Effects of Transcranial Direct Current Stimulation of Bilateral Supplementary Motor Area on the Lower Limb Motor Function in a Stroke Patient with Severe Motor Paralysis: A Case Study
Abstract
:1. Introduction
2. Materials and Methods
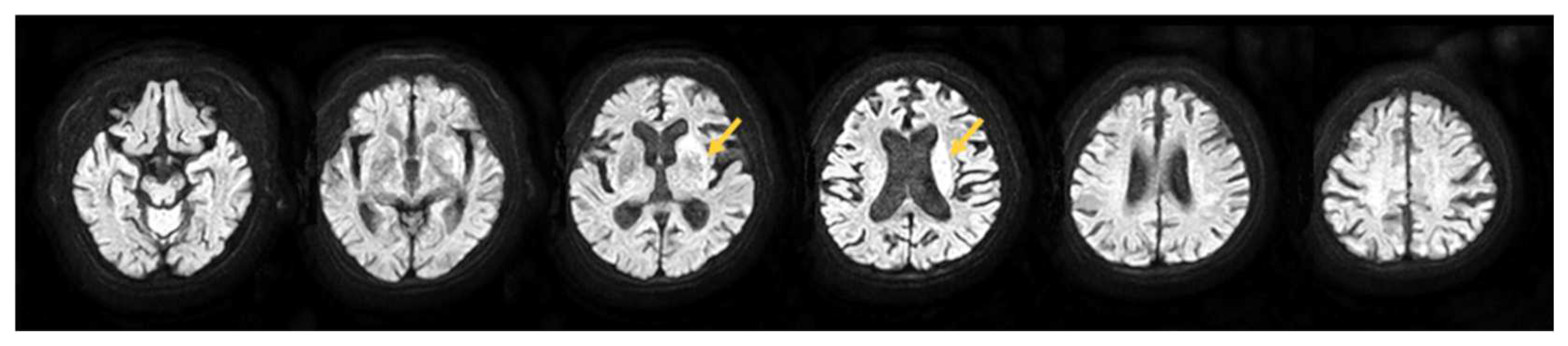
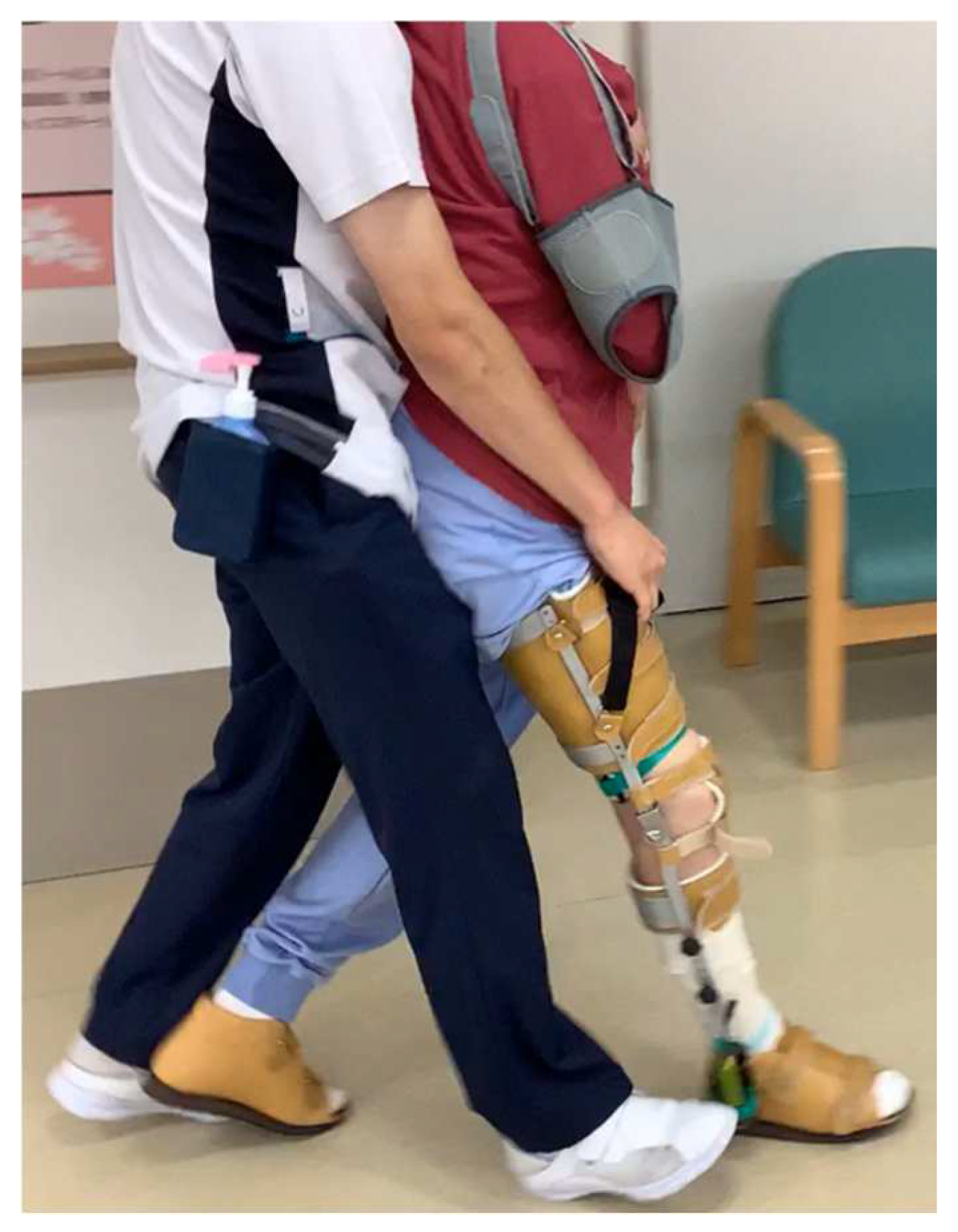
