Anterior or Posterior Ankle Foot Orthoses for Ankle Spasticity: Which One Is Better?
Abstract
:1. Introduction
- Description of the advantages of an anterior plastic AFO for use while barefoot at home over a posterior plastic AFO, which causes discomfort, imbalance, or increased spasticity, and
- Incorporation of dynamic EMG as a bedside or clinical tool for quantifying spasticity and other neuromuscular alterations, such as weakness.
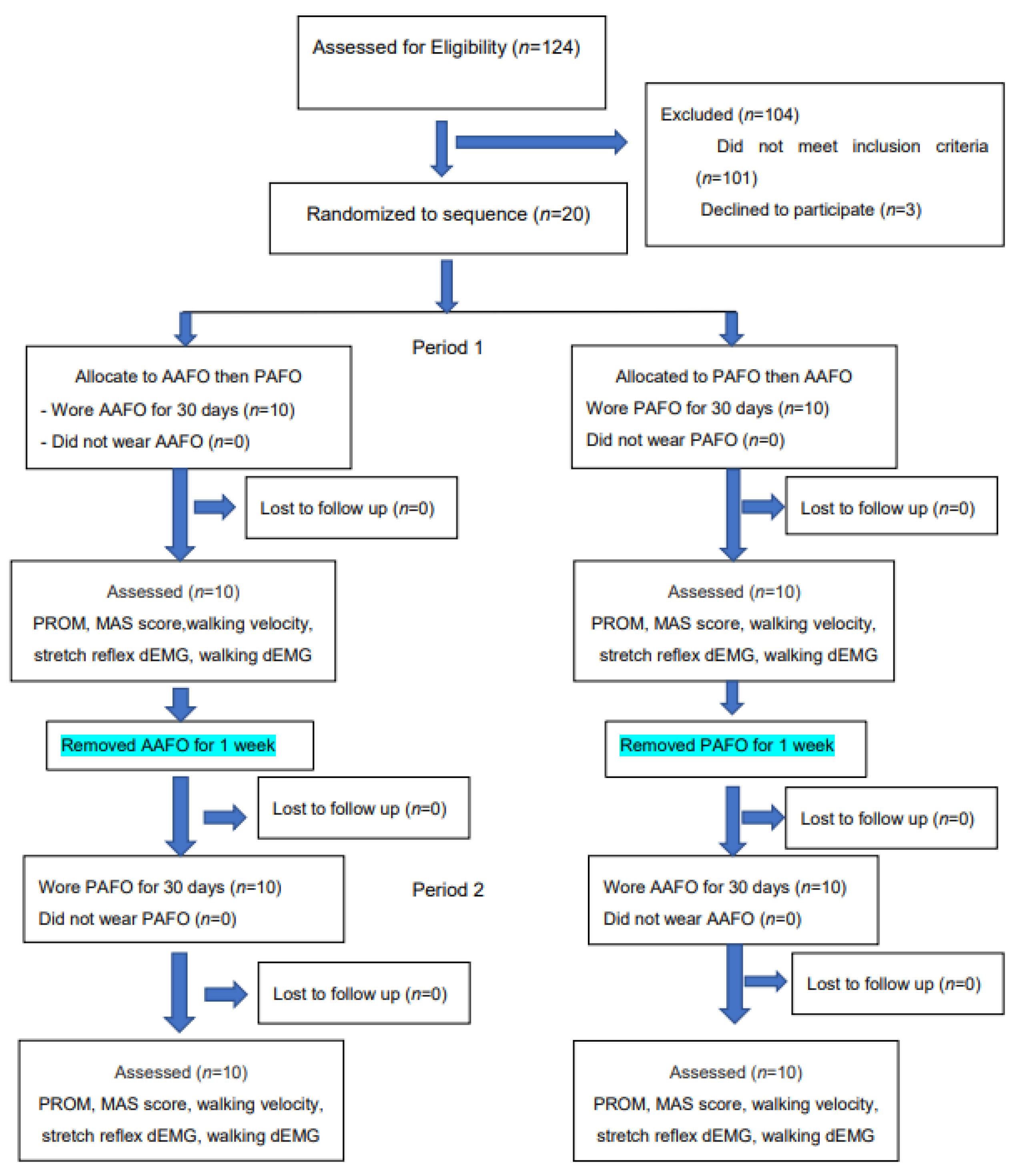
2. Materials and Methods
2.1. Ethics and Clinical Trial Registry
2.2. Subjects
2.3. Procedure
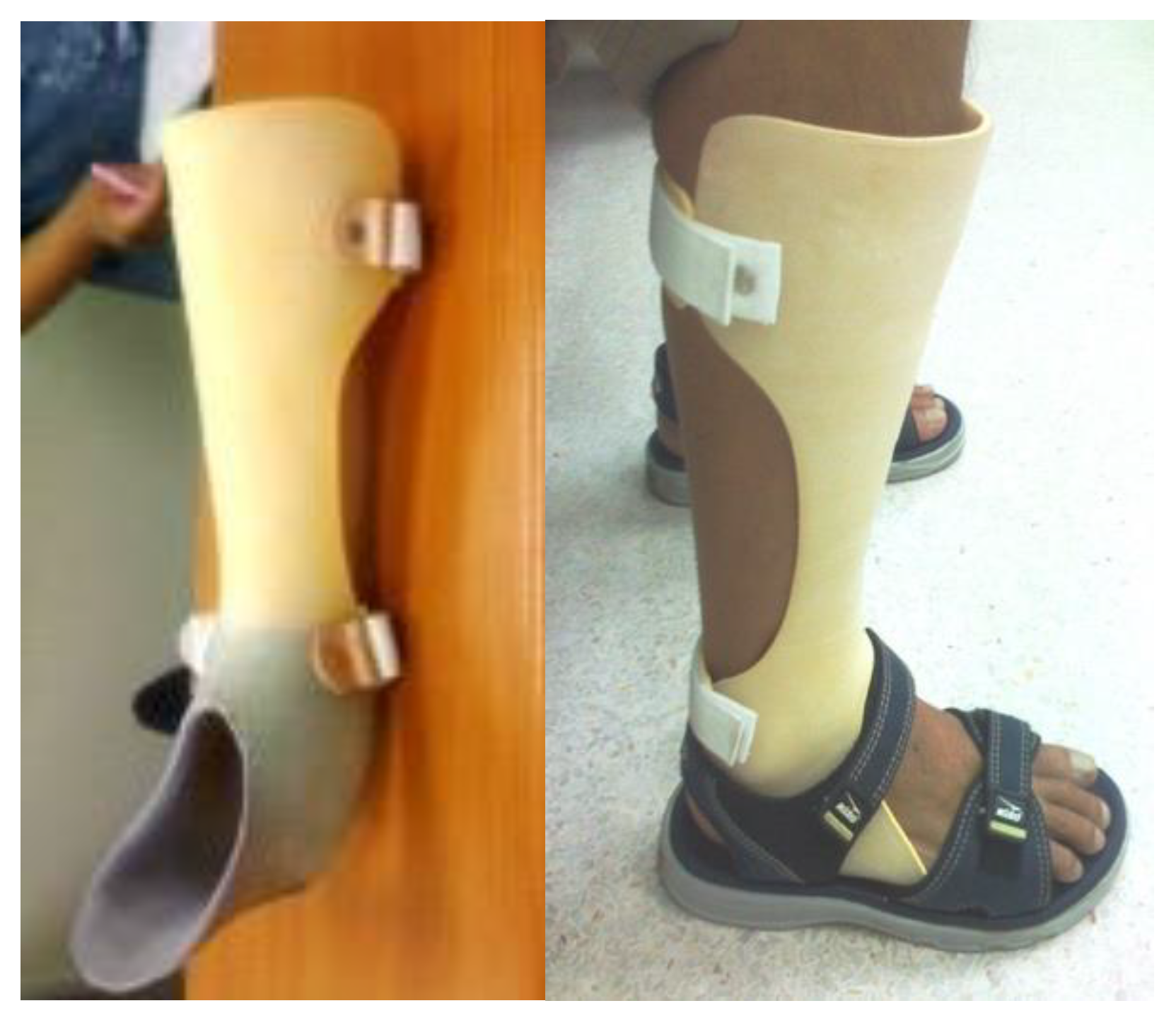
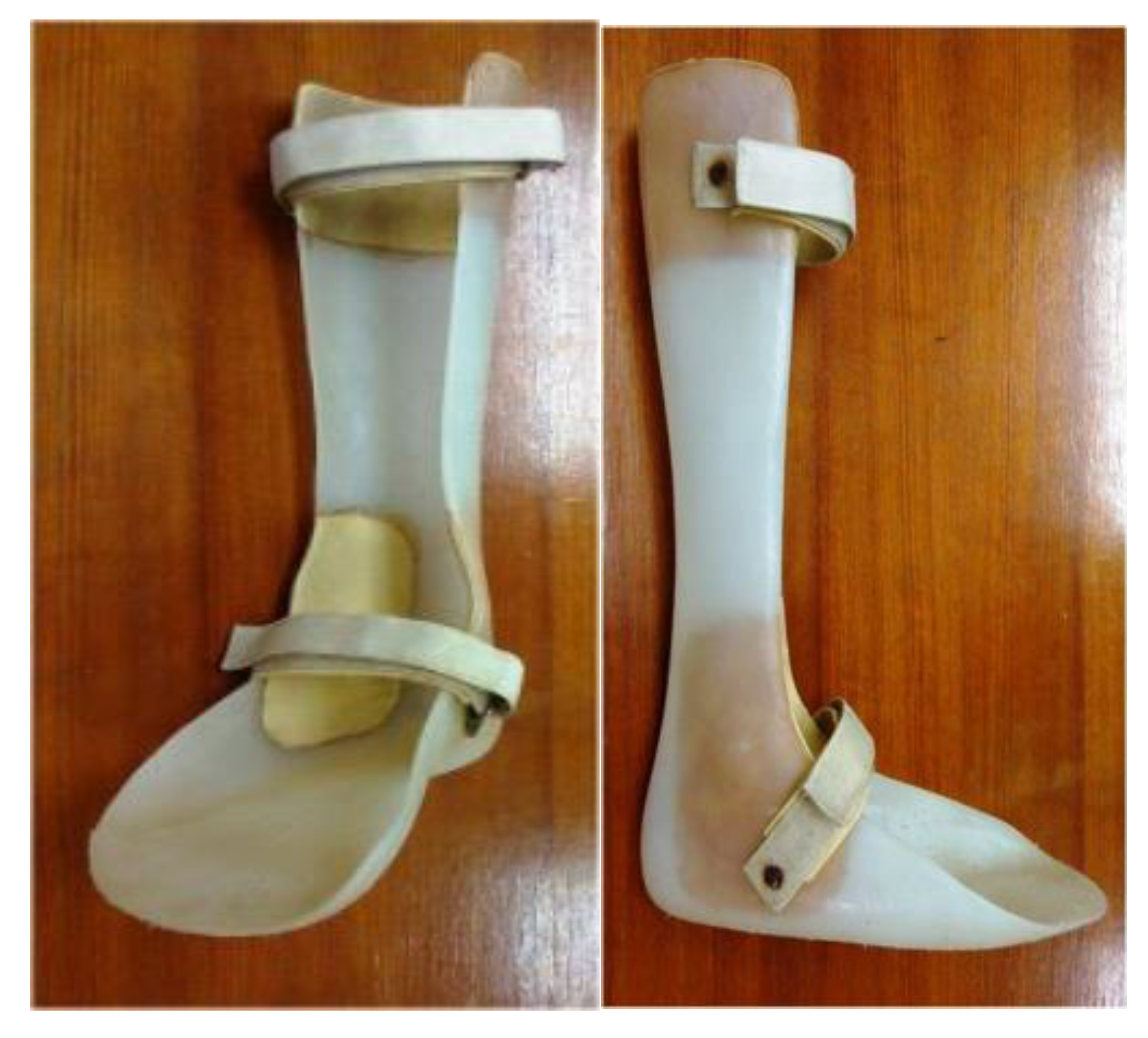
2.4. Plastic AFOs
2.5. Outcome Measurements
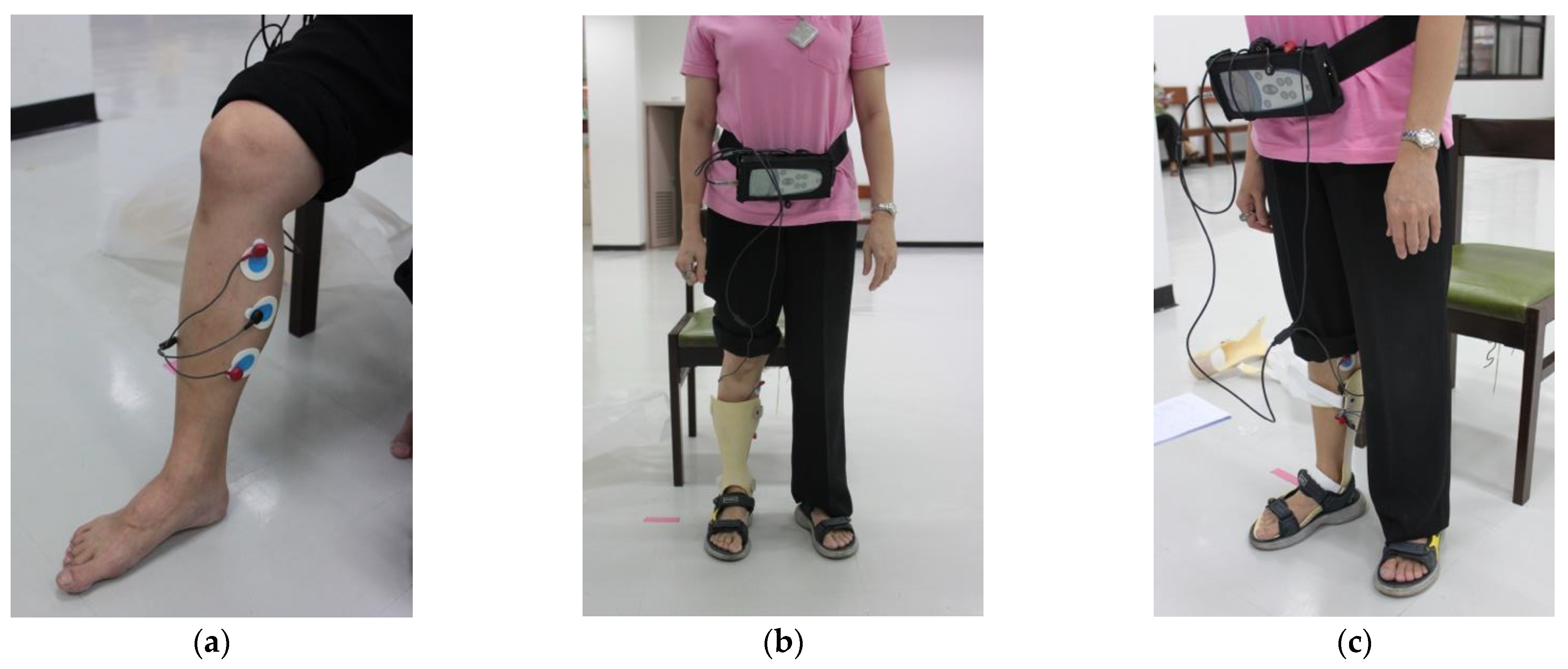
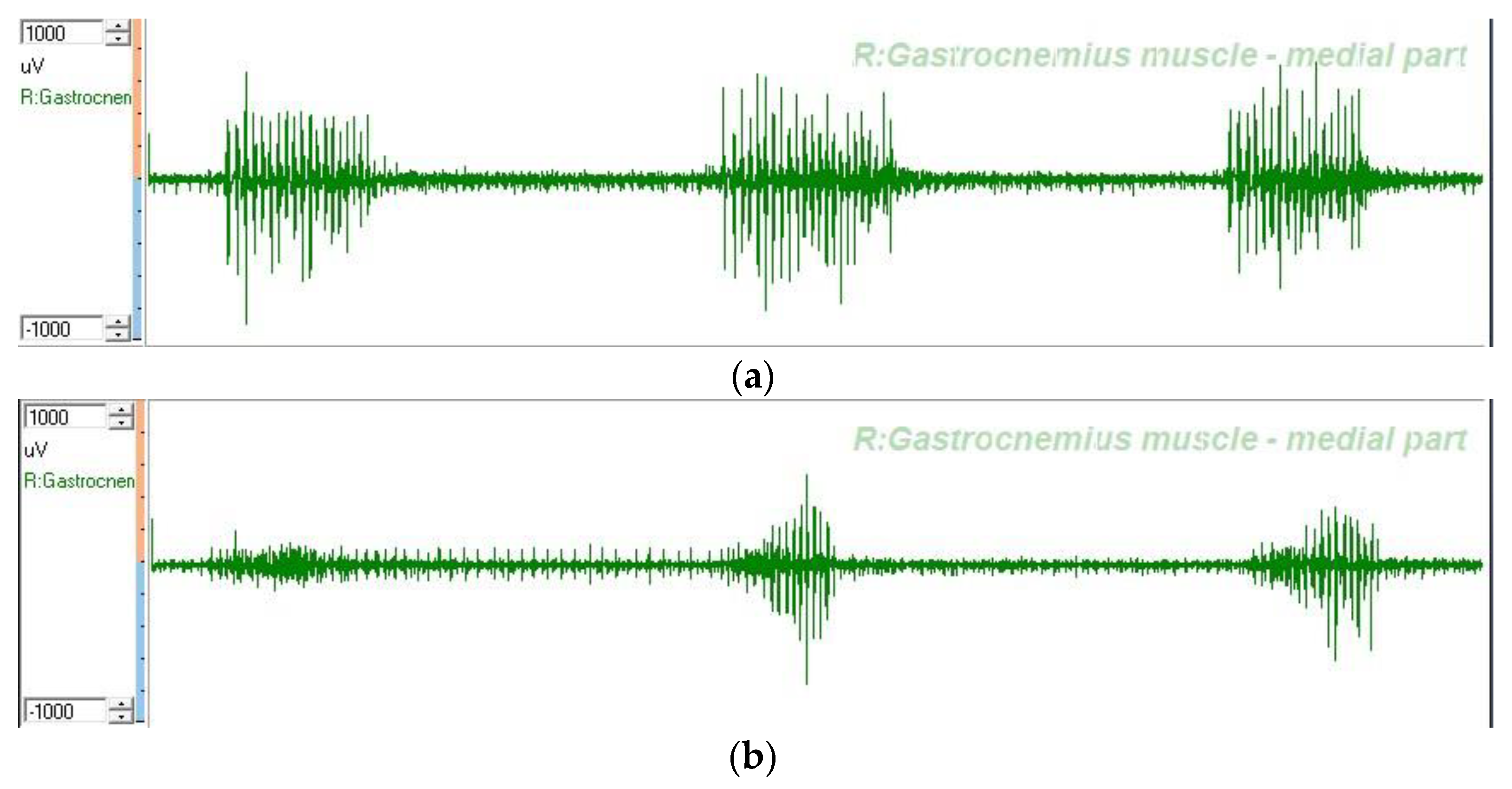
2.6. Wireless dEMG Equipment
2.7. dEMG Measurement
2.8. Statistical Analysis
3. Results
4. Discussion
Limitations of the Study and Potential Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tyson, S.F.; Thornton, H.A. The effect of a hinged ankle foot orthosis on hemiplegic gait: Objective measures and users’ opinions. Clin. Rehabil. 2001, 15, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Choo, Y.J.; Chang, M.C. Effectiveness of an ankle–foot orthosis on walking in patients with stroke: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 15879. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.; Moseley, A. Impact of Ankle-foot Orthoses on Gait and Leg Muscle Activity in Adults with Hemiplegia: Systematic literature review. Physiotherapy 2003, 89, 39–55. [Google Scholar] [CrossRef]
