Inter-Individual Variability in tDCS Effects: A Narrative Review on the Contribution of Stable, Variable, and Contextual Factors
Abstract
1. Introduction
2. Stable Individual Factors of Inter-Individual Variability
2.1. Anatomical Features: Skull Thickness, Cortex Morphology, and Gyrification
2.2. Neurochemical Factors
2.3. Genetic Profile
2.4. Age and Gender Effects
3. Variable Individual Factors of Inter-Individual Variability
3.1. Endogenous Hormonal Variation
3.2. Exogenous Non-Medical Substances Assumption
3.3. Exogenous Medical Substances Consumption
4. Contextual and Experimental Features as a Source of Inter-Individual Variability
4.1. Baseline Capacity Levels as a Source of Inter-Individual Variability
4.2. Task Difficulty as a Source of Inter-Individual Variability
5. Discussion
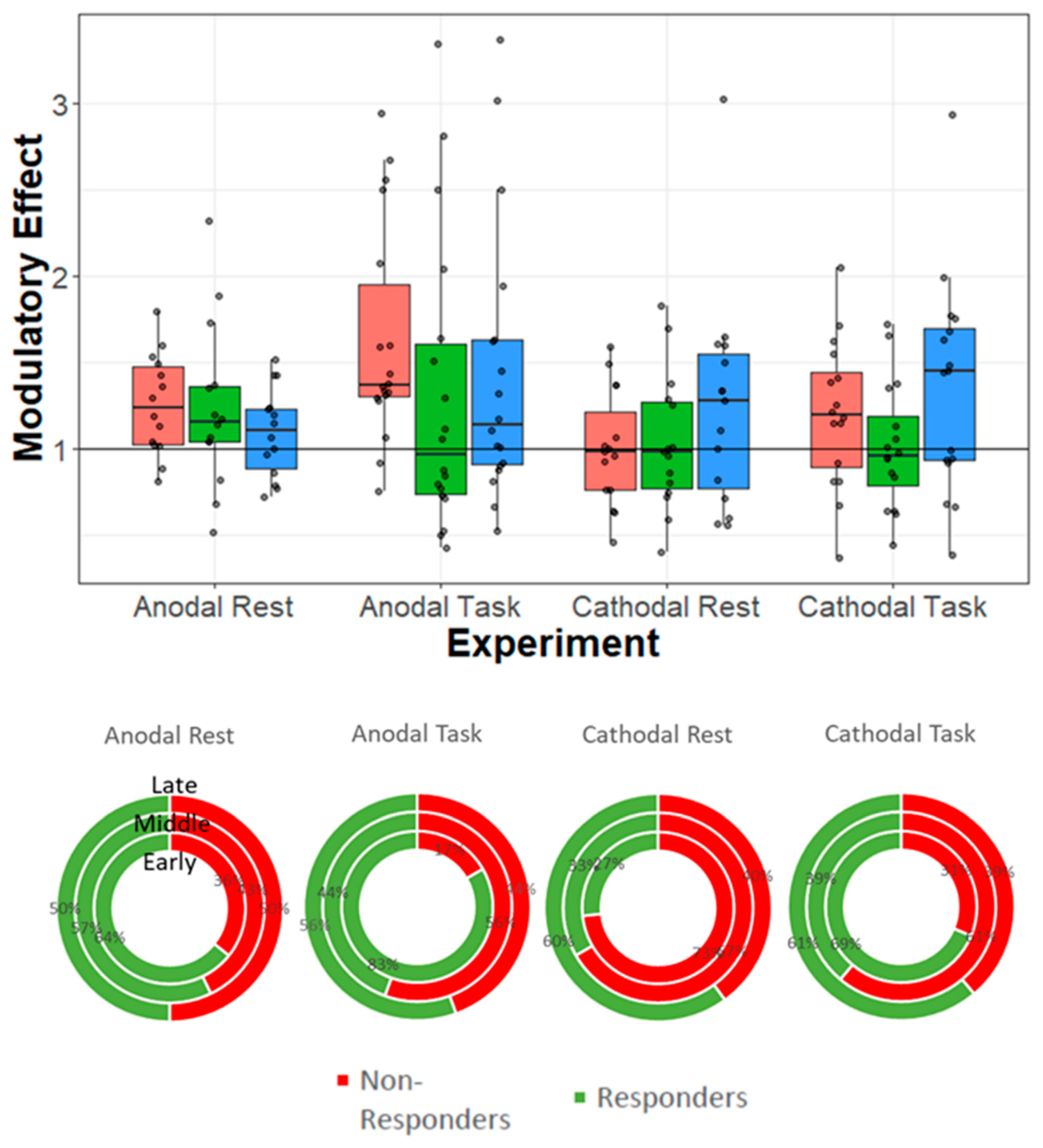
5.1. Data Analysis and Results Presentation
5.2. Computational Modeling to Set tDCS Parameters
5.3. Task and Outcome Measure Selection
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2020, 527, 633. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Paulus, W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 2001, 57, 1899. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Cohen, L.G.; Wassermann, E.M.; Priori, A.; Lang, N.; Antal, A.; Paulus, W.; Hummel, F.; Boggio, P.S.; Fregni, F.; et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008, 1, 206–223. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, G.; Cirillo, G.; di Pino, G.; Capone, F.; Ranieri, F.; Florio, L.; Todisco, V.; Tedeschi, G.; Funke, K.; di Lazzaro, V. Neurobiological after-effects of non-invasive brain stimulation. Brain Stimul. 2017, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lauro, L.J.R.; Rosanova, M.; Mattavelli, G.; Convento, S.; Pisoni, A.; Opitz, A.; Bolognini, N.; Vallar, G. TDCS increases cortical excitability: Direct evidence from TMS-EEG. Cortex 2014, 58, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Lauro, L.J.R.; Pisoni, A.; Rosanova, M.; Casarotto, S.; Mattavelli, G.; Bolognini, N.; Vallar, G. Localizing the effects of anodal tDCS at the level of cortical sources: A Reply to Bailey et al., 2015. Cortex 2016, 74, 323–328. [Google Scholar] [CrossRef]
- Pisoni, A.; Mattavelli, G.; Papagno, C.; Rosanova, M.C.E.; Casali, A.G.; Lauro, L.J.R. Cognitive Enhancement Induced by Anodal tDCS Drives Circuit-Specific Cortical Plasticity. Cereb. Cortex 2018, 28, 1132–1140. [Google Scholar] [CrossRef]
- Varoli, E.; Pisoni, A.; Mattavelli, G.C.; Vergallito, A.; Gallucci, A.; Mauro, L.D.; Rosanova, M.; Bolognini, N.; Vallar, G.; Lauro, L.J.R. Tracking the effect of cathodal transcranial direct current stimulation on cortical excitability and connectivity by means of TMS-EEG. Front. Neurosci. 2018, 12, 319. [Google Scholar] [CrossRef]
- Fregni, F.; El-Hagrassy, M.M.; Pacheco-Barrios, K.; Carvalho, S.; Leite, J.; Simis, M.; Brunelin, J.; Nakamura-Palacios, E.M.; Marangolo, P.; Venkatasubramanian, G. Evidence-based guidelines and secondary meta-analysis for the use of transcranial direct current stimulation in neurological and psychiatric disorders. Int. J. Neuropsychopharmacol. 2021, 24, 256–313. [Google Scholar] [CrossRef]
- Vergallito, A.; Gallucci, A.; Pisoni, A.; Punzi, M.; Caselli, G.; Ruggiero, G.M.; Sassaroli, S.; Romero Lauro, L.J. Effectiveness of noninvasive brain stimulation in the treatment of anxiety disorders: A meta-analysis of sham or behaviour-controlled studies. J. Psychiatry Neurosci. 2021, 46, E592–E614. [Google Scholar] [CrossRef]
- Razza, L.B.; Palumbo, P.; Moffa, A.H.; Carvalho, A.F.; Solmi, M.; Loo, C.K.; Brunoni, A.R. A systematic review and meta-analysis on the effects of transcranial direct current stimulation in depressive episodes. Depress. Anxiety 2020, 37, 594–608. [Google Scholar] [CrossRef] [PubMed]
- Bocci, T.; Ferrucci, R.; Priori, A. Neurophysiological Bases and Mechanisms of Action of Transcranial Direct Current Stimulation (tDCS). In Non Invasive Brain Stimulation in Psychiatry and Clinical Neurosciences; Springer: Berlin/Heidelberg, Germany, 2020; pp. 19–29. [Google Scholar]
- Stagg, C.J.; Antal, A.; Nitsche, M.A. Physiology of Transcranial Direct Current Stimulation. J. ECT 2018, 34, 3. Available online: https://journals.lww.com/ectjournal/Fulltext/2018/09000/Physiology_of_Transcranial_Direct_Current.3.aspx (accessed on 16 December 2021). [CrossRef] [PubMed]
- Nitsche, M.A.; Nitsche, M.S.; Klein, C.C.; Tergau, F.; Rothwell, J.; Paulus, W. Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Clin. Neurophysiol. 2003, 114, 600–604. [Google Scholar] [CrossRef]
- Jacobson, L.; Koslowsky, M.; Lavidor, M. tDCS polarity effects in motor and cognitive domains: A meta-analytical review. Exp. Brain Res. 2012, 216, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.T.; Fitzgerald, P.; Hoy, K. Effects of Anodal Transcranial Direct Current Stimulation on Working Memory: A Systematic Review and Meta-Analysis of Findings from Healthy and Neuropsychiatric Populations. Brain Stimul. 2016, 9, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Horvath, J.C.; Forte, J.D.; Carter, O. Evidence that transcranial direct current stimulation (tDCS) generates little-to-no reliable neurophysiologic effect beyond MEP amplitude modulation in healthy human subjects: A systematic review. Neuropsychologia 2015, 66, 213–236. [Google Scholar] [CrossRef] [PubMed]
- Dedoncker, J.; Brunoni, A.R.; Baeken, C.; Vanderhasselt, M.A. A systematic review and meta-analysis of the effects of transcranial direct current stimulation (tDCS) over the dorsolateral prefrontal cortex in healthy and neuropsychiatric samples: Influence of stimulation parameters. Brain Stimul. 2016, 9, 501–517. [Google Scholar] [CrossRef]
- Antal, A.; Keeser, D.; Priori, A.; Padberg, F.; Nitsche, M.A. Conceptual and procedural shortcomings of the systematic review ‘evidence that transcranial direct current stimulation (tDCS) generates little-to-no reliable neurophysiologic effect beyond MEP amplitude modulation in healthy human subjects: A systematic review’ by Horvath and co-workers. Brain Stimul. 2015, 8, 846–849. [Google Scholar]
- Chhatbar, P.Y.; Feng, W. Data synthesis in meta-analysis may conclude differently on cognitive effect from transcranial direct current stimulation. Brain Stimul. 2015, 8, 974–976. [Google Scholar] [CrossRef]
- Gurevitch, J.; Koricheva, J.; Nakagawa, S.; Stewart, G. Meta-analysis and the science of research synthesis. Nature 2018, 555, 175–182. [Google Scholar] [CrossRef]
- Ridding, M.C.; Ziemann, U. Determinants of the induction of cortical plasticity by non-invasive brain stimulation in healthy subjects. J. Physiol. 2010, 588, 2291–2304. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Uehara, K.; Hanakawa, T. The contribution of interindividual factors to variability of response in transcranial direct current stimulation studies. Front. Cell. Neurosci. 2015, 9, 181. [Google Scholar] [CrossRef] [PubMed]
