gold, P.W.; Higley, J.D. Neurobiological characteristics of rhesus macaque abusive mothers and their relation to social and maternal behavior. Neurosci. Biobehav. Rev. 2005, 29, 51–57. [Google Scholar] [CrossRef]
- Maestripieri, D.; Lindell, S.G.; Higley, J.D. Intergenerational transmission of maternal behavior in rhesus macaques and its underlying mechanisms. Dev. Psychobiol. 2007, 49, 165–171. [Google Scholar] [CrossRef]
- Suomi, S.J. Attachment in rhesus monkeys. In Handbook of Attachment: Theory, Research and Clinical Application; Cassidy, J., Shaver, P., Eds.; Guilford Press: New York, NY, USA, 1999; pp. 181–197. [Google Scholar]
- Liu, D.; Diorio, J.; Tannenbaum, B.; Caldji, C.; Francis, D.; Freedman, A.; Sharma, S.; Pearson, D.; Plotsky, P.M.; Meaney, M.J. Maternal Care, Hippocampal Glucocorticoid Receptors, and Hypothalamic-Pituitary-Adrenal Responses to Stress. Science 1997, 277, 1659–1662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caldji, C.; Diorio, J.; Meaney, M.J. Variations in maternal care in infancy regulate the development of stress reactivity. Biol. Psychiatry 2000, 48, 1164–1174. [Google Scholar] [CrossRef]
- Francis, D.; Diorio, J.; Liu, D.; Meaney, M.J. Nongenomic Transmission across Generations of Maternal Behavior and Stress Responses in the Rat. Science 1999, 286, 1155–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lovic, V.; Fleming, A.S. Propagation of maternal behavior across generations is associated with changes in non-maternal cognitive and behavioral processes. Behav. Process. 2015, 117, 42–47. [Google Scholar] [CrossRef]
- Lomanowska, A.M.; Melo, A.I. Deconstructing the function of maternal stimulation in offspring development: Insights from the artificial rearing model in rats. Horm. Behav. 2016, 77, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Melo, A.I.; Lovic, V.; Gonzalez, A.; Madden, M.; Sinopoli, K.; Fleming, A.S. Maternal and littermate deprivation disrupts maternal behavior and social-learning of food preference in adulthood: Tactile stimulation, nest odor, and social rearing prevent these effects. Dev. Psychobiol. 2006, 48, 209–219. [Google Scholar] [CrossRef]
- Palombo, D.J.; Nowoslawski, M.; Fleming, A.S. Motherless rats show deficits in maternal behavior towards fostered pups. Dev. Psychobiol. 2010, 52, 142–148. [Google Scholar] [CrossRef]
- Afonso, V.M.; King, S.J.; Novakov, M.; Burton, C.; Fleming, A.S. Accumbal dopamine function in postpartum rats that were raised without their mothers. Horm. Behav. 2011, 60, 632–643. [Google Scholar] [CrossRef]
- Lovic, V.; Keen, D.; Fletcher, P.J.; Fleming, A.S. Early-life maternal separation and social isolation produce an increase in impulsive action but not impulsive choice. Behav. Neurosci. 2011, 125, 481–491. [Google Scholar] [CrossRef] [Green Version]
- Shams, S.; Pawluski, J.; Chatterjee-Chakraborty, M.; Oatley, H.; Mastroianni, A.; Fleming, A.S. Dendritic morphology in the striatum and hypothalamus differentially exhibits experience-dependent changes in response to maternal care and early social isolation. Behav. Brain Res. 2012, 233, 79–89. [Google Scholar] [CrossRef]
- Mathews, C.A.; Kaur, N.; Stein, M.B. Childhood trauma and obsessive-compulsive symptoms. Depress. Anxiety 2008, 25, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.; Brown, J.; Smailes, E. Child abuse and neglect and the development of mental disorders in the general population. Dev. Psychopathol. 2001, 13, 981–999. [Google Scholar] [CrossRef]
- Kroska, E.; Miller, M.; Roche, A.I.; Kroska, S.K.; O’Hara, M.W. Effects of traumatic experiences on obsessive-compulsive and internalizing symptoms: The role of avoidance and mindfulness. J. Affect. Disord. 2018, 225, 326–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pears, K.C.; Capaldi, D.M. Intergenerational transmission of abuse: A two-generational prospective study of an at-risk sample. Child Abus. Negl. 2001, 25, 1439–1461. [Google Scholar] [CrossRef]
- Oliver, J.E. Intergenerational transmission of child abuse: Rates, research, and clinical implications. Am. J. Psychiatry 1993, 150, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.; Zigler, E. The intergenerational transmission of abuse is overstated. In Current Controversies on Family Violence; Gelles, R.J., Loseke, D.R., Eds.; Sage: Newbury Park, CA, USA, 1993; pp. 209–221. [Google Scholar]
- Chacon, P.; Bernardes, E.; Faggian, L.; Batistuzzo, M.; Moriyama, T.; Miguel, E.C.; Polanczyk, G.V. Obsessive-compulsive symptoms in children with first degree relatives diagnosed with obsessive-compulsive disorder. Rev. Bras. Psiquiatr. 2018, 40, 388–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, J.P. Breaking the Links in Intergenerational Violence: An Emotional Regulation Perspective. Fam. Process 2013, 52, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Challacombe, F.L.; Salkovskis, P.M.; Woolgar, M.; Wilkinson, E.L.; Read, J.; Acheson, R. Parenting and mother-infant interactions in the context of maternal postpartum obsessive-compulsive disorder: Effects of obsessional symptoms and mood. Infant Behav. Dev. 2016, 44, 11–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratzoni, N.; Doron, G.; Frenkel, T.I. Initial Evidence for Symptoms of Postpartum Parent-Infant Relationship Obsessive Compulsive Disorder (PI-ROCD) and Associated Risk for Perturbed Maternal Behavior and Infant Social Disengagement from Mother. Front. Psychiatry 2021, 12, 589949. [Google Scholar] [CrossRef] [PubMed]
