SIRT1 Interacts with Prepro-Orexin in the Hypothalamus in SOD1G93A Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Western Blotting
2.3. Immunofluorescence
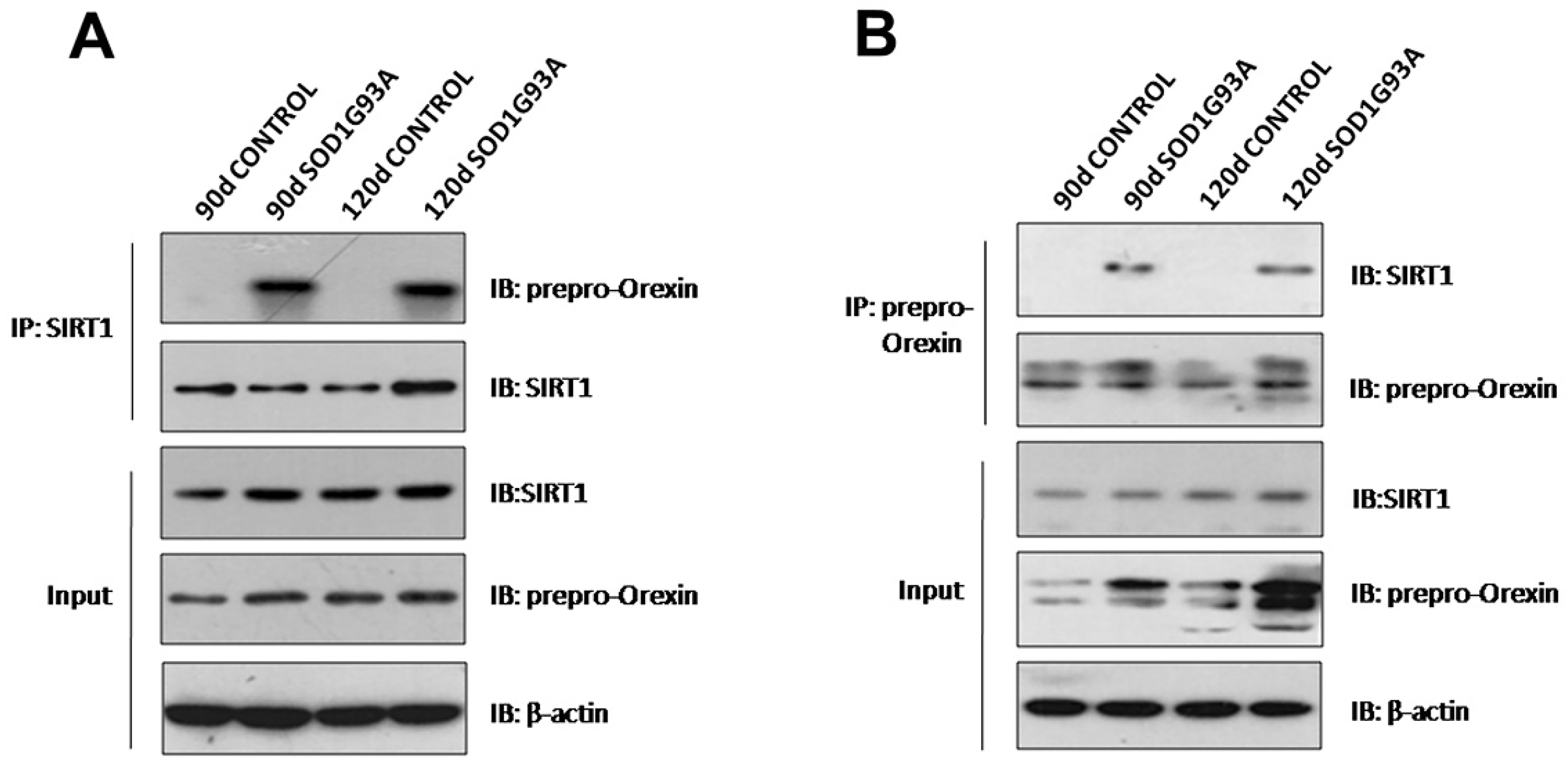
2.4. Co-Immunoprecipitation
2.5. Statistical Analysis
3. Results
3.1. SIRT1 Levels Are Increased in the Hypothalami of Symptomatic SOD1G93A Transgenic Mice
3.2. Prepro-Orexin Interacts with SIRT1 in the Hypothalamus in SOD1G93A Transgenic Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Rowland, L.P.; Shneider, N.A. Amyotrophic lateral sclerosis. N. Engl. J. Med. 2001, 344, 1688–1700. [Google Scholar] [CrossRef] [PubMed]
- Finegan, E.; Shing, S.L.H.; Chipika, R.H.; Doherty, M.A.; Hengeveld, J.C.; Vajda, A.; Donaghy, C.; Pender, N.; McLaughlin, R.L.; Hardiman, O.; et al. Widespread subcortical grey matter degeneration in primary lateral sclerosis: A multimodal imaging study with genetic profiling. Neuroimage Clin. 2019, 24, 102089. [Google Scholar] [CrossRef] [PubMed]
- Westeneng, H.J.; Walhout, R.; Straathof, M.; Schmidt, R.; Hendrikse, J.; Veldink, J.H.; van den Heuvel, M.P.; van den Berg, L.H. Widespread structural brain involvement in ALS is not limited to the C9orf72 repeat expansion. J. Neurol. Neurosurg. Psychiatry 2016, 87, 1354–1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cykowski, M.D.; Takei, H.; Schulz, P.E.; Appel, S.H.; Powell, S.Z. TDP-43 pathology in the basal forebrain and hypothalamus of patients with amyotrophic lateral sclerosis. Acta Neuropathol. Commun. 2014, 2, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalkiadaki, A.; Guarente, L. Sirtuins mediate mammalian metabolic responses to nutrient availability. Nat. Rev. Endocrinol. 2012, 8, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Shin, J.H.; Park, B.W.; Kim, G.S.; Kim, J.C.; Kang, K.S.; Cha, C.I. Region-specific changes in the immunoreactivity of SIRT1 expression in the central nervous system of SOD1(G93A) transgenic mice as an in vivo model of amyotrophic lateral sclerosis. Brain Res. 2012, 1433, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Gabery, S.; Ahmed, R.M.; Caga, J.; Kiernan, M.C.; Halliday, G.M.; Petersen, A. Loss of the metabolism and sleep regulating neuronal populations expressing orexin and oxytocin in the hypothalamus in amyotrophic lateral sclerosis. Neuropathol. Appl. Neurobiol. 