- Abe, H.; Michimata, A.; Sugawara, K.; Sugaya, N.; Izumi, S.-I. Improving Gait Stability in Stroke Hemiplegic Patients with a Plastic Ankle-Foot Orthosis. Tohoku J. Exp. Med. 2009, 218, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, J.F.; Condon, S.M.; Price, R.; De Lateur, B.J. Gait abnormalities in hemiplegia: Their correction by ankle-foot orthoses. Arch. Phys. Med. Rehabil. 1987, 68, 763–771. [Google Scholar]
- Shahabi, S.; Shabaninejad, H.; Kamali, M.; Jalali, M.; Ahmadi Teymourlouy, A. The effects of ankle-foot orthoses on walking speed in patients with stroke: A systematic review and meta-analysis of randomized controlled trials. Clin. Rehabil. 2020, 34, 145–159. [Google Scholar] [CrossRef]
- Lehmann, J.F. Biomechanics of ankle-foot orthoses: Prescription and design. Arch. Phys. Med. Rehabil. 1979, 60, 200–207. [Google Scholar]
- Lehmann, J.F.; Esselman, P.C.; Ko, M.J.; Smith, J.C.; DeLateur, B.J.; Dralle, A.J. Plastic ankle-foot orthoses: Evaluation of function. Arch. Phys. Med. Rehabil. 1983, 64, 402–407. [Google Scholar]
- Chen, C.-K.; Hong, W.-H.; Chu, N.-K.; Lau, Y.-C.; Lew, H.L.; Tang, S.F. Effects of an Anterior Ankle–Foot Orthosis on Postural Stability in Stroke Patients with Hemiplegia. Am. J. Phys. Med. Rehabil. 2008, 87, 815–820. [Google Scholar] [CrossRef]
