Intraoperative Brain Mapping in Multilingual Patients: What Do We Know and Where Are We Going?
Abstract
1. Background from Philosophy to Intraoperative Brain Mapping
2. A Key Issue: The Degree of Anatomical-Functional Integration or Separation of Two or More Languages in a Multilingual Brain
3. How Age of L2-Acquisition Affects Language Organization in the Human Brain
4. Language Switching: A Higher-Order Cognitive Process
“I can not comunicare con you; Oggi I can not say il mio nome to you; I am a disastro today”.[81]
5. Awake Intraoperative Mapping: A Look towards the Future
5.1. The Conceptual Evolution of Broca’s and Wernicke’s Areas Is Needed to Understand Multilingualism
5.2. Subcortical Pathways in Multilingualism: A Matter of Vital and Yet Partially Unknown Importance
5.3. Fatigue and Time Limitation: A Multilinguism-Brain Mapping Challenge
5.4. Topographical Anatomy and/or fMRI-Guided Surgery alone Cannot Predict the Language-Related Areas
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hayakawa, S.; Marian, V. Consequences of Multilingualism for Neural Architecture. Behav. Brain Funct. 2019, 15, 6. [Google Scholar] [CrossRef] [PubMed]
- Friederici, A.D.; Chomsky, N.; Berwick, R.C.; Moro, A.; Bolhuis, J.J. Language, Mind and Brain. Nat. Hum. Behav. 2017, 1, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Suárez Delucchi, N.; Fossa Arcila, P. Vygotsky’s Inner Language and Its Relation to the Unconscious System of Freud. Int. J. Psychoanal. 2020, 101, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Fabbro, F. The Bilingual Brain: Bilingual Aphasia. Brain Lang. 2001, 79, 201–210. [Google Scholar] [CrossRef]
- De Houwer, A. Taalontwikkeling BiJ. Meertalige Kinderen. In Boekblok Handboek Stem-Spraak-en Taalpathologie; Springer: New York, NY, USA, 1999; pp. 274–282. [Google Scholar]
- Price, C.J. The Anatomy of Language: A Review of 100 FMRI Studies Published in 2009. Ann. N. Y. Acad. Sci. 2010, 1191, 62–88. [Google Scholar] [CrossRef]
- Fernandez-Coello, A.; Gil-Robles, S.; Carreiras, M. Multilingual Naming. In Intraoperative Mapping of Cognitive Networks; Springer: New York, NY, USA, 2021; pp. 219–231. [Google Scholar]
- Hämäläinen, S.; Sairanen, V.; Leminen, A.; Lehtonen, M. Bilingualism Modulates the White Matter Structure of Language-Related Pathways. Neuroimage 2017, 152, 249–257. [Google Scholar] [CrossRef]
- Li, P.; Legault, J.; Litcofsky, K.A. Neuroplasticity as a Function of Second Language Learning: Anatomical Changes in the Human Brain. Cortex 2014, 58, 301–324. [Google Scholar] [CrossRef]
- Mechelli, A.; Crinion, J.T.; Noppeney, U.; O’Doherty, J.; Ashburner, J.; Frackowiak, R.S.; Price, C.J. Structural Plasticity in the Bilingual Brain. Nature 2004, 431, 757. [Google Scholar] [CrossRef]
- Stein, M.; Federspiel, A.; Koenig, T.; Wirth, M.; Strik, W.; Wiest, R.; Brandeis, D.; Dierks, T. Structural Plasticity in the Language System Related to Increased Second Language Proficiency. Cortex 2012, 48, 458–465. [Google Scholar] [CrossRef]
- Abutalebi, J.; Canini, M.; della Rosa, P.A.; Sheung, L.P.; Green, D.W.; Weekes, B.S. Bilingualism Protects Anterior Temporal Lobe Integrity in Aging. Neurobiol. Aging 2014, 35, 2126–2133. [Google Scholar] [CrossRef]
- Pliatsikas, C.; Johnstone, T.; Marinis, T. Grey Matter Volume in the Cerebellum Is Related to the Processing of Grammatical Rules in a Second Language: A Structural Voxel-Based Morphometry Study. Cerebellum 2014, 13, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Luk, G.; Bialystok, E.; Craik, F.I.M.; Grady, C.L. Lifelong Bilingualism Maintains White Matter Integrity in Older Adults. J. Neurosci. 2011, 31, 16808–16813. [Google Scholar] [CrossRef] [PubMed]
- Coggins Iii, P.E.; Kennedy, T.J.; Armstrong, T.A. Bilingual Corpus Callosum Variability. Brain Lang. 2004, 89, 69–75. [Google Scholar] [CrossRef]