2021, 47, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Gorges, M.; Vercruysse, P.; Muller, H.P.; Huppertz, H.J.; Rosenbohm, A.; Nagel, G.; Weydt, P.; Petersen, A.; Ludolph, A.C.; Kassubek, J.; et al. Hypothalamic atrophy is related to body mass index and age at onset in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 2017, 88, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Sheng, Z.F.; Cai, B.; Zhang, Y.H.; Fan, D.S. Increased orexin expression promotes sleep/wake disturbances in the SOD1-G93A mouse model of amyotrophic lateral sclerosis. Chin. Med. J. 2015, 128, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Gurney, M.E.; Pu, H.; Chiu, A.Y.; Dal Canto, M.C.; Polchow, C.Y.; Alexander, D.D.; Caliendo, J.; Hentati, A.; Kwon, Y.W.; Deng, H.X.; et al. Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science 1994, 264, 1772–1775. [Google Scholar] [CrossRef] [PubMed]
- George, P.; Keith, F. The Mouse Brain in Stereotaxic Coordinates, 2nd ed.; Acdemic Press: Cambridge, MA, USA, 2001. [Google Scholar]
- Sakurai, T. The neural circuit of orexin (hypocretin): Maintaining sleep and wakefulness. Nat. Rev. Neurosci. 2007, 8, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Goforth, P.B.; Myers, M.G. Roles for Orexin/Hypocretin in the control of energy balance and metabolism. Curr. Top. Behav. Neurosci. 2017, 33, 137–156. [Google Scholar] [PubMed]
- Satoh, A.; Brace, C.S.; Rensing, N.; Cliften, P.; Wozniak, D.F.; Herzog, E.D.; Yamada, K.A.; Imai, S. Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab. 2013, 18, 416–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satoh, A.; Brace, C.S.; Ben-Josef, G.; West, T.; Wozniak, D.F.; Holtzman, D.M.; Herzog, E.D.; Imai, S. SIRT1 promotes the central adaptive response to diet restriction through activation of the dorsomedial and lateral nuclei of the hypothalamus. J. Neurosci. 2010, 30, 10220–10232. [Google Scholar] [CrossRef] [PubMed]
- Panossian, L.; Fenik, P.; Zhu, Y.; Zhan, G.; McBurney, M.W.; Veasey, S. SIRT1 regulation of wakefulness and senescence-like phenotype in wake neurons. J. Neurosci. 2011, 31, 4025–4036. [Google Scholar] [CrossRef] [PubMed]