- Daryabor, A.; Arazpour, M.; Aminian, G. Effect of different designs of ankle-foot orthoses on gait in patients with stroke: A systematic review. Gait Posture 2018, 62, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Chun, M.H.; Ahn, J.S.; Yu, J.Y.; Kang, S.H. Comparison of Gait Analysis Between Anterior and Posterior Ankle Foot Orthosis in Hemiplegic Patients. Am. J. Phys. Med. Rehabil. 2009, 88, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.M.K.; Tang, F.-T.; Wu, S.-H.; Chen, C.-M. Clinical trial of a low-temperature plastic anterior ankle foot orthosis. Am. J. Phys. Med. Rehabil. 1992, 71, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Hung, J.-W.; Chen, P.-C.; Yu, M.-Y.; Hsieh, Y.-W. Long-Term Effect of an Anterior Ankle-Foot Orthosis on Functional Walking Ability of Chronic Stroke Patients. Am. J. Phys. Med. Rehabil. 2011, 90, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-L.; Teng, Y.-L.; Lou, S.-Z.; Chang, H.-Y.; Chen, F.-F.; Yeung, K.-T. Effects of an Anterior Ankle-Foot Orthosis on Walking Mobility in Stroke Patients: Get Up and Go and Stair Walking. Arch. Phys. Med. Rehabil. 2014, 95, 2167–2171. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Hong, W.-H.; Wang, C.-M.; Wu, K.P.-H.; Kang, C.-F.; Tang, S.F. Kinematic Features of Rear-Foot Motion Using Anterior and Posterior Ankle-Foot Orthoses in Stroke Patients With Hemiplegic Gait. Arch. Phys. Med. Rehabil. 2010, 91, 1862–1868. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Shimatani, K.; Hasegawa, M.; Kurita, Y. Effects of Varying Plantarflexion Stiffness of Ankle-Foot Orthosis on Achilles Tendon and Propulsion Force During Gait. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 2194–2202. [Google Scholar] [CrossRef]