- Mohades, S.G.; Struys, E.; van Schuerbeek, P.; Mondt, K.; van de Craen, P.; Luypaert, R. DTI Reveals Structural Differences in White Matter Tracts between Bilingual and Monolingual Children. Brain Res. 2012, 1435, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Cummine, J.; Boliek, C.A. Understanding White Matter Integrity Stability for Bilinguals on Language Status and Reading Performance. Brain Struct. Funct. 2013, 218, 595–601. [Google Scholar] [CrossRef]
- Gold, B.T.; Johnson, N.F.; Powell, D.K. Lifelong Bilingualism Contributes to Cognitive Reserve against White Matter Integrity Declines in Aging. Neuropsychologia 2013, 51, 2841–2846. [Google Scholar] [CrossRef]
- Kuhl, P.K.; Stevenson, J.; Corrigan, N.M.; van den Bosch, J.J.F.; Can, D.D.; Richards, T. Neuroimaging of the Bilingual Brain: Structural Brain Correlates of Listening and Speaking in a Second Language. Brain Lang. 2016, 162, 1–9. [Google Scholar] [CrossRef]
- Gislén, A.; Dacke, M.; Kröger, R.H.H.; Abrahamsson, M.; Nilsson, D.-E.; Warrant, E.J. Superior Underwater Vision in a Human Population of Sea Gypsies. Curr. Biol. 2003, 13, 833–836. [Google Scholar] [CrossRef]
- Maguire, E.A.; Gadian, D.G.; Johnsrude, I.S.; Good, C.D.; Ashburner, J.; Frackowiak, R.S.J.; Frith, C.D. Navigation-Related Structural Change in the Hippocampi of Taxi Drivers. Proc. Natl. Acad. Sci. USA 2000, 97, 4398–4403. [Google Scholar] [CrossRef]
- Green, D.W.; Abutalebi, J. Language Control in Bilinguals: The Adaptive Control Hypothesis. J. Cogn. Psychol. 2013, 25, 515–530. [Google Scholar] [CrossRef]
- Abutalebi, J.; Green, D.W. Neuroimaging of Language Control in Bilinguals: Neural Adaptation and Reserve. Biling. Lang. Cogn. 2016, 19, 689–698. [Google Scholar] [CrossRef]
- Hernandez, A.E.; Martinez, A.; Kohnert, K. In Search of the Language Switch: An FMRI Study of Picture Naming in Spanish–English Bilinguals. Brain Lang. 2000, 73, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.E. Language Switching in the Bilingual Brain: What’s next? Brain Lang. 2009, 109, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Garbin, G.; Costa, A.; Sanjuan, A.; Forn, C.; Rodriguez-Pujadas, A.; Ventura, N.; Belloch, V.; Hernandez, M.; Ávila, C. Neural Bases of Language Switching in High and Early Proficient Bilinguals. Brain Lang. 2011, 119, 129–135. [Google Scholar] [CrossRef]
- Seo, R.; Stocco, A.; Prat, C.S. The Bilingual Language Network: Differential Involvement of Anterior Cingulate, Basal Ganglia and Prefrontal Cortex in Preparation, Monitoring, and Execution. Neuroimage 2018, 174, 44–56. [Google Scholar] [CrossRef]
- Zou, L.; Ding, G.; Abutalebi, J.; Shu, H.; Peng, D. Structural Plasticity of the Left Caudate in Bimodal Bilinguals. Cortex 2012, 48, 1197–1206. [Google Scholar] [CrossRef]
- Penfield, W.; Roberts, L. Speech & Brain Mechanisms; Princeton Legacy Library: Princeton, NJ, USA, 1959. [Google Scholar]
- Ojemann, G.; Ojemann, J.; Lettich, E.; Berger, M. Cortical Language Localization in Left, Dominant Hemisphere: An Electrical Stimulation Mapping Investigation in 117 Patients. J. Neurosurg. 1989, 71, 316–326. [Google Scholar] [CrossRef]
- Broca, P. Remarks on the Seat of the Faculty of Articulated Language, Following an Observation of Aphemia (Loss of Speech). Bull. De La Société Anat. 1861, 6, 330–357. [Google Scholar]
- Wernicke, C. The Aphasic Symptom Complex: A Psychological Study on a Neurological Basis. In Breslau: Kohn and Weigert; Cohen, R.S., Wartofsky, M.W., Eds.; Boston Studies in the Philosophy of Science: Boston, MA, USA, 1874; p. 4. [Google Scholar]
- Giussani, C.; Roux, F.-E.; Lubrano, V.; Gaini, S.M.; Bello, L. Review of Language Organisation in Bilingual Patients: What Can We Learn from Direct Brain Mapping? Acta Neurochir. 2007, 149, 1109–1116. [Google Scholar] [CrossRef]
- Roux, F.-E.; Trémoulet, M. Organization of Language Areas in Bilingual Patients: A Cortical Stimulation Study. J. Neurosurg. 2002, 97, 857–864. [Google Scholar] [CrossRef]