- Doron, G.; Derby, D.; Szepsenwol, O. “I can’t stop thinking about my child’s flaws”: An investigation of parental preoccupation with their children’s perceived flaws. J. Obsessive-Compulsive Relat. Disord. 2017, 14, 106–111. [Google Scholar] [CrossRef]
- Rice, F.; Harold, G.; Thapar, A. The genetic aetiology of childhood depression: A review. J. Child Psychol. Psychiatry 2002, 43, 65–79. [Google Scholar] [CrossRef]
- Garcia, M.; Montalvo, I.; Creus, M.; Cabezas, Á.; Solé, M.; Algora, M.J.; Moreno, I.; Gutiérrez-Zotes, A.; Labad, J. Sex differences in the effect of childhood trauma on the clinical expression of early psychosis. Compr. Psychiatry 2016, 68, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Pruessner, M.; King, S.; Vracotas, N.; Abadi, S.; Iyer, S.; Malla, A.K.; Shah, J.; Joober, R. Gender differences in childhood trauma in first episode psychosis: Association with symptom severity over two years. Schizophr. Res. 2019, 205, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Breslau, N.; Chilcoat, H.D.; Kessler, R.C.; Peterson, E.L.; Lucia, V.C. Vulnerability to assaultive violence: Further specification of the sex difference in post-traumatic stress disorder. Psychol. Med. 1999, 29, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Sonnega, A.; Bromet, E.; Hughes, M.; Nelson, C.B. Posttraumatic Stress Disorder in the National Comorbidity Survey. Arch. Gen. Psychiatry 1995, 52, 1048–1060. [Google Scholar] [CrossRef] [PubMed]
- Saez, I.; Zhu, L.; Set, E.; Kayser, A.; Hsu, M. Dopamine Modulates Egalitarian Behavior in Humans. Curr. Biol. 2015, 25, 912–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez-Lallement, J.; Van Wingerden, M.; Marx, C.; Srejic, M.; Kalenscher, T. Rats prefer mutual rewards in a prosocial choice task. Front. Neurosci. 2015, 8, 443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perkeybile, A.M.; Bales, K.L. Intergenerational transmission of sociality: The role of parents in shaping social behavior in monogamous and non-monogamous species. J. Exp. Biol. 2017, 220, 114–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Germain, A.; Kupfer, D.J. Circadian rhythm disturbances in depression. Hum. Psychopharmacol. Clin. Exp. 2008, 23, 571–585. [Google Scholar] [CrossRef] [Green Version]
- Nezlek, J.B.; Imbrie, M.; Shean, G.D. Depression and everyday social interaction. J. Pers. Soc. Psychol. 1994, 67, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Bahi, A.; Dreyer, J.-L. Dopamine transporter (DAT) knockdown in the nucleus accumbens improves anxiety- and depression-related behaviors in adult mice. Behav. Brain Res. 2019, 359, 104–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tibbitts, G.M. Sleep Disorders: Causes, Effects, and Solutions. Prim. Care Clin. Off. Pract. 2008, 35, 817–837. [Google Scholar] [CrossRef] [PubMed]

| Abbreviation | Explanation |
|---|---|
| WT = wild-type | Most frequent genotype, for dopamine transporter (DAT), in the natural population |
| KO = knock-out | Homozygote knocked-out for the DAT gene, is usually obtained as sibling of other epi-genotypes |
| HET = heterozygous | Inheriting one healthy and one knocked-out allele from each parent |
| MAT = maternal | Heterozygous specimens, offspring of a KO male and a WT female |
| PAT = paternal | Heterozygous specimens, offspring of a WT male and a KO female |
| K-MAT (see text) | MAT rats, but adopted and cared (maltreated) from KO dams |
| MIX = mixed with KO siblings | Heterozygous offspring of a MAT female and a KO male |
| MUX = mixed, and born from KO uterus | Heterozygous offspring of a KO female and a MAT male |
| MYX = mixed, and its dam in KO uterus | Offspring from KO male and MUX female, that is in turn heterozygous offspring from a KO female and a MAT male (i.e., the maternal grandmother was KO and dam was born from KO uterus) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manoni, G.; Puzzo, C.; Gigantesco, A.; Adriani, W. Behavioral Phenotype in Heterozygous DAT Rats: Transgenerational Transmission of Maternal Impact and the Role of Genetic Asset. Brain Sci. 2022, 12, 469. https://doi.org/10.3390/brainsci12040469
Manoni G, Puzzo C, Gigantesco A, Adriani W. Behavioral Phenotype in Heterozygous DAT Rats: Transgenerational Transmission of Maternal Impact and the Role of Genetic Asset. Brain Sciences. 2022; 12(4):469. https://doi.org/10.3390/brainsci12040469
Chicago/Turabian StyleManoni, Greta, Concetto Puzzo, Antonella Gigantesco, and Walter Adriani. 2022. "Behavioral Phenotype in Heterozygous DAT Rats: Transgenerational Transmission of Maternal Impact and the Role of Genetic Asset" Brain Sciences 12, no. 4: 469. https://doi.org/10.3390/brainsci12040469
APA StyleManoni, G., Puzzo, C., Gigantesco, A., & Adriani, W. (2022). Behavioral Phenotype in Heterozygous DAT Rats: Transgenerational Transmission of Maternal Impact and the Role of Genetic Asset. Brain Sciences, 12(4), 469. https://doi.org/10.3390/brainsci12040469