- Imburgio, M.J.; Orr, J.M. Effects of prefrontal tDCS on executive function: Methodological considerations revealed by meta-analysis. Neuropsychologia 2018, 117, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Brunoni, A.R.; Vanderhasselt, M.-A. Working memory improvement with non-invasive brain stimulation of the dorsolateral prefrontal cortex: A systematic review and meta-analysis. Brain Cogn. 2014, 86, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Alonso, V.; Cheeran, B.; Río-Rodríguez, D.; Fernández-Del-Olmo, M. Inter-individual variability in response to non-invasive brain stimulation paradigms. Brain Stimul. 2014, 7, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Wiethoff, S.; Hamada, M.; Rothwell, J.C. Variability in response to transcranial direct current stimulation of the motor cortex. Brain Stimul. 2014, 7, 468–475. [Google Scholar] [CrossRef]
- Pellegrini, M.; Zoghi, M.; Jaberzadeh, S. Cluster analysis and subgrouping to investigate inter-individual variability to non-invasive brain stimulation: A systematic review. Rev. Neurosci. 2018, 29, 675–697. [Google Scholar] [CrossRef]
- Ammann, C.; Lindquist, M.A.; Celnik, P.A. Response variability of different anodal transcranial direct current stimulation intensities across multiple sessions. Brain Stimul. 2017, 10, 757–763. [Google Scholar] [CrossRef]
- Chew, T.; Ho, K.-A.; Loo, C.K. Inter- and intra-individual variability in response to transcranial direct current stimulation (tDCS) at varying current intensities. Brain Stimul. 2015, 8, 1130–1137. [Google Scholar] [CrossRef]
- Labruna, L.; Jamil, A.; Fresnoza, S.; Batsikadze, G.; Kuo, M.F.; Vanderschelden, B.; Ivry, R.B.; Nitsche, M.A. Efficacy of Anodal Transcranial Direct Current Stimulation is Related to Sensitivity to Transcranial Magnetic Stimulation. Brain Stimul. 2016, 9, 8–15. [Google Scholar] [CrossRef]
- Lopez-Alonso, V.; del Olmo, M.F.; Costantini, A.; Henríquez, J.J.G.; Cheeran, B. Intra-individual variability in the response to anodal transcranial direct current stimulation. Clin. Neurophysiol. 2015, 126, 2342–2347. [Google Scholar] [CrossRef] [PubMed]
- Puri, R.; Hinder, M.; Fujiyama, H.; Gomez, R.; Carson, R.; Summers, J.J. Duration-dependent effects of the BDNF Val66Met polymorphism on anodal tDCS induced motor cortex plasticity in older adults: A group and individual perspective. Front. Aging Neurosci. 2015, 7, 107. [Google Scholar] [CrossRef] [PubMed]
- Puri, R.; Hinder, M.R.; Canty, A.J.; Summers, J.J. Facilitatory non-invasive brain stimulation in older adults: The effect of stimulation type and duration on the induction of motor cortex plasticity. Exp. Brain Res. 2016, 234, 3411–3423. [Google Scholar] [CrossRef] [PubMed]
- Strube, W.; Bunse, T.; Malchow, B.; Hasan, A. Efficacy and interindividual variability in motor-cortex plasticity following anodal tDCS and paired-associative stimulation. Neural Plast. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Strube, W.; Bunse, T.; Nitsche, M.A.; Nikolaeva, A.; Palm, U.; Padberg, F.; Falkai, P.; Hasan, A. Bidirectional variability in motor cortex excitability modulation following 1 mA transcranial direct current stimulation in healthy participants. Physiol. Rep. 2016, 4, e12884. [Google Scholar] [CrossRef]
- Luque-Casado, A.; Fogelson, N.; Iglesias-Soler, E.; Fernandez-Del-Olmo, M. Exploring the effects of Transcranial Direct Current Stimulation over the prefrontal cortex on working memory: A cluster analysis approach. Behav. Brain Res. 2019, 375, 112144. [Google Scholar] [CrossRef]
- Luque-Casado, A.; Rodríguez-Freiría, R.; Fogelson, N.; Iglesias-Soler, E.; Fernández-Del-Olmo, M. An Integrative Clustering Approach to tDCS Individual Response Variability in Cognitive Performance: Beyond a Null Effect on Working Memory. Neuroscience 2020, 443, 120–130. [Google Scholar] [CrossRef]
- Tremblay, S.; Larochelle-Brunet, F.; LaFleur, L.-P.; El Mouderrib, S.; Lepage, J.-F.; Théoret, H. Systematic assessment of duration and intensity of anodal transcranial direct current stimulation on primary motor cortex excitability. Eur. J. Neurosci. 2016, 44, 2184–2190. [Google Scholar] [CrossRef]
- Guerra, A.; Lopez-Alonso, V.; Cheeran, B.; Suppa, A. Variability in non-invasive brain stimulation studies: Reasons and results. Neurosci. Lett. 2020, 719, 133330. [Google Scholar] [CrossRef]
- Benwell, C.S.; Learmonth, G.; Miniussi, C.; Harvey, M.; Thut, G. Non-linear effects of transcranial direct current stimulation as a function of individual baseline performance: Evidence from biparietal tDCS influence on lateralized attention bias. Cortex 2015, 69, 152–165. [Google Scholar] [CrossRef]
- Jamil, A.; Nitsche, M.A. What Effect Does tDCS Have on the Brain? Basic Physiology of tDCS. Curr. Behav. Neurosci. Rep. 2017, 4, 331–340. [Google Scholar] [CrossRef]
- Opitz, A.; Paulus, W.; Will, S.; Antunes, A.; Thielscher, A. Determinants of the electric field during transcranial direct current stimulation. Neuroimage 2015, 109, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Bikson, M.; Rahman, A.; Datta, A. Computational models of transcranial direct current stimulation. Clin. EEG Neurosci. 2012, 43, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Wang, H.; Zhang, J.; Yan, T.; Pei, G. Multi-layer skull modeling and importance for tDCS simulation. In Proceedings of the 2021 International Conference on Bioinformatics and Intelligent Computing, BIC 2021, Harbin, China, 22–24 January 2021; pp. 250–256. [Google Scholar] [CrossRef]
- Pellegrini, M.; Zoghi, M.; Jaberzadeh, S. Biological and anatomical factors influencing interindividual variability to noninvasive brain stimulation of the primary motor cortex: A systematic review and meta-analysis. Rev. Neurosci. 2018, 29, 199–222. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.; Kim, H.-I.; Jun, S.C. The Effect of a Transcranial Channel as a Skull/Brain Interface in High-Definition Transcranial Direct Current Stimulation—A Computational Study. Sci. Rep. 2017, 7, 40612. [Google Scholar] [CrossRef]
- Horvath, J.C.; Carter, O.; Forte, J.D. Transcranial direct current stimulation: Five important issues we aren’t discussing (but probably should be). Front. Syst. Neurosci. 2014, 8, 2014. Available online: https://www.frontiersin.org/article/10.3389/fnsys.2014.00002 (accessed on 16 December 2021). [CrossRef]
- Miranda, P.C.; Mekonnen, A.; Salvador, R.; Ruffini, G. The electric field in the cortex during transcranial current stimulation. Neuroimage 2013, 70, 48–58. [Google Scholar] [CrossRef]
- Datta, A. Inter-individual variation during transcranial direct current stimulation and normalization of dose using MRI-derived computational models. Front. Psychiatry 2012, 3, 91. [Google Scholar] [CrossRef]
- Suh, H.S.; Lee, W.H.; Kim, T.-S. Influence of anisotropic conductivity in the skull and white matter on transcranial direct current stimulation via an anatomically realistic finite element head model. Phys. Med. Biol. 2012, 57, 6961–6980. [Google Scholar] [CrossRef]
- Miranda, P.C.; Lomarev, M.; Hallett, M. Modeling the current distribution during transcranial direct current stimulation. Clin. Neurophysiol. 2006, 117, 1623–1629. [Google Scholar] [CrossRef]