- Roux, F.-E.; Boulanouar, K.; Lotterie, J.-A.; Mejdoubi, M.; LeSage, J.P.; Berry, I. Language Functional Magnetic Resonance Imaging in Preoperative Assessment of Language Areas: Correlation with Direct Cortical Stimulation. Neurosurgery 2003, 52, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Lucas, T.H.; McKhann, G.M.; Ojemann, G.A. Functional Separation of Languages in the Bilingual Brain: A Comparison of Electrical Stimulation Language Mapping in 25 Bilingual Patients and 117 Monolingual Control Patients. J. Neurosurg. 2004, 101, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Kroll, J.F.; Stewart, E. Category Interference in Translation and Picture Naming: Evidence for Asymmetric Connections between Bilingual Memory Representations. J. Mem. Lang. 1994, 33, 149–174. [Google Scholar] [CrossRef]
- Dijkstra, T.; van Heuven, W.J.B. The Architecture of the Bilingual Word Recognition System: From Identification to Decision. Biling. Lang. Cogn. 2002, 5, 175–197. [Google Scholar] [CrossRef]
- Van Hell, J.G.; de Groot, A.M.B. Conceptual Representation in Bilingual Memory: Effects of Concreteness and Cognate Status in Word Association. Biling. Lang. Cogn. 1998, 1, 193–211. [Google Scholar] [CrossRef]
- Van de Putte, E.; de Baene, W.; Brass, M.; Duyck, W. Neural Overlap of L1 and L2 Semantic Representations in Speech: A Decoding Approach. Neuroimage 2017, 162, 106–116. [Google Scholar] [CrossRef]
- Klein, D.; Milner, B.; Zatorre, R.J.; Meyer, E.; Evans, A.C. The Neural Substrates Underlying Word Generation: A Bilingual Functional-Imaging Study. Proc. Natl. Acad. Sci. USA 1995, 92, 2899–2903. [Google Scholar] [CrossRef]
- Illes, J.; Francis, W.S.; Desmond, J.E.; Gabrieli, J.D.E.; Glover, G.H.; Poldrack, R.; Lee, C.J.; Wagner, A.D. Convergent Cortical Representation of Semantic Processing in Bilinguals. Brain Lang. 1999, 70, 347–363. [Google Scholar] [CrossRef]
- Klein, D.; Milner, B.; Zatorre, R.J.; Visca, R.; Olivier, A. Cerebral Organization in a Right-Handed Trilingual Patient with Right-Hemisphere Speech: A Positron Emission Tomography Study. Neurocase 2002, 8, 369–375. [Google Scholar] [CrossRef]
- Binder, J.R. The Wernicke Area: Modern Evidence and a Reinterpretation. Neurology 2015, 85, 2170–2175. [Google Scholar] [CrossRef]
- Turk-Browne, N.B.; Yi, D.-J.; Chun, M.M. Linking Implicit and Explicit Memory: Common Encoding Factors and Shared Representations. Neuron 2006, 49, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Li, D.; Li, P. Brain Decoding in Multiple Languages: Can Cross-Language Brain Decoding Work? Brain Lang. 2021, 215, 104922. [Google Scholar] [CrossRef] [PubMed]
- Rossell, S.L.; Price, C.J.; Nobre, A.C. The Anatomy and Time Course of Semantic Priming Investigated by FMRI and ERPs. Neuropsychologia 2003, 41, 550–564. [Google Scholar] [CrossRef]
- Mummery, C.J.; Shallice, T.; Price, C.J. Dual-Process Model in Semantic Priming: A Functional Imaging Perspective. Neuroimage 1999, 9, 516–525. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chee, M.W.L.; Soon, C.S.; Lee, H.L. Common and Segregated Neuronal Networks for Different Languages Revealed Using Functional Magnetic Resonance Adaptation. J. Cogn. Neurosci. 2003, 15, 85–97. [Google Scholar] [CrossRef]
- Haxby, J.V. Multivariate Pattern Analysis of FMRI: The Early Beginnings. Neuroimage 2012, 62, 852–855. [Google Scholar] [CrossRef]
- Rodd, J.M.; Vitello, S.; Woollams, A.M.; Adank, P. Localising Semantic and Syntactic Processing in Spoken and Written Language Comprehension: An Activation Likelihood Estimation Meta-Analysis. Brain Lang. 2015, 141, 89–102. [Google Scholar] [CrossRef]
- Van Doren, L.; Dupont, P.; de Grauwe, S.; Peeters, R.; Vandenberghe, R. The Amodal System for Conscious Word and Picture Identification in the Absence of a Semantic Task. Neuroimage 2010, 49, 3295–3307. [Google Scholar] [CrossRef]