- Cakar, E.; Durmus, O.; Tekin, L.; Dincer, U.; Kiralp, M.Z. The ankle-foot orthosis improves balance and reduces fall risk of chronic spastic hemiparetic patients. Eur. J. Phys. Rehabil. Med. 2010, 46, 363–368. [Google Scholar]
- Tyson, S.F.; Kent, R.M. Effects of an Ankle-Foot Orthosis on Balance and Walking After Stroke: A Systematic Review and Pooled Meta-Analysis. Arch. Phys. Med. Rehabil. 2013, 94, 1377–1385. [Google Scholar] [CrossRef]
- Nikamp-Simons, C.D.M.; Buurke, J.H.; Van Der Palen, J.A.M.; Hermens, H.J.; Rietman, J.S. Early or delayed provision of an ankle-foot orthosis in patients with acute and subacute stroke: A randomized controlled trial. Clin. Rehabil. 2016, 31, 798–808. [Google Scholar] [CrossRef]
- Tyson, S.F.; Sadeghi-Demneh, E.; Nester, C.J. A systematic review and meta-analysis of the effect of an ankle-foot orthosis on gait biomechanics after stroke. Clin. Rehabil. 2013, 27, 879–891. [Google Scholar] [CrossRef]
- Nikamp, C.D.; Hobbelink, M.S.; van der Palen, J.; Hermens, H.J.; Rietman, J.S.; Buurke, J.H. A randomized controlled trial on providing ankle-foot orthoses in patients with (sub-)acute stroke: Short-term kinematic and spatiotemporal effects and effects of timing. Gait Posture 2017, 55, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Nikamp, C.D.; Van