- Datta, A.; Bansal, V.; Diaz, J.; Patel, J.; Reato, D.; Bikson, M. Gyri-precise head model of transcranial direct current stimulation: Improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimul. 2009, 2, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Laakso, I.; Tanaka, S.; Koyama, S.; De Santis, V.; Hirata, A. Inter-subject variability in electric fields of motor cortical tDCS. Brain Stimul. 2015, 8, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Tatti, E.; Rossi, S.; Innocenti, I.; Rossi, A.; Santarnecchi, E. Non-invasive brain stimulation of the aging brain: State of the art and future perspectives. Ageing Res. Rev. 2016, 29, 66–89. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, S.; Towhidkhah, F. Computational human head models of tDCS: Influence of brain atrophy on current density distribution. Brain Stimul. 2018, 11, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Hanley, C.J.; Tales, A. Anodal tDCS improves attentional control in older adults. Exp. Gerontol. 2019, 115, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Datta, A.; Bikson, M.; Fregni, F. Transcranial direct current stimulation in patients with skull defects and skull plates: High-resolution computational FEM study of factors altering cortical current flow. Neuroimage 2010, 52, 1268–1278. [Google Scholar] [CrossRef]
- Wagner, T.; Fregni, F.; Fecteau, S.; Grodzinsky, A.; Zahn, M.; Pascual-Leone, A. Transcranial direct current stimulation: A computer-based human model study. Neuroimage 2007, 35, 1113–1124. [Google Scholar] [CrossRef]
- Indahlastari, A.; Albizu, A.; O’Shea, A.; Forbes, M.A.; Nissim, N.R.; Kraft, J.N.; Evangelista, N.D.; Hausman, H.K.; Woods, A.J. Modeling transcranial electrical stimulation in the aging brain. Brain Stimul. 2020, 13, 664–674. [Google Scholar] [CrossRef]
- Dahnke, R.; Yotter, R.A.; Gaser, C. Cortical thickness and central surface estimation. Neuroimage 2013, 65, 336–348. [Google Scholar] [CrossRef]
- Mahdavi, S.; Yavari, F.; Gharibzadeh, S.; Towhidkhah, F. Modeling studies for designing transcranial direct current stimulation protocol in Alzheimer’s disease. Front. Comput. Neurosci. 2014, 8, 72. [Google Scholar] [CrossRef]
- Unal, G.; Ficek, B.; Webster, K.; Shahabuddin, S.; Truong, D.; Hampstead, B.; Bikson, M.; Tsapkini, K. Impact of brain atrophy on tDCS and HD-tDCS current flow: A modeling study in three variants of primary progressive aphasia. Neurol. Sci. 2020, 41, 1781–1789. [Google Scholar] [CrossRef] [PubMed]
- Metwally, M.K.; Han, S.M.; Kim, T.-S. The effect of tissue anisotropy on the radial and tangential components of the electric field in transcranial direct current stimulation. Med. Biol. Eng. Comput. 2015, 53, 1085–1101. [Google Scholar] [CrossRef] [PubMed]
- Shahid, S.; Wen, P.; Ahfock, T. Effects of model complexity and tissue anisotropic conductivity on cortical modulation during transcranial direct current stimulation. IET Sci. Meas. Technol. 2012, 6, 464–473. [Google Scholar] [CrossRef]
- Russell, M.J.; Goodman, T.; Pierson, R.; Shepherd, S.; Wang, Q.; Groshong, B.; Wiley, D.F. Individual differences in transcranial electrical stimulation current density. J. Biomed. Res. 2013, 27, 495–508. [Google Scholar] [CrossRef]
- Shahid, S.; Wen, P.; Ahfock, T. Assessment of electric field distribution in anisotropic cortical and subcortical regions under the influence of tDCS. Bioelectromagnetics 2014, 35, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Rademacher, J.; Caviness, V.S., Jr.; Steinmetz, H.; Galaburda, A.M. Topographical variation of the human primary cortices: Implications for neuroimaging, brain mapping, and neurobiology. Cereb. Cortex 1993, 3, 313–329. [Google Scholar] [CrossRef]
- Ono, S.M.; Kubik, C.D. Abernathey. In Atlas of the Cerebral Sulci; Thieme: New York, NY, USA, 1990. [Google Scholar]
- Filmer, H.L.; Ehrhardt, S.E.; Shaw, T.B.; Mattingley, J.B.; Dux, P.E. The efficacy of transcranial direct current stimulation to prefrontal areas is related to underlying cortical morphology. Neuroimage 2019, 196, 41–48. [Google Scholar] [CrossRef]
- Datta, A.; Baker, J.M.; Bikson, M.; Fridriksson, J. Individualized model predicts brain current flow during transcranial direct-current stimulation treatment in responsive stroke patient. Brain Stimul. 2011, 4, 169–174. [Google Scholar] [CrossRef]
- Rawji, V.; Ciocca, M.; Zacharia, A.; Soares, D.; Truong, D.; Bikson, M.; Rothwell, J.; Bestmann, S. TDCS changes in motor excitability are specific to orientation of current flow. Brain Stimul. 2018, 11, 289–298. [Google Scholar] [CrossRef]
- Bindman, L.J.; Lippold, O.C.J.; Redfearn, J.W.T. Long-lasting changes in the level of the electrical activity of the cerebral cortex produced by polarizing currents. Nature 1962, 196, 584–585. [Google Scholar] [CrossRef]
- Wagner, S.; Rampersad, S.M.; Aydin, Ü.; Vorwerk, J.; Oostendorp, T.F.; Neuling, T.; Herrmann, C.S.; Stegeman, D.F.; Wolters, C.H. Investigation of tDCS volume conduction effects in a highly realistic head model. J. Neural Eng. 2014, 11, 016002. [Google Scholar] [CrossRef] [PubMed]
- Bikson, M.; Inoue, M.; Akiyama, H.; Deans, J.K.; Fox, J.E.; Miyakawa, H.; Jefferys, J.G.R. Effects of uniform extracellular DC electric fields on excitability in rat hippocampal slices in vitro. J. Physiol. 2004, 557, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Reato, D.; Arlotti, M.; Gasca, F.; Datta, A.; Parra, L.C.; Bikson, M. Cellular effects of acute direct current stimulation: Somatic and synaptic terminal effects. J. Physiol. 2013, 591, 2563–2578. [Google Scholar] [CrossRef] [PubMed]
- Komarov, M.; Malerba, P.; Golden, R.; Nunez, P.; Halgren, E.; Bazhenov, M. Selective recruitment of cortical neurons by electrical stimulation. PLoS Comput. Biol. 2019, 15, e1007277. [Google Scholar] [CrossRef]
- Stagg, C.J.; Best, J.G.; Stephenson, M.C.; O’Shea, J.; Wylezinska, M.; Kineses, Z.T.; Morris, P.G.; Matthews, P.M.; Johansen-Berg, H. Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J. Neurosci. 2009, 29, 5202–5206. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Müller-Dahlhaus, F.; Paulus, W.; Ziemann, U. The pharmacology of neuroplasticity induced by non-invasive brain stimulation: Building models for the clinical use of CNS active drugs. J. Physiol. 2012, 590, 4641–4662. [Google Scholar] [CrossRef]
- Stagg, C.J.; Bachtiar, V.; Amadi, U.; Gudberg, C.A.; Ilie, A.S.; Sampaio-Baptista, C.; O’Shea, J.; Woolrich, M.; Smith, S.M.; Filippini, N.; et al. Local GABA concentration is related to network-level resting functional connectivity. Elife 2014, 3, e01465. [Google Scholar] [CrossRef]
- Krause, B.; Márquez-Ruiz, J.; Kadosh, R.C. The effect of transcranial direct current stimulation: A role for cortical excitation/inhibition balance? Front. Hum. Neurosci. 2013, 7, 602. Available online: https://www.frontiersin.org/article/10.3389/fnhum.2013.00602 (accessed on 16 December 2021). [CrossRef]
- Fresnoza, S.; Stiksrud, E.; Klinker, F.; Liebetanz, D.; Paulus, W.; Kuo, M.-F.; Nitsche, M.A. Dosage-dependent effect of dopamine D2 receptor activation on motor cortex plasticity in humans. J. Neurosci. 2014, 34, 10701–10709. [Google Scholar] [CrossRef]
- Monte-Silva, K.; Liebetanz, D.; Grundey, J.; Paulus, W.; Nitsche, M.A. Dosage-dependent non-linear effect of l-dopa on human motor cortex plasticity. J. Physiol. 2010, 588, 3415–3424. [Google Scholar] [CrossRef]
- Fritsch, B.; Reis, J.; Martinowich, K.; Schambra, H.M.; Ji, Y.; Cohen, L.G.; Lu, B. Direct current stimulation promotes BDNF-dependent synaptic plasticity: Potential implications for motor learning. Neuron 2010, 66, 198–204. [Google Scholar] [CrossRef]