- Lubrano, V.; Prod’homme, K.; Démonet, J.-F.; Köpke, B. Language Monitoring in Multilingual Patients Undergoing Awake Craniotomy: A Case Study of a German–English–French Trilingual Patient with a WHO Grade II Glioma. J. Neurolinguistics 2012, 25, 567–578. [Google Scholar] [CrossRef]
- Kim, K.H.S.; Relkin, N.R.; Lee, K.-M.; Hirsch, J. Distinct Cortical Areas Associated with Native and Second Languages. Nature 1997, 388, 171–174. [Google Scholar] [CrossRef]
- Połczyńska, M.M.; Bookheimer, S.Y. Factors Modifying the Amount of Neuroanatomical Overlap between Languages in Bilinguals—A Systematic Review of Neurosurgical Language Mapping Studies. Brain Sci. 2020, 10, 983. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Coello, A.; Havas, V.; Juncadella, M.; Sierpowska, J.; Rodríguez-Fornells, A.; Gabarrós, A. Age of Language Acquisition and Cortical Language Organization in Multilingual Patients Undergoing Awake Brain Mapping. J. Neurosurg. 2016, 126, 1912–1923. [Google Scholar] [CrossRef] [PubMed]
- Abutalebi, J.; Cappa, S.F.; Perani, D. The Bilingual Brain as Revealed by Functional Neuroimaging. Biling. Lang. Cogn. 2001, 4, 179–190. [Google Scholar] [CrossRef]
- Abzianidze, L.; Bjerva, J.; Evang, K.; Haagsma, H.; van Noord, R.; Ludmann, P.; Nguyen, D.-D.; Bos, J. The Parallel Meaning Bank: Towards a Multilingual Corpus of Translations Annotated with Compositional Meaning Representations. arXiv 2017, arXiv:1702.03964. [Google Scholar]
- Boas, H.C. Semantic Frames as Interlingual Representations for Multilingual Lexical Databases. Int. J. Lexicogr. 2005, 18, 445–478. [Google Scholar] [CrossRef]
- Murphy, E.; Woolnough, O.; Rollo, P.S.; Roccaforte, Z.J.; Segaert, K.; Hagoort, P.; Tandon, N. Minimal Phrase Composition Revealed by Intracranial Recordings. J. Neurosci. 2022, 42, 3216–3227. [Google Scholar] [CrossRef]
- Tate, M.C.; Herbet, G.; Moritz-Gasser, S.; Tate, J.E.; Duffau, H. Probabilistic Map of Critical Functional Regions of the Human Cerebral Cortex: Broca’s Area Revisited. Brain 2014, 137, 2773–2782. [Google Scholar] [CrossRef]
- Kilbride, R.D. Intraoperative Functional Cortical Mapping of Language. J. Clin. Neurophysiol. 2013, 30, 591–596. [Google Scholar] [CrossRef]
- Duffau, H. Lessons from Brain Mapping in Surgery for Low-Grade Glioma: Insights into Associations between Tumour and Brain Plasticity. Lancet Neurol. 2005, 4, 476–486. [Google Scholar] [CrossRef]
- Ojemann, G.A.; Whitaker, H.A. The Bilingual Brain. Arch. Neurol. 1978, 35, 409–412. [Google Scholar] [CrossRef]
- Bloch, C.; Kaiser, A.; Kuenzli, E.; Zappatore, D.; Haller, S.; Franceschini, R.; Luedi, G.; Radue, E.-W.; Nitsch, C. The Age of Second Language Acquisition Determines the Variability in Activation Elicited by Narration in Three Languages in Broca’s and Wernicke’s Area. Neuropsychologia 2009, 47, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Fabbro, F.; Naatanen, R.; Kujala, T. The Neurolinguistics of Bilingualism. Nature 1999, 398, 577. [Google Scholar]
- Harley, B.; Wang, W. The Critical Period Hypothesis: Where Are We Now. In Tutorials in Bilingualism: Psycholinguistic Perspectives; Psychology Press: Hove, UK, 1997; pp. 19–51. [Google Scholar]
- Johnson, J.S.; Newport, E.L. Critical Period Effects in Second Language Learning: The Influence of Maturational State on the Acquisition of English as a Second Language. Cogn. Psychol. 1989, 21, 60–99. [Google Scholar] [CrossRef]
- Meschyan, G.; Hernandez, A.E. Impact of Language Proficiency and Orthographic Transparency on Bilingual Word Reading: An FMRI Investigation. Neuroimage 2006, 29, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Perani, D.; Abutalebi, J.; Paulesu, E.; Brambati, S.; Scifo, P.; Cappa, S.F.; Fazio, F. The Role of Age of Acquisition and Language Usage in Early, High-proficient Bilinguals: An FMRI Study during Verbal Fluency. Hum. Brain Mapp. 2003, 19, 170–182. [Google Scholar] [CrossRef]