Der Palen, J.; Hermens, H.J.; Rietman, J.S.; Buurke, J.H. The influence of early or delayed provision of ankle-foot orthoses on pelvis, hip and knee kinematics in patients with sub-acute stroke: A randomized controlled trial. Gait Posture 2018, 63, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Tyson, S.F.; Vail, A.; Thomas, N.A.; Woodward-Nutt, K.; Plant, S.; Tyrrell, P.J. Bespoke versus off-the-shelf ankle-foot orthosis for people with stroke: Randomized controlled trial. Clin. Rehabil. 2018, 32, 367–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zissimopoulos, A.; Fatone, S.; Gard, S. The effect of ankle–foot orthoses on self-reported balance confidence in persons with chronic poststroke hemiplegia. Prosthetics Orthot. Int. 2014, 38, 148–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikamp, C.D.M.; Hobbelink, M.S.H.; van der Palen, J.; Hermens, H.J.; Rietman, J.S.; Buurke, J.H. The effect of ankle-foot orthoses on fall/near fall incidence in patients with (sub-)acute stroke: A randomized controlled trial. PLoS ONE 2019, 14, e0213538. [Google Scholar] [CrossRef]
- Totah, D.; Menon, M.; Jones-Hershinow, C.; Barton, K.; Gates, D.H. The impact of ankle-foot orthosis stiffness on gait: A systematic literature review. Gait Posture 2019, 69, 101–111. [Google Scholar] [CrossRef]