- Teo, J.T.H.; Bentley, G.; Lawrence, P.; Soltesz, F.; Miller, S.; Willé, D.; Mchugh, S.; Dodds, C.; Lu, B.; Croft, R.J.; et al. Late cortical plasticity in motor and auditory cortex: Role of met-allele in BDNF Val66Met polymorphism. Int. J. Neuropsychopharmacol. 2014, 17, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Soltesz, F.; Suckling, J.; Lawrence, P.; Tait, R.; Ooi, C.; Bentley, G.; Dodds, C.M.; Miller, S.R.; Wille, D.R.; Byrne, M.; et al. Identification of BDNF sensitive electrophysiological markers of synaptic activity and their structural correlates in healthy subjects using a genetic approach utilizing the functional BDNF Val66Met polymorphism. PLoS ONE 2014, 9, e95558. [Google Scholar] [CrossRef] [PubMed]
- Antal, A.; Chaieb, L.; Moliadze, V.; Zarrouki, D.; Shoukier, M.; Paulus, W. BDNF gene polymorphisms and motor cortical plasticity in healthy humans: When should we consider it. J. Neurosci. Rehabil. 2014, 1, 1–13. [Google Scholar] [CrossRef]
- Nieratschker, V.; Kiefer, C.; Giel, K.; Krüger, R.; Plewnia, C. The COMT Val/Met polymorphism modulates effects of tDCS on response inhibition. Brain Stimul. 2015, 8, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Plewnia, C.; Zwissler, B.; Längst, I.; Maurer, B.; Giel, K.; Krüger, R. Effects of transcranial direct current stimulation (tDCS) on executive functions: Influence of COMT Val/Met polymorphism. Cortex 2013, 49, 1801–1807. [Google Scholar] [CrossRef]
- Farcito, S.; Puonti, O.; Montanaro, H.; Saturnino, G.B.; Nielsen, J.D.; Madsen, C.G.; Siebner, H.R.; Neufeld, E.; Kuster, N.; Lloyd, B.A. Accurate anatomical head segmentations: A data set for biomedical simulations. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 6118–6123. [Google Scholar]
- Russell, M.; Goodman, T.; Wang, Q.; Groshong, B.; Lyeth, B.G. Gender differences in current received during transcranial electrical stimulation. Front. Psychiatry 2014, 5, 104. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Kashyap, R.; Goodwill, A.M.; O’Brien, B.A.; Rapp, B.; Oishi, K.; Desmond, J.E.; Chen, S.H.A. Sex difference in tDCS current mediated by changes in cortical anatomy: A study across young, middle and older adults. Brain Stimul. 2022, 15, 125–140. [Google Scholar] [CrossRef]
- Hunold, A.; Haueisen, J.; Freitag, C.M.; Siniatchkin, M.; Moliadze, V. Chapter 2—Cortical current density magnitudes during transcranial direct current stimulation correlate with skull thickness in children, adolescent and young adults. In Progress in Brain Research; Kadosh, R.C., Zaehle, T., Krauel, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 264, pp. 41–56. [Google Scholar] [CrossRef]
- Kessler, S.K.; Minhas, P.; Woods, A.J.; Rosen, A.; Gorman, C.; Bikson, M. Dosage Considerations for Transcranial Direct Current Stimulation in Children: A Computational Modeling Study. PLoS ONE 2013, 8, e76112. [Google Scholar] [CrossRef]
- Moliadze, V.; Schmanke, T.; Andreas, S.; Lyzhko, E.; Freitag, C.M.; Siniatchkin, M. Stimulation intensities of transcranial direct current stimulation have to be adjusted in children and adolescents. Clin. Neurophysiol. 2015, 126, 1392–1399. [Google Scholar] [CrossRef]
- Fujiyama, H.; Hyde, J.; Hinder, M.R.; Kim, S.-J.; McCormack, G.H.; Vickers, J.C.; Summers, J.J. Delayed plastic responses to anodal tDCS in older adults. Front. Aging Neurosci. 2014, 6, 115. [Google Scholar] [CrossRef]
- Salat, D.H.; Buckner, R.L.; Snyder, A.Z.; Greve, D.N.; Desikan, R.S.R.; Busa, E.; Morris, J.C.; Dale, A.M.; Fischl, B. Thinning of the cerebral cortex in aging. Cereb. Cortex 2004, 14, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Rettmann, M.E.; Kraut, M.A.; Prince, J.L.; Resnick, S.M. Cross-sectional and longitudinal analyses of anatomical sulcal changes associated with aging. Cereb. Cortex 2006, 16, 1584–1594. [Google Scholar] [CrossRef] [PubMed]
- Thambisetty, M.; Wan, J.; Carass, A.; An, Y.; Prince, J.; Resnick, S.M. Longitudinal changes in cortical thickness associated with normal aging. Neuroimage 2010, 52, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Heidegger, T.; Hansen-Goos, O.; Batlaeva, O.; Annak, O.; Ziemann, U.; Lötsch, J. data-driven approach to responder subgroup identification after paired continuous theta burst stimulation. Front. Hum. Neurosci. 2017, 11, 382. [Google Scholar] [CrossRef] [PubMed]
- Hanley, C.J.; Alderman, S.L.; Clemence, E. Optimising cognitive enhancement: Systematic assessment of the effects of tdcs duration in older adults. Brain Sci. 2020, 10, 304. [Google Scholar]
- Smith, M.J.; Keel, J.C.; Greenberg, B.D.; Adams, L.F.; Schmidt, P.J.; Rubinow, D.A.; Wassermann, E.M. Menstrual cycle effects on cortical excitability. Neurology 1999, 53, 2069. [Google Scholar] [CrossRef]
- Inghilleri, M.; Conte, A.; Curra, A.; Frasca, V.; Lorenzano, C.; Berardelli, A. Ovarian hormones and cortical excitability. An rTMS study in humans. Clin. Neurophysiol. 2004, 115, 1063–1068. [Google Scholar] [CrossRef]
- Sale, M.; Ridding, M.C.; Nordstrom, M.A. Cortisol inhibits neuroplasticity induction in human motor cortex. J. Neurosci. 2008, 28, 8285–8293. [Google Scholar] [CrossRef]
- Sale, M.V.; Ridding, M.C.; Nordstrom, M.A. Factors influencing the magnitude and reproducibility of corticomotor excitability changes induced by paired associative stimulation. Exp. Brain Res. 2007, 181, 615–626. [Google Scholar] [CrossRef]
- Labruna, L.; Stark-Inbar, A.; Breska, A.; Dabit, M.; Vanderschelden, B.; Nitsche, M.A.; Ivry, R.B. Individual differences in TMS sensitivity influence the efficacy of tDCS in facilitating sensorimotor adaptation. Brain Stimul. 2019, 12, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M.F.; Paulus, W.; Nitsche, M.A. Sex di¡erences in cortical neuroplasticity in humans. Neuroreport 2006, 17, 1703–1707. Available online: http://journals.lww.com/neuroreport (accessed on 16 December 2021). [CrossRef] [PubMed]
- Rothwell, J.C.; Thompson, P.D.; Day, B.L.; Dick, J.P.R.; Kachi, T.; Cowan, J.M.A.; Marsden, C.D. Motor cortex stimulation in intact man: 1. General characteristics of EMG responses in different muscles. Brain 1987, 110, 1173–1190. [Google Scholar] [CrossRef] [PubMed]
- Ranjit, N.; Young, E.A.; Raghunathan, T.E.; Kaplan, G.A. Modeling cortisol rhythms in a population-based study. Psychoneuroendocrinology 2005, 30, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Nader, N.; Chrousos, G.P.; Kino, T. Interactions of the circadian CLOCK system and the HPA axis. Trends Endocrinol. Metab. 2010, 21, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Clow, A.; Law, R.; Evans, P.; Vallence, A.-M.; Hodyl, N.A.; Goldsworthy, M.R.; Rothwell, J.R.; Ridding, M.C. Day differences in the cortisol awakening response predict day differences in synaptic plasticity in the brain. Stress 2014, 17, 219–223. [Google Scholar] [CrossRef]
- Chagas, A.P.; Monteiro, M.; Mazer, V.; Baltar, A.; Marques, D.; Carneiro, M.; de Araújo, M.; das, G.R.; Piscitelli, D.; Monte-Silva, K. Cortical excitability variability: Insights into biological and behavioral characteristics of healthy individuals. J. Neurol. Sci. 2018, 390, 172–177. [Google Scholar] [CrossRef]
- Smith, M.J.; Adams, L.F.; Schmidt, P.J.; Rubinow, D.R.; Wassermann, E.M. Effects of ovarian hormones on human cortical excitability. Ann. Neurol. 2002, 51, 599–603. [Google Scholar] [CrossRef]
- Zoghi, M.; Vaseghi, B.; Bastani, A.; Jaberzadeh, S.; Galea, M.P. The effects of sex hormonal fluctuations during menstrual cycle on cortical excitability and manual dexterity (a pilot study). PLoS ONE 2015, 10, e0136081. [Google Scholar]
- Hattemer, K.; Knake, S.; Reis, J.; Rochon, J.; Oertel, W.H.; Rosenow, F.; Hamer, H.M. Excitability of the motor cortex during ovulatory and anovulatory cycles: A transcranial magnetic stimulation study. Clin. Endocrinol. 2007, 66, 387–393. [Google Scholar] [CrossRef]