- Perani, D.; Paulesu, E.; Galles, N.S.; Dupoux, E.; Dehaene, S.; Bettinardi, V.; Cappa, S.F.; Fazio, F.; Mehler, J. The Bilingual Brain. Proficiency and Age of Acquisition of the Second Language. Brain J. Neurol. 1998, 121, 1841–1852. [Google Scholar] [CrossRef]
- Abutalebi, J.; Green, D. Bilingual Language Production: The Neurocognition of Language Representation and Control. J. Neurolinguist. 2007, 20, 242–275. [Google Scholar] [CrossRef]
- Vingerhoets, G.; van Borsel, J.; Tesink, C.; van den Noort, M.; Deblaere, K.; Seurinck, R.; Vandemaele, P.; Achten, E. Multilingualism: An FMRI Study. Neuroimage 2003, 20, 2181–2196. [Google Scholar] [CrossRef]
- Yetkin, O.; Zerrin Yetkin, F.; Haughton, V.M.; Cox, R.W. Use of Functional MR to Map Language in Multilingual Volunteers. Am. J. Neuroradiol. 1996, 17, 473–477. [Google Scholar]
- Giussani, C.; Roux, F.-E.; Ojemann, J.; Sganzerla, E.P.; Pirillo, D.; Papagno, C. Is Preoperative Functional Magnetic Resonance Imaging Reliable for Language Areas Mapping in Brain Tumor Surgery? Review of Language Functional Magnetic Resonance Imaging and Direct Cortical Stimulation Correlation Studies. Neurosurgery 2010, 66, 113–120. [Google Scholar] [CrossRef]
- Chee, M.W.L.; Tan, E.W.L.; Thiel, T. Mandarin and English Single Word Processing Studied with Functional Magnetic Resonance Imaging. J. Neurosci. 1999, 19, 3050–3056. [Google Scholar] [CrossRef] [PubMed]
- Jasinska, K.K.; Petitto, L. How Age of Bilingual Exposure Can Change the Neural Systems for Language in the Developing Brain: A Functional near Infrared Spectroscopy Investigation of Syntactic Processing in Monolingual and Bilingual Children. Dev. Cogn. Neurosci. 2013, 6, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Kuchcinski, G.; Mellerio, C.; Pallud, J.; Dezamis, E.; Turc, G.; Rigaux-Viodé, O.; Malherbe, C.; Roca, P.; Leclerc, X.; Varlet, P. Three-Tesla Functional MR Language Mapping: Comparison with Direct Cortical Stimulation in Gliomas. Neurology 2015, 84, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Leonard, M.K.; Torres, C.; Travis, K.E.; Brown, T.T.; Hagler, D.J., Jr.; Dale, A.M.; Elman, J.L.; Halgren, E. Language Proficiency Modulates the Recruitment of Non-Classical Language Areas in Bilinguals. PLoS ONE 2011, 6, e18240. [Google Scholar] [CrossRef]
- Połczyńska, M.M.; Benjamin, C.F.A.; Japardi, K.; Frew, A.; Bookheimer, S.Y. Language System Organization in a Quadrilingual with a Brain Tumor: Implications for Understanding of the Language Network. Neuropsychologia 2016, 86, 167–175. [Google Scholar] [CrossRef]
- Abutalebi, J.; Miozzo, A.; Cappa, S.F. Do Subcortical Structures Control ‘Language Selection’ in Polyglots? Evidence from Pathological Language Mixing. Neurocase 2000, 6, 51–56. [Google Scholar]
- Fabbro, F.; Skrap, M.; Aglioti, S. Pathological Switching between Languages after Frontal Lesions in a Bilingual Patient. J. Neurol. Neurosurg. Psychiatry 2000, 68, 650–652. [Google Scholar] [CrossRef]
- Rodriguez-Fornells, A.; Rotte, M.; Helnze, H.J.; Nösseit, T.; Münte, T.F. Brain Potential and Functional MRI Evidence for How to Handle Two Languages with One Brain. Nature 2002, 415, 1026–1029. [Google Scholar] [CrossRef]
- Branzi, F.M.; Calabria, M.; Boscarino, M.L.; Costa, A. On the Overlap between Bilingual Language Control and Domain-General Executive Control. Acta Psychol. 2016, 166, 21–30. [Google Scholar] [CrossRef]
- Robles, S.G.; Gatignol, P.; Capelle, L.; Mitchell, M.C.; Duffau, H. The Role of Dominant Striatum in Language: A Study Using Intraoperative Electrical Stimulations. J. Neurol. Neurosurg. Psychiatry 2005, 76, 940–946. [Google Scholar] [CrossRef]
- McCormick, L.M.; Ziebell, S.; Nopoulos, P.; Cassell, M.; Andreasen, N.C.; Brumm, M. Anterior Cingulate Cortex: An MRI-Based Parcellation Method. Neuroimage 2006, 32, 1167–1175. [Google Scholar] [CrossRef]