2.1. Participant
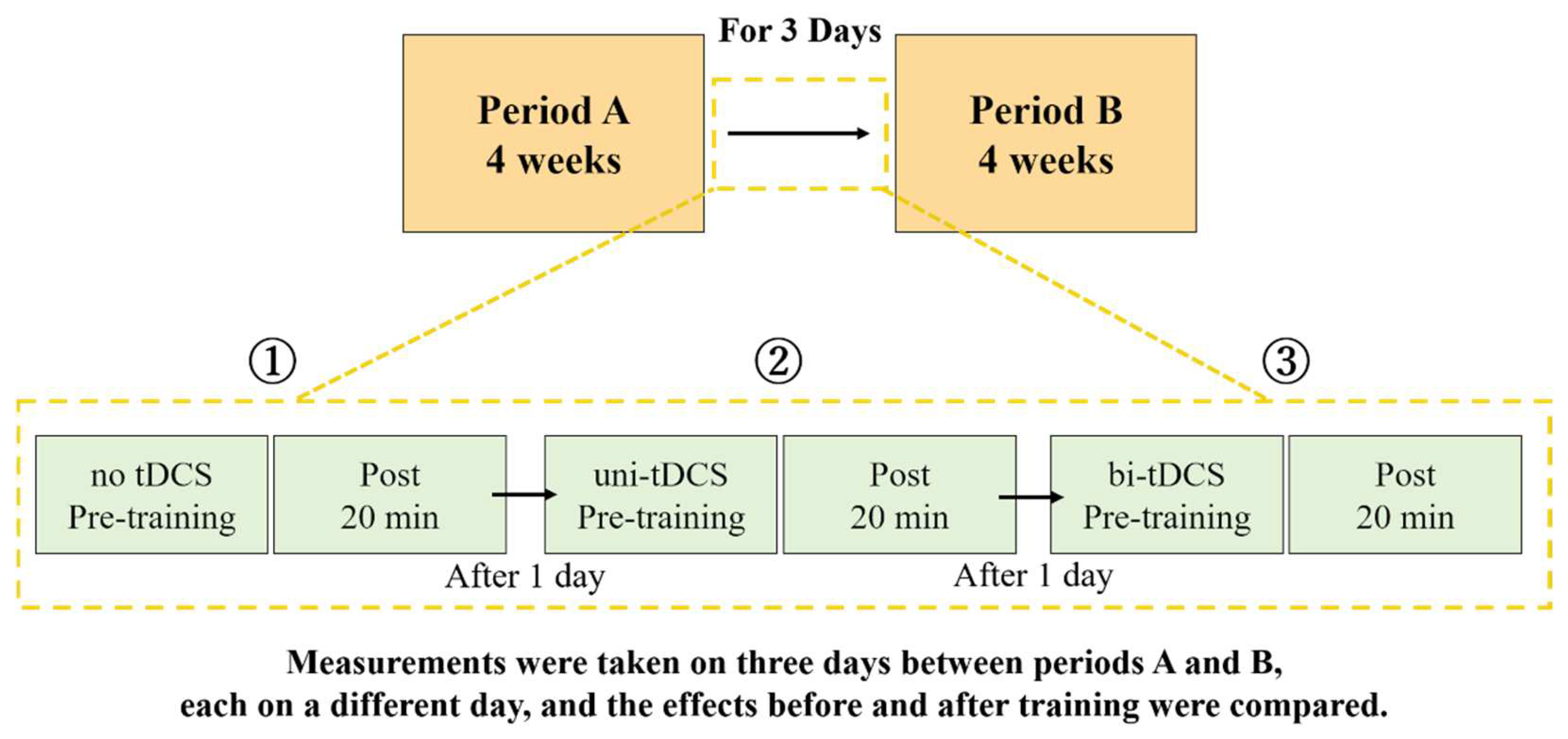
2.2. Study Design
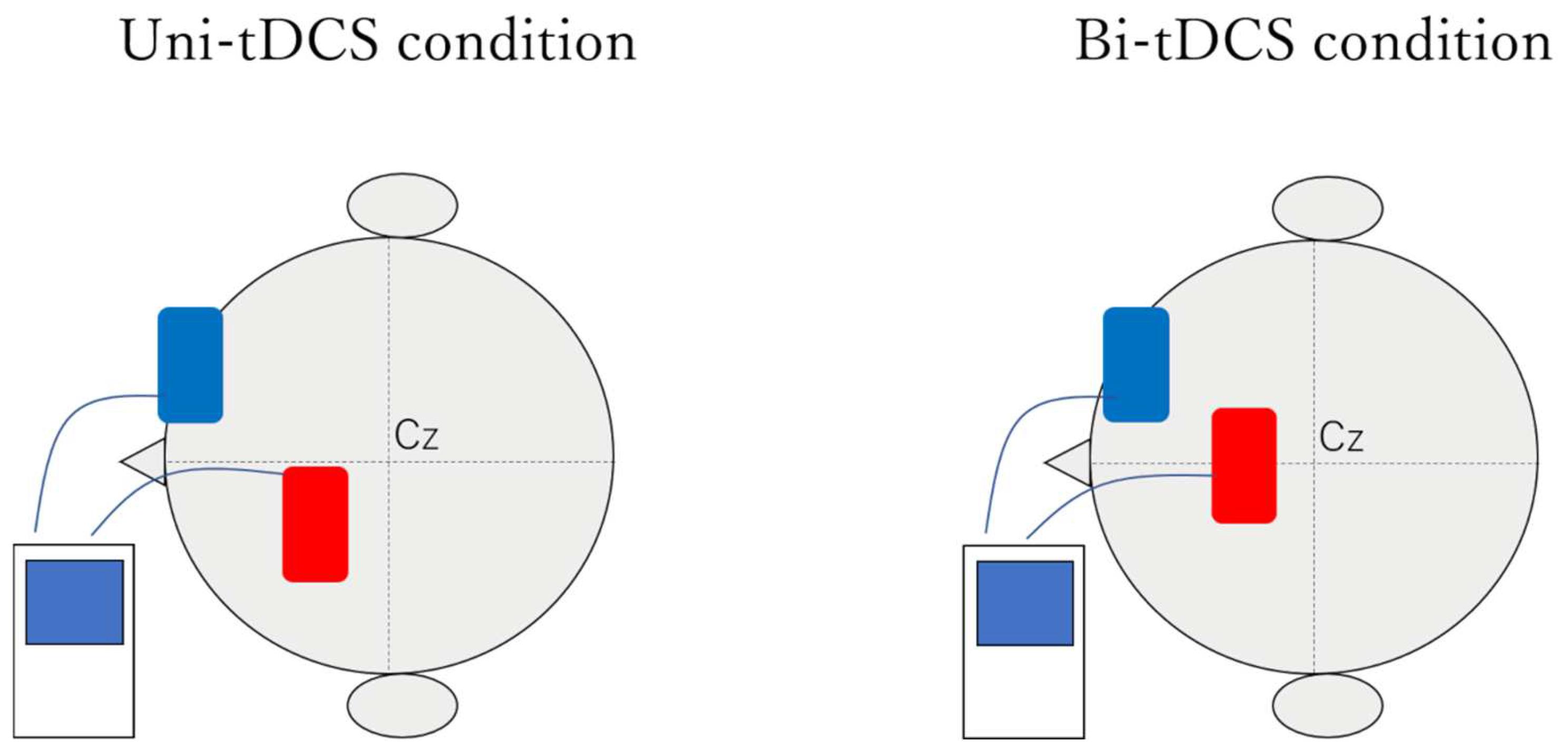
2.3. Setting up tDCS
2.4. Clinical Assessment and Measurement Items
2.5. Data Analysis
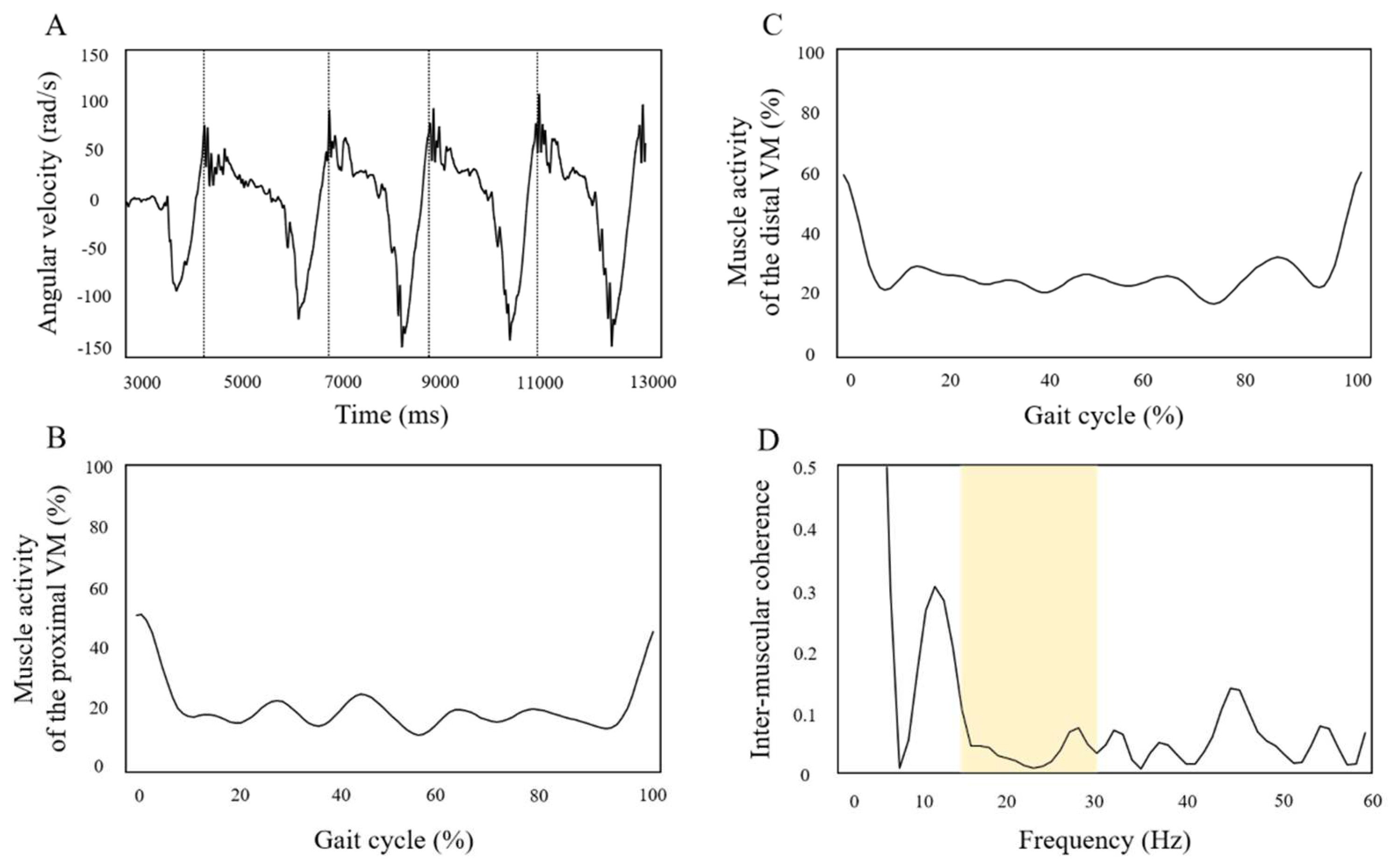
3. Results
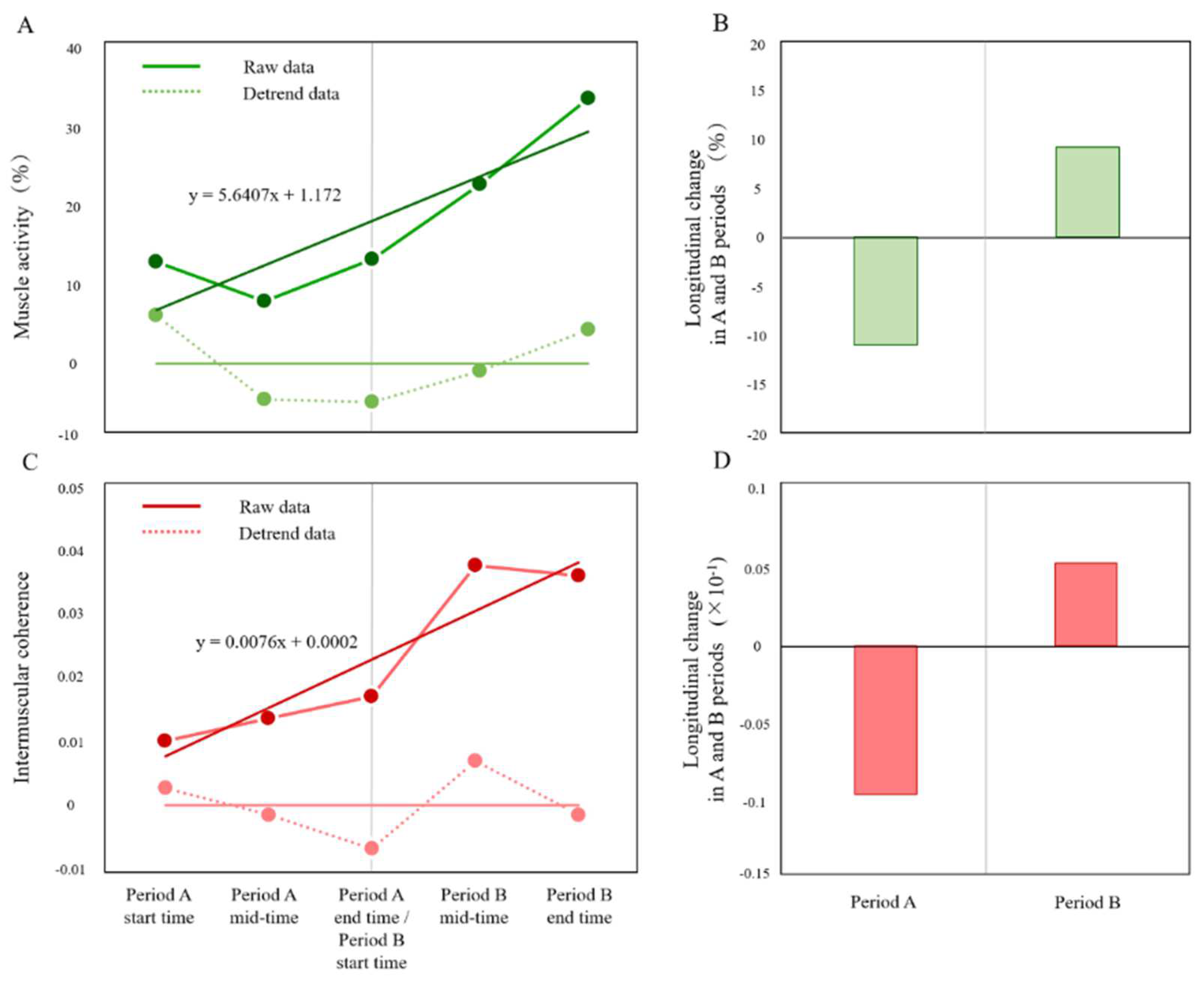