- Tecchio, F.; Zappasodi, F.; Pasqualetti, P.; de Gennaro, L.; Pellicciari, M.C.; Ercolani, M.; Squitti, R.; Rossini, P.M. Age dependence of primary motor cortex plasticity induced by paired associative stimulation. Clin. Neurophysiol. 2008, 119, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Specterman, M.; Bhuiya, A.; Kuppuswamy, A.; Strutton, P.; Catley, M.; Davey, N. The effect of an energy drink containing glucose and caffeine on human corticospinal excitability. Physiol. Behav. 2005, 83, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Lattari, E.; Vieira, L.A.F.; Oliveira, B.R.R.; Unal, G.; Bikson, M.; de Mello Pedreiro, R.C.; Marques Neto, S.R.; Machado, S.; Maranhão-Neto, G.A. Effects of Transcranial Direct Current Stimulation with Caffeine Intake on Muscular Strength and Perceived Exertion. J. Strength Cond. Res. 2019, 33, 1237–1243. Available online: https://journals.lww.com/nsca-jscr/Fulltext/2019/05000/Effects_of_Transcranial_Direct_Current_Stimulation.10.aspx (accessed on 16 December 2021). [CrossRef] [PubMed]
- Thirugnanasambandam, N.; Grundey, J.; Adam, K.; Drees, A.; Skwirba, A.C.; Lang, N.; Paulus, W.; Nitsche, M.A. Nicotinergic impact on focal and non-focal neuroplasticity induced by non-invasive brain stimulation in non-smoking humans. Neuropsychopharmacology 2011, 36, 879–886. [Google Scholar] [CrossRef]
- Batsikadze, G.; Paulus, W.; Grundey, J.; Kuo, M.-F.; Nitsche, M.A. Effect of the nicotinic α4β2-receptor partial agonist varenicline on non-invasive brain stimulation-induced neuroplasticity in the human motor cortex. Cereb. Cortex 2015, 25, 3249–3259. [Google Scholar] [CrossRef]
- Grundey, J.; Thirugnanasambandam, N.; Kaminsky, K.; Drees, A.; Skwirba, A.; Lang, N.; Paulus, W.; Nitsche, M.A. Rapid effect of nicotine intake on neuroplasticity in non-smoking humans. Front. Pharmacol. 2012, 3, 186. [Google Scholar] [CrossRef] [PubMed]
- Grundey, J.; Barlay, J.; Batsikadze, G.; Kuo, M.-F.; Paulus, W.; Nitsche, M. Nicotine modulates human brain plasticity via calcium-dependent mechanisms. J. Physiol. 2018, 596, 5429–5441. [Google Scholar] [CrossRef]
- Lucke, C.; Heidegger, T.; Röhner, M.; Toennes, S.W.; Krivanekova, L.; Müller-Dahlhaus, F.; Ziemann, U. Deleterious effects of a low amount of ethanol on LTP-like plasticity in human cortex. Neuropsychopharmacology 2014, 39, 1508–1518. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Grundey, J.; Liebetanz, D.; Lang, N.; Tergau, F.; Paulus, W. Catecholaminergic consolidation of motor cortical neuroplasticity in humans. Cereb. Cortex 2004, 14, 1240–1245. [Google Scholar] [CrossRef]
- Naish, K.R.; Vedelago, L.; MacKillop, J.; Amlung, M. Effects of neuromodulation on cognitive performance in individuals exhibiting addictive behaviors: A systematic review. Drug Alcohol Depend. 2018, 192, 338–351. [Google Scholar] [CrossRef]
- McLaren, M.E.; Nissim, N.R.; Woods, A.J. The effects of medication use in transcranial direct current stimulation: A brief review. Brain Stimul. 2018, 11, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Liebetanz, D.; Nitsche, M.A.; Tergau, F.; Paulus, W. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain 2002, 125, 2238–2247. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Fricke, K.; Henschke, U.; Schlitterlau, A.; Liebetanz, D.; Lang, N.; Henning, S.; Tergau, F.; Paulus, W. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J. Physiol. 2003, 553, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Lugon, M.D.M.V.; Batsikadze, G.; Fresnoza, S.; Grundey, J.; Kuo, M.-F.; Paulus, W.; Nakamura-Palacios, E.M.; Nitsche, M.A. Mechanisms of Nicotinic Modulation of Glutamatergic Neuroplasticity in Humans. Cereb. Cortex 2017, 27, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Monte-Silva, K.; Kuo, M.F.; Hessenthaler, S.; Fresnoza, S.; Liebetanz, D.; Paulus, W.; Nitsche, M.A. Induction of late LTP-like plasticity in the human motor cortex by repeated non-invasive brain stimulation. Brain Stimul. 2013, 6, 424–432. [Google Scholar] [CrossRef]
- Kuo, M.-F.; Paulus, W.; Nitsche, M.A. Boosting focally-induced brain plasticity by dopamine. Cereb. Cortex 2008, 18, 648–651. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Liebetanz, D.; Schlitterlau, A.; Henschke, U.; Fricke, K.; Frommann, K.; Lang, N.; Henning, S.; Paulus, W.; Tergau, F. GABAergic modulation of DC stimulation-induced motor cortex excitability shifts in humans. Eur. J. Neurosci. 2004, 19, 2720.e6. [Google Scholar] [CrossRef]
- Holbrook, A.M.; Crowther, R.; Lotter, A.; Cheng, C.; King, D. Meta-analysis of benzodiazepine use in the treatment of insomnia. Can. Med. Assoc. J. 2000, 162, 225–233. [Google Scholar]
- Nitsche, M.A.; Kuo, M.-F.; Karrasch, R.; Wächter, B.; Liebetanz, D.; Paulus, W. Serotonin Affects Transcranial Direct Current-Induced Neuroplasticity in Humans. Biol. Psychiatry 2009, 66, 503–508. [Google Scholar] [CrossRef]
- Mojtabai, R.; Olfson, M. National trends in long-term use of antidepressant medications: Results from the US National Health and Nutrition Examination Survey. J. Clin. Psychiatry 2013, 74, 12452. [Google Scholar] [CrossRef]
- Fertonani, A.; Miniussi, C. Transcranial electrical stimulation: What we know and do not know about mechanisms. Neuroscientist 2017, 23, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Romei, V.; Thut, G.; Silvanto, J. Information-based approaches of noninvasive transcranial brain stimulation. Trends Neurosci. 2016, 39, 782–795. [Google Scholar] [CrossRef] [PubMed]
- Miniussi, C.; Harris, J.; Ruzzoli, M. Modelling non-invasive brain stimulation in cognitive neuroscience. Neurosci. Biobehav. Rev. 2013, 37, 1702–1712. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Orgogozo, V. The loci of repeated evolution: A catalog of genetic hotspots of phenotypic variation. Evolution 2013, 67, 1235–1250. [Google Scholar] [CrossRef]
- Ohn, S.H.; Park, C.-I.; Yoo, W.-K.; Ko, M.-H.; Choi, K.P.; Kim, G.-M.; Lee, Y.T.; Kim, Y.-H. Time-dependent effect of transcranial direct current stimulation on the enhancement of working memory. Neuroreport 2008, 19, 43–47. [Google Scholar] [CrossRef]
- Reis, J.; Fritsch, B. Modulation of motor performance and motor learning by transcranial direct current stimulation. Curr. Opin. Neurol. 2011, 24, 590–596. [Google Scholar] [CrossRef]
- Stagg, C.; Jayaram, G.; Pastor, D.; Kincses, Z.; Matthews, P.; Johansen-Berg, H. Johansen-Berg. Polarity and timing-dependent effects of transcranial direct current stimulation in explicit motor learning. Neuropsychologia 2011, 49, 800–804. [Google Scholar] [CrossRef]
- Bikson, M.; Rahman, A. Origins of specificity during tDCS: Anatomical, activity-selective, and input-bias mechanisms. Front. Hum. Neurosci. 2013, 7, 688. [Google Scholar] [CrossRef]
- De Almeida, L.R.; Pope, P.A.; Hansen, P.C. Task load modulates tDCS effects on brain network for phonological processing. Cogn. Process. 2020, 21, 341–363. [Google Scholar] [CrossRef]
- Varoli, E.; Pisoni, A.; Mattavelli, G.; Vergallito, A.; del Mauro, L.; Vallar, G.; Lauro, L.J.R. P74 TMS-EEG: A promising tool to study the tDCS effects on cortical excitability. Clin. Neurophysiol. 2020, 131, e53. [Google Scholar] [CrossRef]