- Kho, K.H.; Duffau, H.; Gatignol, P.; Leijten, F.S.S.; Ramsey, N.F.; van Rijen, P.C.; Rutten, G.-J.M. Involuntary Language Switching in Two Bilingual Patients during the Wada Test and Intraoperative Electrocortical Stimulation. Brain Lang. 2007, 101, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Moritz-Gasser, S.; Duffau, H. Cognitive Processes and Neural Basis of Language Switching: Proposal of a New Model. Neuroreport 2009, 20, 1577–1580. [Google Scholar] [CrossRef] [PubMed]
- Moritz-Gasser, S.; Duffau, H. Evidence of a Large-Scale Network Underlying Language Switching: A Brain Stimulation Study: Case Report. J. Neurosurg. 2009, 111, 729–732. [Google Scholar] [CrossRef]
- Sierpowska, J.; Gabarrós, A.; Ripollés, P.; Juncadella, M.; Castañer, S.; Camins, Á.; Plans, G.; Rodríguez-Fornells, A. Intraoperative Electrical Stimulation of Language Switching in Two Bilingual Patients. Neuropsychologia 2013, 51, 2882–2892. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Fornells, A.; de Diego Balaguer, R.; Münte, T.F. Executive Control in Bilingual Language Processing. Lang. Learn. 2006, 56, 133–190. [Google Scholar] [CrossRef]
- Sierpowska, J.; Fernandez-Coello, A.; Gomez-Andres, A.; Camins, À.; Castaner, S.; Juncadella, M.; Gabarrós, A.; Rodriguez-Fornells, A. Involvement of the Middle Frontal Gyrus in Language Switching as Revealed by Electrical Stimulation Mapping and Functional Magnetic Resonance Imaging in Bilingual Brain Tumor Patients. Cortex 2018, 99, 78–92. [Google Scholar] [CrossRef]
- Hernandez, A.E.; Dapretto, M.; Mazziotta, J.; Bookheimer, S. Language Switching and Language Representation in Spanish–English Bilinguals: An FMRI Study. Neuroimage 2001, 14, 510–520. [Google Scholar] [CrossRef] [PubMed]
- De Martino, M.; Talacchi, A.; Capasso, R.; Mazzotta, A.; Miceli, G. Language Assessment in Multilingualism and Awake Neurosurgery. Front. Hum. Neurosci. 2021, 15, 15. [Google Scholar] [CrossRef]
- Antoniou, M.; Wright, S.M. Uncovering the Mechanisms Responsible for Why Language Learning May Promote Healthy Cognitive Aging. Front. Psychol. 2017, 8, 2217. [Google Scholar] [CrossRef]
- Quinteros Baumgart, C.; Billick, S.B. Positive Cognitive Effects of Bilingualism and Multilingualism on Cerebral Function: A Review. Psychiatr. Q. 2018, 89, 273–283. [Google Scholar] [CrossRef]
- Aronin, L.; Singleton, D. Multilingualism as a New Linguistic Dispensation. Int. J. Multiling. 2008, 5, 1–16. [Google Scholar] [CrossRef]
- Ojemann, G.A. Brain Organization for Language from the Perspective of Electrical Stimulation Mapping. Behav. Brain Sci. 1983, 6, 189–206. [Google Scholar] [CrossRef]
- Haglund, M.M.; Berger, M.S.; Shamseldin, M.; Lettich, E.; Ojemann, G.A. Cortical Localization of Temporal Lobe Language Sites in Patients with Gliomas. Neurosurgery 1994, 34, 567–576. [Google Scholar] [PubMed]
- Duffau, H.; Capelle, L.; Sichez, N.; Denvil, D.; Lopes, M.; Sichez, J.; Bitar, A.; Fohanno, D. Intraoperative Mapping of the Subcortical Language Pathways Using Direct Stimulations: An Anatomo-functional Study. Brain 2002, 125, 199–214. [Google Scholar] [CrossRef]
- Bilotta, F.; Rosa, G. ‘Anesthesia’ for Awake Neurosurgery. Curr. Opin. Anesthesiol. 2009, 22, 560–565. [Google Scholar] [CrossRef]
- Stevanovic, A.; Rossaint, R.; Veldeman, M.; Bilotta, F.; Coburn, M. Anaesthesia Management for Awake Craniotomy: Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0156448. [Google Scholar] [CrossRef]
- Kanno, A.; Mikuni, N. Evaluation of Language Function under Awake Craniotomy. Neurol. Med. Chir. 2014, 55, 367–373. [Google Scholar] [CrossRef]
- Dronkers, N.F. A New Brain Region for Coordinating Speech Articulation. Nature 1996, 384, 159–161. [Google Scholar] [CrossRef]
- Tettamanti, M.; Weniger, D. Broca’s Area: A Supramodal Hierarchical Processor? Cortex 2006, 42, 491–494. [Google Scholar] [CrossRef]
- Duffau, H. New Concepts in Surgery of WHO Grade II Gliomas: Functional Brain Mapping, Connectionism and Plasticity—A Review. J. Neurooncol. 2006, 79, 77–115. [Google Scholar] [CrossRef] [PubMed]