- Becker, S.; Von Werder, S.C.F.A.; Lassek, A.-K.; Disselhorst-Klug, C. Time-frequency coherence of categorized sEMG data during dynamic contractions of biceps, triceps, and brachioradialis as an approach for spasticity detection. Med Biol. Eng. Comput. 2018, 57, 703–713. [Google Scholar] [CrossRef]
- Cooper, A.; Musa, I.M.; van Deursen, R.; Wiles, C.M. Electromyography characterization of stretch responses in hemiparetic stroke patients and their relationship with the Modified Ashworth Scale. Clin. Rehabil. 2005, 19, 760–766. [Google Scholar] [CrossRef]
- Hu, B.; Zhang, X.; Mu, J.; Wu, M.; Wang, Y. Spasticity assessment based on the Hilbert-Huang transform marginal spectrum entropy and the root mean square of surface electromyography signals: A preliminary study. Biomed. Eng. Online 2018, 17, 27. [Google Scholar] [CrossRef] [Green Version]
- Li, X.P.; Cheng, K.; Zhou, J. Clinical assessment of muscle spasticity in stroke patients with surface electromyography and isokinetic assessment. China J. Mod Med. 2010, 20, 605–608. [Google Scholar]
- Onishi, H.; Yagi, R.; Akasaka, K.; Momose, K.; Ihashi, K.; Handa, Y. Relationship between EMG signals and force in human vastus lateralis muscle using multiple bipolar wire electrodes. J. Electromyogr. Kinesiol. 2000, 10, 59–67. [Google Scholar] [CrossRef]
- Frenkel-Toledo, S.; Solomon, J.M.; Shah, A.; Baniña, M.C.; Berman, S.; Soroker, N.; Liebermann, D.G.; Levin, M.F. Tonic stretch reflex threshold as a measure of spasticity after stroke: Reliability, minimal detectable change and responsiveness. Clin. Neurophysiol. 