- Sathappan, A.V.; Luber, B.M.; Lisanby, S.H. The dynamic duo: Combining noninvasive brain stimulation with cognitive interventions. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 89, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Hussey, E.K.; Ward, N.; Christianson, K.; Kramer, A. Language and memory improvements following tDCS of left lateral prefrontal cortex. PLoS ONE 2015, 10, e0141417. [Google Scholar] [CrossRef]
- Nozari, N.; Woodard, K.; Thompson-Schill, S.L. Consequences of cathodal stimulation for behavior: When does it help and when does it hurt performance? PLoS ONE 2014, 9, e84338. [Google Scholar] [CrossRef] [PubMed]
- Pope, P.A.; Brenton, J.W.; Miall, R.C. Task-specific facilitation of cognition by anodal transcranial direct current stimulation of the prefrontal cortex. Cereb. Cortex 2015, 25, 4551–4558. [Google Scholar] [CrossRef]
- Tseng, P.; Hsu, T.Y.; Chang, C.F.; Tzeng, O.J.L.; Hung, D.L.; Muggleton, N.G.; Walsh, V.; Liang, W.K.; Cheng, S.K.; Juan, C.H. Unleashing potential: Transcranial direct current stimulation over the right posterior parietal cortex improves change detection in low-performing individuals. J. Neurosci. 2012, 32, 10554–10561. [Google Scholar] [CrossRef]
- Learmonth, G.; Thut, G.; Benwell, C.; Harvey, M. The implications of state-dependent tDCS effects in aging: Behavioural response is determined by baseline performance. Neuropsychologia 2015, 74, 108–119. [Google Scholar] [CrossRef]
- Hsu, T.-Y.; Juan, C.-H.; Tseng, P. Individual differences and state-dependent responses in transcranial direct current stimulation. Front. Hum. Neurosci. 2016, 10, 643. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, J.; Yuan, Z. An ERP investigation on the second language and emotion perception: The role of emotion word type. Int. J. Biling. Educ. Biling. 2019, 25, 539–551. [Google Scholar] [CrossRef]
- Splittgerber, M.; Salvador, R.; Brauer, H.; Breitling-Ziegler, C.; Prehn-Kristensen, A.; Krauel, K.; Nowak, R.; Ruffini, G.; Moliadze, V.; Siniatchkin, M. Individual baseline performance and electrode montage impact on the effects of anodal tDCS over the left dorsolateral prefrontal cortex. Front. Hum. Neurosci. 2020, 14, 349. [Google Scholar] [CrossRef]
- Wu, D.; Zhou, Y.; Lv, H.; Liu, N.; Zhang, P. The initial visual performance modulates the effects of anodal transcranial direct current stimulation over the primary visual cortex on the contrast sensitivity function. Neuropsychologia 2021, 156, 107854. [Google Scholar] [CrossRef]
- Wu, D.; Zhou, Y.; Xu, P.; Liu, N.; Sun, K.; Xiao, W. Initial performance modulates the effects of cathodal transcranial direct current stimulation (tDCS) over the right dorsolateral prefrontal cortex on inhibitory control. Brain Res. 2021, 1774, 147722. [Google Scholar] [CrossRef]
- Mizuguchi, N.; Katayama, T.; Kanosue, K. The Effect of Cerebellar Transcranial Direct Current Stimulation on A Throwing Task Depends on Individual Level of Task Performance. Neuroscience 2018, 371, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Heinen, K.; Sagliano, L.; Candini, M.; Husain, M.; Cappelletti, M.; Zokaei, N. Cathodal transcranial direct current stimulation over posterior parietal cortex enhances distinct aspects of visual working memory. Neuropsychologia 2016, 87, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.T.; Berryhill, M.E. Parietal Contributions to Visual Working Memory Depend on Task Difficulty. Front. Psychiatry 2012, 3, 81. Available online: https://www.frontiersin.org/article/10.3389/fpsyt.2012.00081 (accessed on 16 December 2021). [CrossRef]
- Gözenman, F.; Berryhill, M.E. Working memory capacity differentially influences responses to tDCS and HD-tDCS in a retro-cue task. Neurosci. Lett. 2016, 629, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.-Y.; Tseng, P.; Liang, W.-K.; Cheng, S.-K.; Juan, C.-H. Transcranial direct current stimulation over right posterior parietal cortex changes prestimulus alpha oscillation in visual short-term memory task. NeuroImage 2014, 98, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Santarnecchi, E.; Muller, T.; Rossi, S.; Sarkar, A.; Polizzotto, N.R.; Rossi, A.; Cohen Kadosh, R. Individual differences and specificity of prefrontal gamma frequency-tACS on fluid intelligence capabilities. Cortex 2016, 75, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Penton, T.; Dixon, L.; Evans, L.J.; Banissy, M.J. Emotion perception improvement following high frequency transcranial random noise stimulation of the inferior frontal cortex. Sci. Rep. 2017, 7, 11278. [Google Scholar] [CrossRef]
- Silvanto, J.; Cattaneo, Z. Common framework for ‘virtual lesion’ and state-dependent TMS: The facilitatory/suppressive range model of online TMS effects on behavior. Brain Cogn. 2017, 119, 32–38. [Google Scholar] [CrossRef]
- Silvanto, J.; Bona, S.; Marelli, M.; Cattaneo, Z. On the Mechanisms of Transcranial Magnetic Stimulation (TMS): How Brain State and Baseline Performance Level Determine Behavioral Effects of TMS. Front. Psychol. 2018, 9, 741. Available online: https://www.frontiersin.org/article/10.3389/fpsyg.2018.00741 (accessed on 16 December 2021). [CrossRef]
- Juan, C.-H.; Tseng, P.; Hsu, T.-Y. Elucidating and Modulating the Neural Correlates of Visuospatial Working Memory via Noninvasive Brain Stimulation. Curr. Dir. Psychol. Sci. 2017, 26, 165–173. [Google Scholar] [CrossRef]
- London, R.E.; Slagter, H.A. No effect of transcranial direct current stimulation over left dorsolateral prefrontal cortex on temporal attention. J. Cogn. Neurosci. 2021, 33, 756–768. [Google Scholar] [CrossRef] [PubMed]
- Reinhart, R.M.; Xiao, W.; McClenahan, L.J.; Woodman, G.F. Electrical Stimulation of Visual Cortex Can Immediately Improve Spatial Vision. Curr. Biol. 2016, 26, 1867–1872. [Google Scholar] [CrossRef] [PubMed]
- Strobach, T.; Antonenko, D.; Abbarin, M.; Escher, M.; Flöel, A.; Schubert, T. Modulation of dual-task control with right prefrontal transcranial direct current stimulation (tDCS). Exp. Brain Res. 2017, 236, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Turi, Z.; Csifcsák, G.; Boayue, N.M.; Aslaksen, P.; Antal, A.; Paulus, W.; Groot, J.; Hawkins, G.E.; Forstmann, B.; Opitz, A. Blinding is compromised for transcranial direct current stimulation at 1 mA for 20 min in young healthy adults. Eur. J. Neurosci. 2019, 50, 3261–3268. [Google Scholar] [CrossRef]
- Braga, M.; Barbiani, D.; Andani, M.E.; Villa-Sánchez, B.; Tinazzi, M.; Fiorio, M. The Role of Expectation and Beliefs on the Effects of Non-Invasive Brain Stimulation. Brain Sci. 2021, 11, 1526. [Google Scholar] [CrossRef]
- Roe, J.; Nesheim, M.; Mathiesen, N.C.; Moberget, T.; Alnæs, D.; Sneve, M.H. The effects of tDCS upon sustained visual attention are dependent on cognitive load. Neuropsychologia 2016, 80, 1–8. [Google Scholar] [CrossRef]
- Pope, P.A.; Miall, R.C. Task-specific facilitation of cognition by cathodal transcranial direct current stimulation of the cerebellum. Brain Stimul. 2012, 5, 84–94. [Google Scholar] [CrossRef]
- Lee, N.K.; Kwon, Y.H.; Kang, K.W.; Son, S.M. Is effect of transcranial direct current stimulation on visuomotor coordination dependent on task difficulty? Neural Regen. Res. 2015, 10, 463–466. [Google Scholar] [CrossRef]
- Fregni, F.; Boggio, P.S.; Nitsche, M.; Bermpohl, F.; Antal, A.; Feredoes, E.; Marcolin, M.A.; Rigonatti, S.P.; Silva, M.T.A.; Paulus, W.; et al. Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Exp. Brain Res. 2005, 166, 23–30. [Google Scholar] [CrossRef]