- Mesulam, M.-M.; Thompson, C.K.; Weintraub, S.; Rogalski, E.J. The Wernicke Conundrum and the Anatomy of Language Comprehension in Primary Progressive Aphasia. Brain 2015, 138, 2423–2437. [Google Scholar] [CrossRef] [PubMed]
- Burdach, K.F. Vom Baue Und Leben Des Gehirns; Dyk: Leipzig, Germany, 1826; Volume 3. [Google Scholar]
- Tate, M.C.; Herbet, G.; Moritz-Gasser, S.; Tate, J.E.; Duffau, H. Reply: Probabilistic Map of Language Regions: Challenge and Implication. Brain 2015, 138, e338. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Herbet, G.; Maheu, M.; Costi, E.; Lafargue, G.; Duffau, H. Mapping Neuroplastic Potential in Brain-Damaged Patients. Brain 2016, 139, 829–844. [Google Scholar] [CrossRef]
- Young, J.S.; Lee, A.T.; Chang, E.F. A Review of Cortical and Subcortical Stimulation Mapping for Language. Neurosurgery 2021, 89, 331–342. [Google Scholar] [CrossRef]
- Kinoshita, K.; Delcroix, M.; Gannot, S.; Habets, E.A.P.; Haeb-Umbach, R.; Kellermann, W.; Leutnant, V.; Maas, R.; Nakatani, T.; Raj, B. A Summary of the REVERB Challenge: State-of-the-Art and Remaining Challenges in Reverberant Speech Processing Research. EURASIP J. Adv. Signal Process. 2016, 2016, 7. [Google Scholar] [CrossRef]
- Mandonnet, E.; Sarubbo, S.; Duffau, H. Proposal of an Optimized Strategy for Intraoperative Testing of Speech and Language during Awake Mapping. Neurosurg. Rev. 2017, 40, 29–35. [Google Scholar] [CrossRef]
- Weng, S.-M.; Fang, S.-Y.; Li, L.-W.; Fan, X.; Wang, Y.-Y.; Jiang, T. Intra-Operative Mapping and Language Protection in Glioma. Chin. Med. J. 2021, 134, 2398–2402. [Google Scholar] [CrossRef]
- Legault, J.; Grant, A.; Fang, S.-Y.; Li, P. A Longitudinal Investigation of Structural Brain Changes during Second Language Learning. Brain Lang. 2019, 197, 104661. [Google Scholar] [CrossRef]
- Yagmurlu, K.; Middlebrooks, E.H.; Tanriover, N.; Rhoton, A.L. Fiber Tracts of the Dorsal Language Stream in the Human Brain. J. Neurosurg. 2016, 124, 1396–1405. [Google Scholar] [CrossRef]
- Liu, X.; Tu, L.; Wang, J.; Jiang, B.; Gao, W.; Pan, X.; Li, M.; Zhong, M.; Zhu, Z.; Niu, M. Onset Age of L2 Acquisition Influences Language Network in Early and Late Cantonese-Mandarin Bilinguals. Brain Lang. 2017, 174, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, Y.; Tang, A.C.; Liu, N.; Wang, X.; Du, Y.; Hu, W. English Spoken Word Segmentation Activates the Prefrontal Cortex and Temporo-Parietal Junction in Chinese ESL Learners: A Functional near-Infrared Spectroscopy (FNIRS) Study. Brain Res. 2020, 1733, 146693. [Google Scholar] [CrossRef] [PubMed]
- Thieba, C.; Long, X.; Dewey, D.; Lebel, C. Young Children in Different Linguistic Environments: A Multimodal Neuroimaging Study of the Inferior Frontal Gyrus. Brain Cogn. 2019, 134, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Rapport, R.L.; Tan, C.T.; Whitaker, H.A. Language Function and Dysfunction among Chinese-and English-Speaking Polyglots: Cortical Stimulation, Wada Testing, and Clinical Studies. Brain Lang. 1983, 18, 342–366. [Google Scholar] [CrossRef]
- Pouratian, N.; Bookheimer, S.Y.; Rex, D.E.; Martin, N.A.; Toga, A.W. Utility of Preoperative Functional Magnetic Resonance Imaging for Identifying Language Cortices in Patients with Vascular Malformations. J. Neurosurg. 2002, 97, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Chee, M.W.L.; Weekes, B.; Lee, K.M.; Soon, C.S.; Schreiber, A.; Hoon, J.J.; Chee, M. Overlap and Dissociation of Semantic Processing of Chinese Characters, English Words, and Pictures: Evidence from FMRI. Neuroimage 2000, 12, 392–403. [Google Scholar] [CrossRef]
- Yetkin, F.Z.; Mueller, W.M.; Morris, G.L.; McAuliffe, T.L.; Ulmer, J.L.; Cox, R.W.; Daniels, D.L.; Haughton, V.M. Functional MR Activation Correlated with Intraoperative Cortical Mapping. Am. J. Neuroradiol. 1997, 18, 1311–1315. [Google Scholar]
- Schlosser, M.J.; Luby, M.; Spencer, D.D.; Awad, I.A.; McCarthy, G. Comparative Localization of Auditory Comprehension by Using Functional Magnetic Resonance Imaging and Cortical Stimulation. J. Neurosurg. 1999, 91, 626–635. [Google Scholar] [CrossRef]