2021, 132, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Marques, I.; Silva, M.B.; Silva, A.N.; Luiz, L.M.D.; Soares, A.B.; Naves, E.L.M. Measurement of post-stroke spasticity based on tonic stretch reflex threshold: Implications of stretch velocity for clinical practice. Disabil. Rehabil. 2017, 41, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Park, S.-J. Prediction of Myoelectric Biomarkers in Post-Stroke Gait. Sensors 2021, 21, 5334. [Google Scholar] [CrossRef] [PubMed]
- Bat-Erdene, B.-O.; Saver, J.L. Automatic Acute Stroke Symptom Detection and Emergency Medical Systems Alerting by Mobile Health Technologies: A Review. J. Stroke Cerebrovasc. Dis. 2021, 30, 105826. [Google Scholar] [CrossRef]
| Parameter | Before AFO | After AFO | D | p | p |
|---|---|---|---|---|---|
| PROM of ankle DF (d) | |||||
| AAFO | 3.00 ± 3.50 | 5.50 ± 3.70 | 2.50 ± 2.64 | 0.015 * | 0.990 |
| PAFO | 3.00 ± 3.50 | 5.50 ± 3.69 | 2.50 ± 2.64 | 0.015 * | |
| MAS of GM | |||||
| AAFO | 1.65 ± 0.24 | 1.30 ± 0.26 | −0.35 ± 0.24 | 0.001 * | 0.343 |
| PAFO | 1.65 ± 0.24 | 1.35 ± 0.34 | −0.30 ± 0.26 | 0.005 * | |
| Walking velocity (m/s) | |||||
| AAFO | 0.26 ± 0.21 | 0.28 ± 0.20 | 0.016 ± 0.017 | 0.015 * | 0.021 ** |
| PAFO | 0.26 ± 0.21 | 0.27 ± 0.19 | 0.008 ± 0.025 | 0.343 | |
| Stretch reflex RMS of GM (µV) | |||||
| AAFO | 45.70 ± 33.59 | 37.60 ± 30.24 | −8.10 ± 7.99 | 0.011 * | 0.017 ** |
| PAFO | 51.20 ± 49.11 | 46.90 ± 46.59 | −4.30 ± 5.33 | 0.311 | |
| Walking RMS of GM (µV) | |||||