- Mancuso, L.E.; Ilieva, I.P.; Hamilton, R.H.; Farah, M.J. Does Transcranial Direct Current Stimulation Improve Healthy Working Memory? A Meta-analytic Review. J. Cogn. Neurosci. 2016, 28, 1063–1089. [Google Scholar] [CrossRef] [PubMed]
- Brehm, J.W.; Self, E.A. The intensity of motivation. Ann. Rev. Psychol. 1989, 40, 109–131. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.; Friedrich, A.; Gendolla, G.H.E. Task difficulty effects on cardiac activity. Psychophysiology 2008, 45, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Berlingeri, M.; Danelli, L.; Bottini, G.; Sberna, M.; Paulesu, E. Reassessing the HAROLD model: Is the hemispheric asymmetry reduction in older adults a special case of compensatory-related utilisation of neural circuits? Exp. Brain Res. 2012, 224, 393–410. [Google Scholar] [CrossRef]
- Howe, P.; Horowitz, T.; Morocz, I.A.; Wolfe, J.; Livingstone, M.S. Using fMRI to distinguish components of the multiple object tracking task. J. Vis. 2009, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Nagel, I.E.; Preuschhof, C.; Li, S.-C.; Nyberg, L.; Bäckman, L.; Lindenberger, U.; Heekeren, H.R. Load Modulation of BOLD Response and Connectivity Predicts Working Memory Performance in Younger and Older Adults. J. Cogn. Neurosci. 2011, 23, 2030–2045. [Google Scholar] [CrossRef] [PubMed]
- Reuter-Lorenz, P.A.; Cappell, K.A. Neurocognitive Aging and the Compensation Hypothesis. Curr. Dir. Psychol. Sci. 2008, 17, 177–182. [Google Scholar] [CrossRef]
- Nyberg, L.; Dahlin, E.; Neely, A.S.; Bäckman, L. Neural correlates of variable working memory load across adult age and skill: Dissociative patterns within the fronto-parietal network. Scand. J. Psychol. 2009, 50, 41–46. [Google Scholar] [CrossRef]
- Schneider-Garces, N.J.; Gordon, B.A.; Brumback-Peltz, C.R.; Shin, E.; Lee, Y.; Sutton, B.P.; Maclin, E.L.; Gratton, G.; Fabiani, M. Span, CRUNCH, and Beyond: Working Memory Capacity and the Aging Brain. J. Cogn. Neurosci. 2010, 22, 655–669. [Google Scholar] [CrossRef]
- Vergallito, A.; Lauro, L.J.R.; Bonandrini, R.; Zapparoli, L.; Danelli, L.; Berlingeri, M. What is difficult for you can be easy for me. Effects of increasing individual task demand on prefrontal lateralization: A tDCS study. Neuropsychologia 2018, 109, 283–294. [Google Scholar] [CrossRef]
- Weiss, M.; Lavidor, M. When Less Is More: Evidence for a Facilitative Cathodal tDCS Effect in Attentional Abilities. 2012. Available online: http://direct.mit.edu/jocn/article-pdf/24/9/1826/1944304/jocn_a_00248.pdf?casa_token=hOhTeFdrNfEAAAAA:3y2kiCqEbruVQneoTWyAkPt-slO266Xe0mCQmD65eGjyoLU5q1K4tRQdgUHOn7C-nqZGUhQz6Q (accessed on 4 January 2022).
- Blumberg, E.J.; Peterson, M.S.; Parasuraman, R. Enhancing multiple object tracking performance with noninvasive brain stimulation: A causal role for the anterior intraparietal sulcus. Front. Syst. Neurosci. 2015, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Paladini, R.; Wieland, F.A.M.; Naert, L.; Bonato, M.; Mosimann, U.P.; Nef, T.; Müri, R.M.; Nyffeler, T.; Cazzoli, D. The Impact of Cognitive Load on the Spatial Deployment of Visual Attention: Testing the Role of Interhemispheric Balance with Biparietal Transcranial Direct Current Stimulation. Front. Neurosci. 2020, 13, 1391. [Google Scholar] [CrossRef]
- Vergallito, A.; Varoli, E.; Giustolisi, B.; Cecchetto, C.; Del Mauro, L.; Lauro, L.J.R. Mind the stimulation site: Enhancing and diminishing sentence comprehension with anodal tDCS. Brain Lang. 2020, 204, 104757. [Google Scholar] [CrossRef] [PubMed]
- Giustolisi, B.; Vergallito, A.; Cecchetto, C.; Varoli, E.; Lauro, L.J.R. Anodal transcranial direct current stimulation over left inferior frontal gyrus enhances sentence comprehension. Brain Lang. 2018, 176, 36–41. [Google Scholar] [CrossRef]
- Pupíková, M.; Šimko, P.; Gajdoš, M.; Rektorová, I. Modulation of Working Memory and Resting-State fMRI by tDCS of the Right Frontoparietal Network. Neural Plast. 2021, 2021, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lovett, M.C.; Daily, L.Z.; Reder, L.M. A source activation theory of working memory: Cross-task prediction of performance in ACT-R. Cogn. Syst. Res. 2000, 1, 99–118. [Google Scholar] [CrossRef]
- Sandrini, M.; Fertonani, A.; Cohen, L.G.; Miniussi, C. Double dissociation of working memory load effects induced by bilateral parietal modulation. Neuropsychologia 2012, 50, 396–402. [Google Scholar] [CrossRef][Green Version]
- Gill, J.; Shah-Basak, P.P.; Hamilton, R. It’s the thought that counts: Examining the task-dependent effects of transcranial direct current stimulation on executive function. Brain Stimul. 2014, 8, 253–259. [Google Scholar] [CrossRef]
- Medina, J.; Cason, S. No evidential value in samples of transcranial direct current stimulation (tDCS) studies of cognition and working memory in healthy populations. Cortex 2017, 94, 131–141. [Google Scholar] [CrossRef]
- Boisgontier, M.P.; Cheval, B. The anova to mixed model transition. Neurosci. Biobehav. Rev. 2016, 68, 1004–1005. [Google Scholar] [CrossRef]
- Baayen, H.; Davidson, D.; Bates, D. Mixed-effects modeling with crossed random effects for subjects and items. J. Mem. Lang. 2008, 59, 390–412. [Google Scholar] [CrossRef]
- Barr, D.J.; Levy, R.; Scheepers, C.; Tily, H.J. Random effects structure for confirmatory hypothesis testing: Keep it maximal. J. Mem. Lang. 2013, 68, 255–278. [Google Scholar] [CrossRef] [PubMed]
- Hair, J.; Anderson, R.; Tatham, R.; Black, W. Multivariate Data Analysis, 5th ed.; Prentice Hall International: Upper Saddle River, NJ, USA, 1998. [Google Scholar]
- Weissgerber, T.L.; Milic, N.M.; Winham, S.; Garovic, V. Beyond Bar and Line Graphs: Time for a New Data Presentation Paradigm. PLoS Biol. 2015, 13, e1002128. [Google Scholar] [CrossRef] [PubMed]
- Rousselet, G.A.; Pernet, C.R.; Wilcox, R.R. Beyond differences in means: Robust graphical methods to compare two groups in neuroscience. Eur. J. Neurosci. 2017, 46, 1738–1748. [Google Scholar] [CrossRef] [PubMed]
- Peterchev, A.V.; Wagner, T.A.; Miranda, P.C.; Nitsche, M.A.; Paulus, W.; Lisanby, S.H.; Pascual-Leone, A.; Bikson, M. Fundamentals of transcranial electric and magnetic stimulation dose: Definition, selection, and reporting practices. Brain Stimul. 2012, 5, 435–453. [Google Scholar] [CrossRef] [PubMed]
- Truong, D.Q.; Magerowski, G.; Blackburn, G.L.; Bikson, M.; Alonso-Alonso, M. Computational modeling of transcranial direct current stimulation (tDCS) in obesity: Impact of head fat and dose guidelines. NeuroImage Clin. 2013, 2, 759–766. [Google Scholar] [CrossRef][Green Version]
- Jung, Y.-J.; Kim, J.-H.; Im, C.-H. COMETS: A MATLAB toolbox for simulating local electric fields generated by transcranial direct current stimulation (tDCS). Biomed. Eng. Lett. 2013, 3, 39–46. [Google Scholar] [CrossRef]
- Thielscher, A.; Antunes, A.; Saturnino, G.B. Field modeling for transcranial magnetic stimulation: A useful tool to understand the physiological effects of TMS? In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 222–225. [Google Scholar] [CrossRef]
- Evans, C.; Bachmann, C.; Lee, J.S.; Gregoriou, E.; Ward, N.; Bestmann, S. Dose-controlled tDCS reduces electric field intensity variability at a cortical target site. Brain Stimul. 2020, 13, 125–136. [Google Scholar] [CrossRef]