- Lurito, J.T.; Lowe, M.J.; Sartorius, C.; Mathews, V.P. Comparison of FMRI and Intraoperative Direct Cortical Stimulation in Localization of Receptive Language Areas. J. Comput. Assist. Tomogr. 2000, 24, 99–105. [Google Scholar] [CrossRef]
- Maknojia, S.; Tam, F.; Das, S.; Schweizer, T.; Graham, S.J. Visualization of Brain Shift Corrected Functional Magnetic Resonance Imaging Data for Intraoperative Brain Mapping. World Neurosurg. X 2019, 2, 100021. [Google Scholar] [CrossRef]
- Sakr, H.M.; Mohamed, M.A.; Jalalod, H.; Abbas, Y.A. Influence of FMRI on Operative Planning of Brain Tumors: Initial Experience in a Histopathologically Variable Subset of Tumors. Egypt. J. Radiol. Nucl. Med. 2011, 42, 215–221. [Google Scholar] [CrossRef][Green Version]
- Benson, R.R.; FitzGerald, D.B.; LeSueur, L.L.; Kennedy, D.N.; Kwong, K.K.; Buchbinder, B.R.; Davis, T.L.; Weisskoff, R.M.; Talavage, T.M.; Logan, W.J. Language Dominance Determined by Whole Brain Functional MRI in Patients with Brain Lesions. Neurology 1999, 52, 798. [Google Scholar] [CrossRef] [PubMed]
- Leung, L.W.L.; Unadkat, P.; Bertotti, M.M.; Bi, W.L.; Essayed, W.I.; Bunevicius, A.; Chavakula, V.; Rigolo, L.; Fumagalli, L.; Tie, Z. Clinical Utility of Preoperative Bilingual Language FMRI Mapping in Patients with Brain Tumors. J. Neuroimaging 2020, 30, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Nimsky, C.; Ganslandt, O.; Cerny, S.; Hastreiter, P.; Greiner, G.; Fahlbusch, R. Quantification of, Visualization of, and Compensation for Brain Shift Using Intraoperative Magnetic Resonance Imaging. Neurosurgery 2000, 47, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.L.G.; Maurer, C.R.; Maciunas, R.J.; Maciunas, R.J.; Barwise, J.A.; Fitzpatrick, J.M.; Wang, M.Y. Measurement of Intraoperative Brain Surface Deformation under a Craniotomy. Neurosurgery 1998, 43, 514–526. [Google Scholar] [CrossRef]
- Gerard, I.J.; Kersten-Oertel, M.; Petrecca, K.; Sirhan, D.; Hall, J.A.; Collins, D.L. Brain Shift in Neuronavigation of Brain Tumors: A Review. Med. Image Anal. 2017, 35, 403–420. [Google Scholar] [CrossRef]
- Ohue, S.; Kumon, Y.; Nagato, S.; Kohno, S.; Harada, H.; Nakagawa, K.; Kikuchi, K.; Miki, H.; Ohnishi, T. Evaluation of Intraoperative Brain Shift Using an Ultrasound-Linked Navigation System for Brain Tumor Surgery. Neurol. Med. Chir. 2010, 50, 291–300. [Google Scholar] [CrossRef]
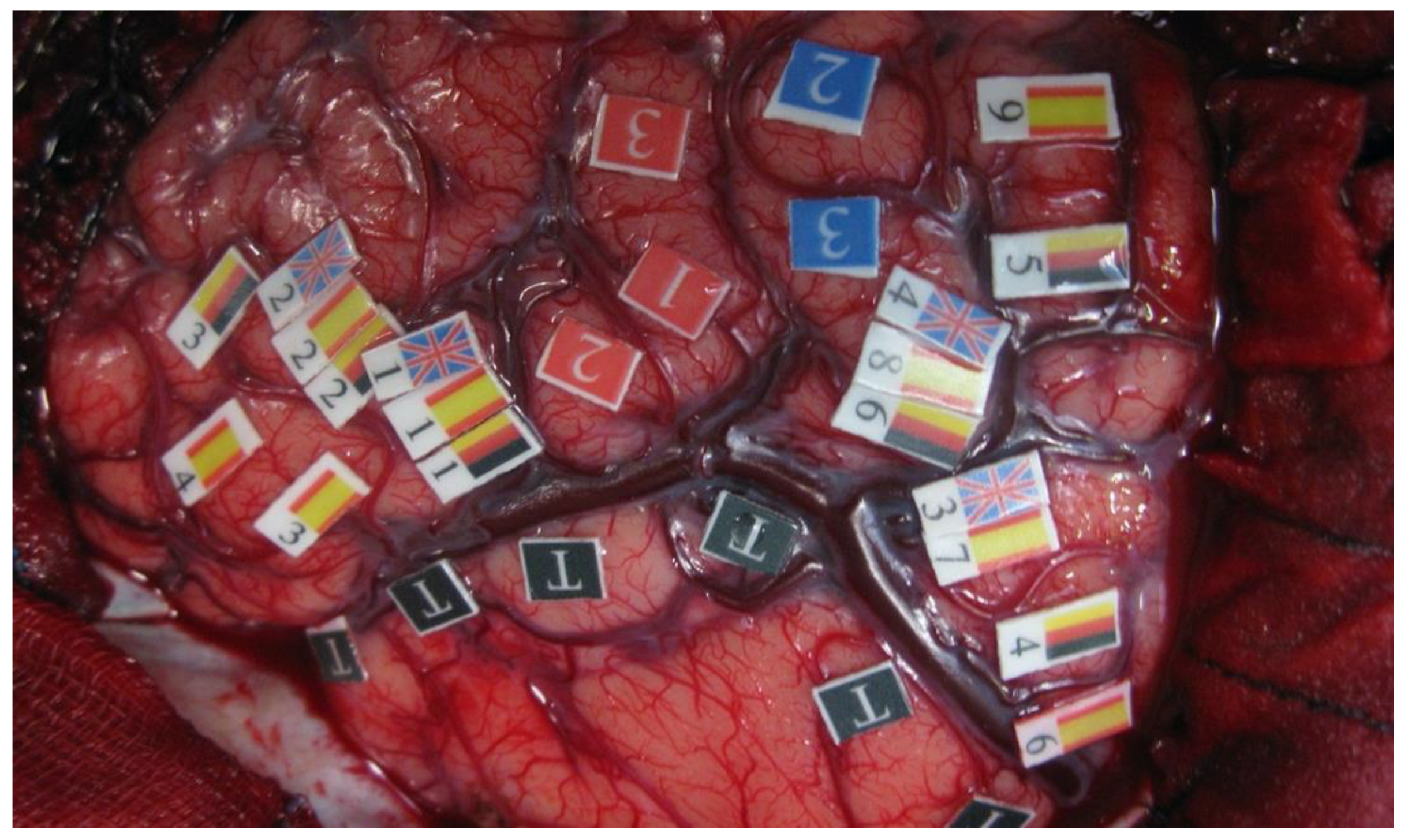
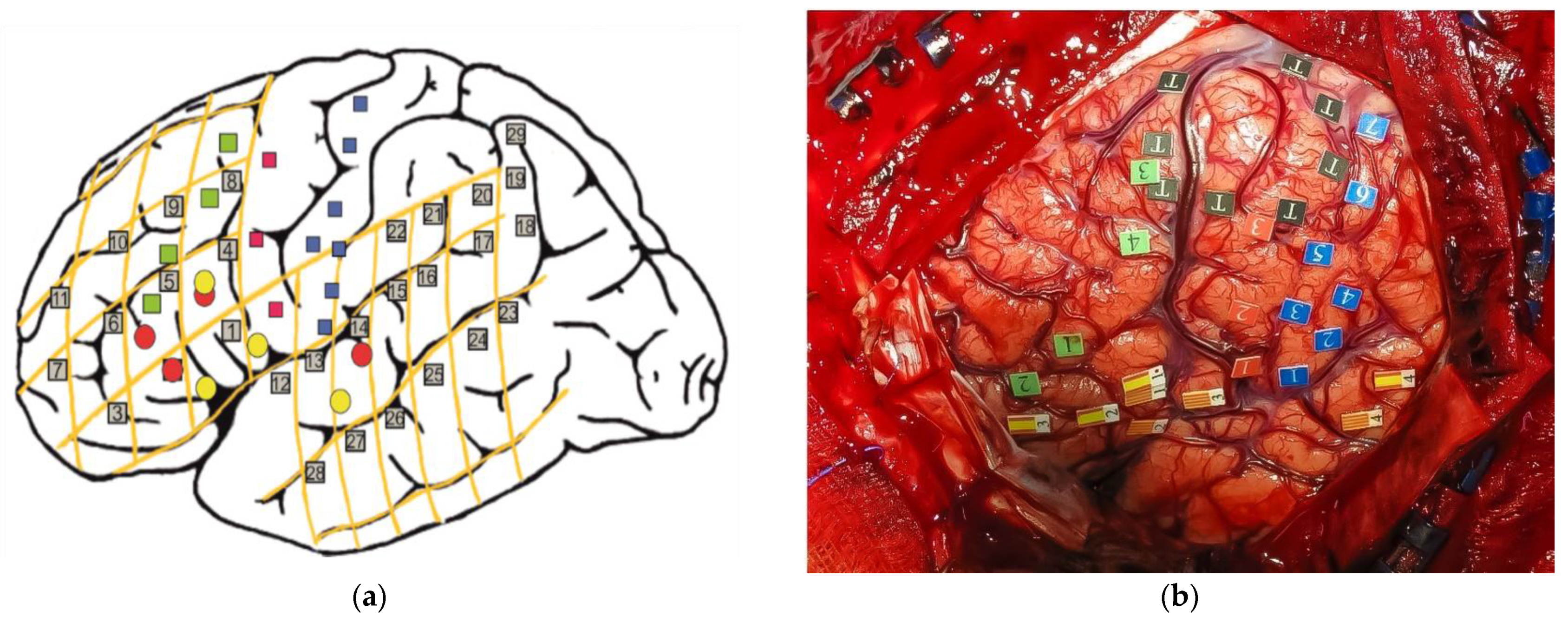
| The degree of anatomical–functional integration or separation of languages |
| How the age of L2-acquisition does affect language organization |
| Neural underpinnings underlying language switching in multilinguals |
| Cortical and subcortical pathways in multilingualism: beyond Broca and Wernicke |
| Multilingual brain mapping technique: a gold standard with limitations |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Fernández, J.; Gabarrós, A.; Fernandez-Coello, A. Intraoperative Brain Mapping in Multilingual Patients: What Do We Know and Where Are We Going? Brain Sci. 2022, 12, 560. https://doi.org/10.3390/brainsci12050560
Martín-Fernández J, Gabarrós A, Fernandez-Coello A. Intraoperative Brain Mapping in Multilingual Patients: What Do We Know and Where Are We Going? Brain Sciences. 2022; 12(5):560. https://doi.org/10.3390/brainsci12050560
Chicago/Turabian StyleMartín-Fernández, Jesús, Andreu Gabarrós, and Alejandro Fernandez-Coello. 2022. "Intraoperative Brain Mapping in Multilingual Patients: What Do We Know and Where Are We Going?" Brain Sciences 12, no. 5: 560. https://doi.org/10.3390/brainsci12050560
APA StyleMartín-Fernández, J., Gabarrós, A., & Fernandez-Coello, A. (2022). Intraoperative Brain Mapping in Multilingual Patients: What Do We Know and Where Are We Going? Brain Sciences, 12(5), 560. https://doi.org/10.3390/brainsci12050560