- Laudati, G.; Mascolo, L.; Guida, N.; Sirabella, R.; Pizzorusso, V.; Bruzzaniti, S.; Serani, A.; Di Renzo, G.; Canzoniero, L.M.T.; Formisano, L. Resveratrol treatment reduces the vulnerability of SH-SY5Y cells and cortical neurons overexpressing SOD1-G93A to thimerosal toxicity through SIRT1/DREAM/PDYN pathway. Neurotoxicology 2019, 71, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y.; Tang, L.; Zhang, N.; Fan, D.S. Protective effects of resveratrol through the up-regulation of SIRT1 expression in the mutant hSOD1-G93A-bearing motor neuron-like cell culture model of amyotrophic lateral sclerosis. Neurosci. Lett. 2011, 503, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, R.; del Valle, J.; Modol, L.; Martinez, A.; Granado-Serrano, A.B.; Ramirez-Nunez, O.; Pallas, M.; Portero-Otin, M.; Osta, R.; Navarro, X. Resveratrol improves motoneuron function and extends survival in SOD1(G93A) ALS mice. Neurotherapeutics 2014, 11, 419–432. [Google Scholar] [PubMed] [Green Version]
- Watanabe, S.; Ageta-Ishihara, N.; Nagatsu, S.; Takao, K.; Komine, O.; Endo, F.; Miyakawa, T.; Misawa, H.; Takahashi, R.; Kinoshita, M.; et al. SIRT1 overexpression ameliorates a mouse model of SOD1-linked amyotrophic lateral sclerosis via HSF1/HSP70i chaperone system. Mol. Brain 2014, 7, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.; Sui, Y.; Gao, W.; Cai, B.; Fan, D.S. Effects of diet on adenosine monophosphate-activated protein kinase activity and disease progression in an amyotrophic lateral sclerosis model. J. Int. Med. Res. 2015, 43, 67–79. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Liu, R.; Sheng, Z.; Zhang, Y.; Fan, D. SIRT1 Interacts with Prepro-Orexin in the Hypothalamus in SOD1G93A Mice. Brain Sci. 2022, 12, 490. https://doi.org/10.3390/brainsci12040490
Zhang G, Liu R, Sheng Z, Zhang Y, Fan D. SIRT1 Interacts with Prepro-Orexin in the Hypothalamus in SOD1G93A Mice. Brain Sciences. 2022; 12(4):490. https://doi.org/10.3390/brainsci12040490
Chicago/Turabian StyleZhang, Gan, Rong Liu, Zhaofu Sheng, Yonghe Zhang, and Dongsheng Fan. 2022. "SIRT1 Interacts with Prepro-Orexin in the Hypothalamus in SOD1G93A Mice" Brain Sciences 12, no. 4: 490. https://doi.org/10.3390/brainsci12040490
APA StyleZhang, G., Liu, R., Sheng, Z., Zhang, Y., & Fan, D. (2022). SIRT1 Interacts with Prepro-Orexin in the Hypothalamus in SOD1G93A Mice. Brain Sciences, 12(4), 490. https://doi.org/10.3390/brainsci12040490