- Guerra, A.; Lopez-Alonso, V.; Cheeran, B.; Suppa, A. Solutions for managing variability in non-invasive brain stimulation studies. Neurosci. Lett. 2020, 719, 133332. [Google Scholar] [CrossRef]
- Laakso, I.; Mikkonen, M.; Koyama, S.; Hirata, A.; Tanaka, S. Can electric fields explain inter-individual variability in transcranial direct current stimulation of the motor cortex? Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Dmochowski, J.P.; Datta, A.; Huang, Y.; Richardson, J.D.; Bikson, M.; Fridriksson, J.; Parra, L.C. Targeted transcranial direct current stimulation for rehabilitation after stroke. NeuroImage 2013, 75, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Bastani, A.; Jaberzadeh, S. Does anodal transcranial direct current stimulation enhance excitability of the motor cortex and motor function in healthy individuals and subjects with stroke: A systematic review and meta-analysis. Clin. Neurophysiol. 2012, 123, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Batsikadze, G.; Moliadze, V.; Paulus, W.; Kuo, M.-F.; Nitsche, M.A. Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J. Physiol. 2013, 591, 1987–2000. [Google Scholar] [CrossRef] [PubMed]
- Teo, F.; Hoy, K.E.; Daskalakis, Z.J.; Fitzgerald, P.B. Investigating the role of current strength in tdcs modulation of working memory performance in healthy controls. Front. Psychiatry 2011, 2, 45. [Google Scholar] [CrossRef] [PubMed]
- Lisman, J.E. Three Ca2+ levels affect plasticity differently: The LTP zone, the LTD zone and no man’s land. J. Physiol. 2001, 532, 285. [Google Scholar] [CrossRef] [PubMed]
- Hoy, K.E.; Emonson, M.R.; Arnold, S.L.; Thomson, R.H.; Daskalakis, Z.J.; Fitzgerald, P.B. Testing the limits: Investigating the effect of tDCS dose on working memory enhancement in healthy controls. Neuropsychologia 2013, 51, 1777–1784. [Google Scholar] [CrossRef]
- Kashyap, R.; Bhattacharjee, S.; Arumugam, R.; Bharath, R.D.; Udupa, K.; Oishi, K.; Desmond, J.E.; Annabel Chen, S.H.; Guan, C. Focality-oriented selection of current dose for transcranial direct current stimulation. J. Pers. Med. 2021, 11, 940. [Google Scholar] [CrossRef]
- Workman, C.; Kamholz, J.; Rudroff, T. The tolerability and efficacy of 4 ma transcranial direct current stimulation on leg muscle fatigability. Brain Sci. 2019, 10, 12. [Google Scholar] [CrossRef]
- Workman, C.D.; Fietsam, A.C.; Kamholz, J.; Rudroff, T. Women report more severe sensations from 2 mA and 4 mA transcranial direct current stimulation than men. Eur. J. Neurosci. 2020, 53, 2696–2702. [Google Scholar] [CrossRef]
- Bortoletto, M.; Pellicciari, M.C.; Rodella, C.; Miniussi, C. The interaction with task-induced activity is more important than polarization: A tDCS study. Brain Stimul. 2015, 8, 269–276. [Google Scholar] [CrossRef]
- Bastani, A.; Jaberzadeh, S. Differential modulation of corticospinal excitability by different current densities of anodal transcranial direct current stimulation. PLoS ONE 2013, 8, e72254. [Google Scholar]
- Kidgell, D.J.; Daly, R.M.; Young, K.; Lum, J.; Tooley, G.; Jaberzadeh, S.; Zoghi, M.; Pearce, A.J. Different Current Intensities of Anodal Transcranial Direct Current Stimulation Do Not Differentially Modulate Motor Cortex Plasticity. Neural Plast. 2013, 2013, 603502. [Google Scholar] [CrossRef] [PubMed]
- Nikolin, S.; Martin, D.; Loo, C.K.; Boonstra, T.W. Effects of TDCS dosage on working memory in healthy participants. Brain Stimul. 2018, 11, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Louviot, S.; Tyvaert, L.; Maillard, L.G.; Colnat-Coulbois, S.; Dmochowski, J.; Koessler, L. Transcranial Electrical Stimulation generates electric fields in deep human brain structures. Brain Stimul. 2021, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ruhnau, P.; Rufener, K.; Heinze, H.-J.; Zaehle, T. Sailing in a sea of disbelief: In vivo measurements of transcranial electric stimulation in human subcortical structures. Brain Stimul. 2018, 11, 241–243. [Google Scholar] [CrossRef] [PubMed]
- Chhatbar, P.Y.; Kautz, S.A.; Takacs, I.; Rowland, N.C.; Revuelta, G.J.; George, M.S.; Bikson, M.; Feng, W. Evidence of transcranial direct current stimulation-generated electric fields at subthalamic level in human brain in vivo. Brain Stimul. 2018, 11, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Opitz, A.; Falchier, A.; Yan, C.G.; Yeagle, E.M.; Linn, G.S.; Megevand, P.; Thielscher, A.; Deborah, R.A.; Milham, M.P.; Mehta, A.D.; et al. Spatiotemporal structure of intracranial electric fields induced by transcranial electric stimulation in humans and nonhuman primates. Sci. Rep. 2016, 6, 31236. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, A.A.; Lafon, B.; Friedman, D.; Dayan, M.; Wang, X.; Bikson, M.; Doyle, W.K.; Devinsky, O.; Parra, L.C. Measurements and models of electric fields in the in vivo human brain during transcranial electric stimulation. eLife 2017, 6, e18834. [Google Scholar] [CrossRef]
- Bjekić, J.; Živanović, M.; Filipović, S.R. Transcranial direct current stimulation (Tdcs) for memory enhancement. J. Vis. Exp. 2021, 2021, e62681. [Google Scholar] [CrossRef]
- Lafon, B.; Rahman, A.; Bikson, M.; Parra, L.C. Direct Current Stimulation Alters Neuronal Input/Output Function. Brain Stimul. 2016, 10, 36–45. [Google Scholar] [CrossRef]
- Corp, D.T.; Bereznicki, H.G.K.; Clark, G.M.; Youssef, G.J.; Fried, P.J.; Jannati, A.; Davies, C.B.; Gomes-Osman, J.; Kirkovski, M.; Albein-Urios, N.; et al. Large-scale analysis of interindividual variability in single and paired-pulse TMS data. Clin. Neurophysiol. 2021, 132, 2639–2653. [Google Scholar] [CrossRef] [PubMed]
- Kasten, F.H.; Duecker, K.; Maack, M.C.; Meiser, A.; Herrmann, C.S. Integrating electric field modeling and neuroimaging to explain inter-individual variability of tACS effects. Nat. Commun. 2019, 10, 5427. [Google Scholar] [CrossRef] [PubMed]
- Moliadze, V.; Fritzsche, G.; Antal, A. Comparing the efficacy of excitatory transcranial stimulation methods measuring motor evoked potentials. Neural Plast. 2014, 2014, 837141. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Li, C.; Liu, N.; Xu, P.; Xiao, W. Visual Motion Perception Improvements Following Direct Current Stimulation over V5 Are Dependent on Initial Performance. Exp. Brain Res. 2020, 238, 2409–2416. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vergallito, A.; Feroldi, S.; Pisoni, A.; Romero Lauro, L.J. Inter-Individual Variability in tDCS Effects: A Narrative Review on the Contribution of Stable, Variable, and Contextual Factors. Brain Sci. 2022, 12, 522. https://doi.org/10.3390/brainsci12050522
Vergallito A, Feroldi S, Pisoni A, Romero Lauro LJ. Inter-Individual Variability in tDCS Effects: A Narrative Review on the Contribution of Stable, Variable, and Contextual Factors. Brain Sciences. 2022; 12(5):522. https://doi.org/10.3390/brainsci12050522
Chicago/Turabian StyleVergallito, Alessandra, Sarah Feroldi, Alberto Pisoni, and Leonor J. Romero Lauro. 2022. "Inter-Individual Variability in tDCS Effects: A Narrative Review on the Contribution of Stable, Variable, and Contextual Factors" Brain Sciences 12, no. 5: 522. https://doi.org/10.3390/brainsci12050522
APA StyleVergallito, A., Feroldi, S., Pisoni, A., & Romero Lauro, L. J. (2022). Inter-Individual Variability in tDCS Effects: A Narrative Review on the Contribution of Stable, Variable, and Contextual Factors. Brain Sciences, 12(5), 522. https://doi.org/10.3390/brainsci12050522