| AAFO | 209.90 ± 233.87 | 146.80 ± 184.12 | −62.50 ± 78.09 | 0.015 * | 0.015 ** |
| PAFO | 197.60 ± 206.30 | 301.30 ± 298.72 | 103.70 ± 129.36 | 0.032 * | |
| Parameter | AAFO | PAFO | p |
|---|---|---|---|
| Beautiful | 3.09 ± 0.30 | 2.55 ± 0.52 | 0.006 * |
| Strong and durable | 2.91 ± 0.54 | 3.00 ± 0.00 | 0.341 |
| Easy to put on | 2.09 ± 0.55 | 2.73 ± 0.47 | 0.046 * |
| Comfort during walking | 3.00 ± 0.00 | 2.54 ± 0.52 | 0.016 * |
| Reduces ankle spasticity | 3.00 ± 0.00 | 2.27 ± 0.47 | <0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.P.C.; Suputtitada, A.; Chatkungwanson, W.; Seehaboot, K. Anterior or Posterior Ankle Foot Orthoses for Ankle Spasticity: Which One Is Better? Brain Sci. 2022, 12, 454. https://doi.org/10.3390/brainsci12040454
Chen CPC, Suputtitada A, Chatkungwanson W, Seehaboot K. Anterior or Posterior Ankle Foot Orthoses for Ankle Spasticity: Which One Is Better? Brain Sciences. 2022; 12(4):454. https://doi.org/10.3390/brainsci12040454
Chicago/Turabian StyleChen, Carl P. C., Areerat Suputtitada, Watchara Chatkungwanson, and Kittikorn Seehaboot. 2022. "Anterior or Posterior Ankle Foot Orthoses for Ankle Spasticity: Which One Is Better?" Brain Sciences 12, no. 4: 454. https://doi.org/10.3390/brainsci12040454
APA StyleChen, C. P. C., Suputtitada, A., Chatkungwanson, W., & Seehaboot, K. (2022). Anterior or Posterior Ankle Foot Orthoses for Ankle Spasticity: Which One Is Better? Brain Sciences, 12(4), 454. https://doi.org/10.3390/brainsci12040454