3.1. Results of Clinical Assessments at the Period A End Time (Period B Start Time) and Period B End Time
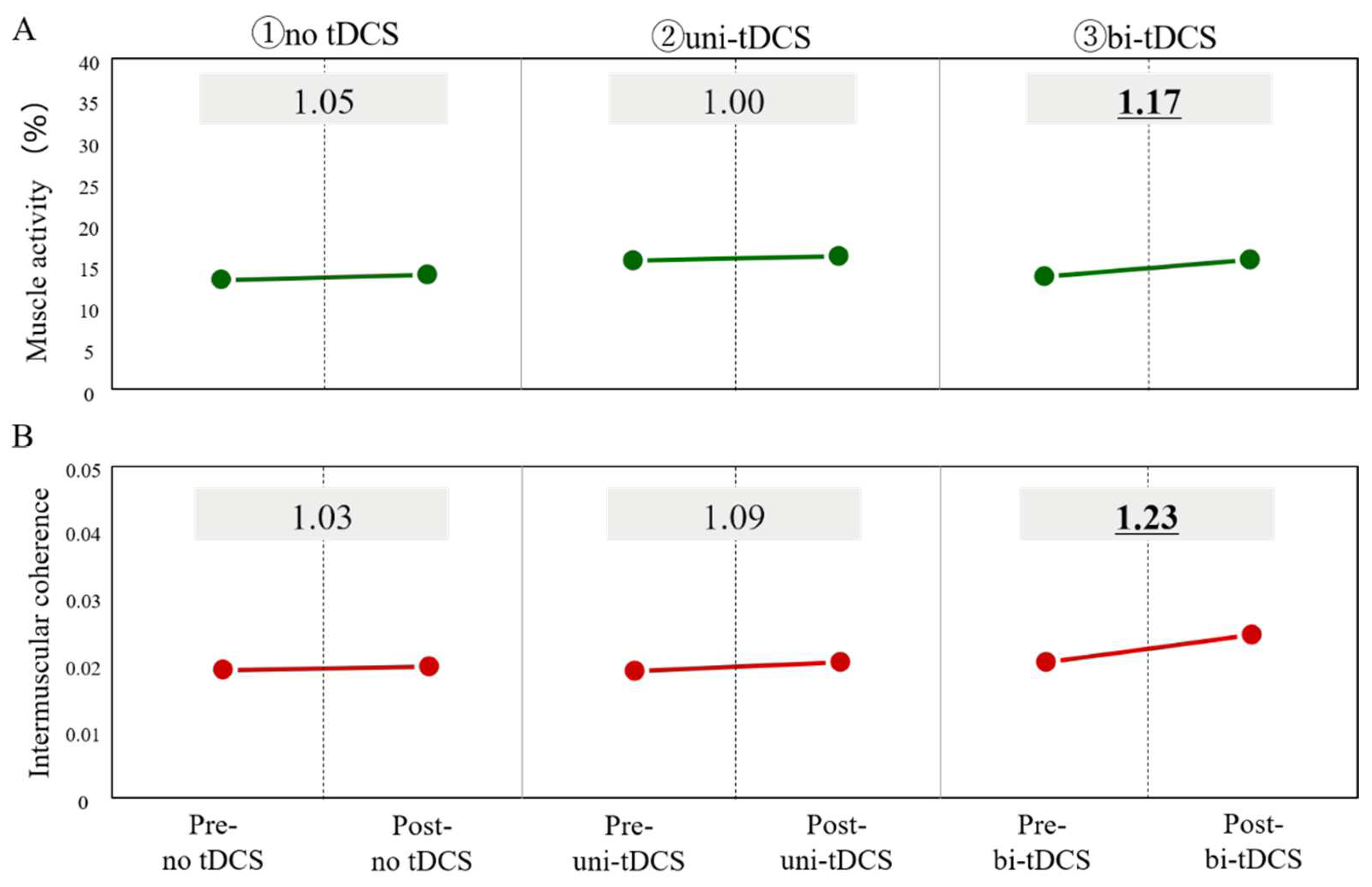
3.2. 20-Minute Short-Term Effects of Different Stimulation Positions of tDCS on Paretic Lower Limb Motor Function
3.3. Effects of 4-Weeks Bi-tDCS Intervention on Paretic Lower Limb Motor Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Veerbeek, J.M.; Winters, C.; van Wegen, E.E.H.; Kwakkel, G. Is the Proportional Recovery Rule Applicable to the Lower Limb after a First-Ever Ischemic Stroke? PLoS ONE 2018, 13, e0189279. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.C.; Byblow, W.D.; Barber, P.A.; Stinear, C.M. Proportional Recovery from Lower Limb Motor Impairment after Stroke. Stroke 2017, 48, 1400–1403. [Google Scholar] [CrossRef] [PubMed]
- Duncan, P.W.; Goldstein, L.B.; Horner, R.D.; Landsman, P.B.; Samsa, G.P.; Matchar, D.B. Similar Motor Recovery of Upper and Lower Extremities after Stroke. Stroke 1994, 25, 1181–1188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.-L.; Chen, B.-B.; Hsueh, P.-I.; Jeng, J.-S.; Koh, C.-L.; Hsieh, C.-L. Prediction of Lower Extremity Motor Recovery in Persons with Severe Lower Extremity Paresis after Stroke. Brain Inj. 2018, 32, 627–633. [Google Scholar] [CrossRef]
- Jorgensen, H.S.; Nakayama, H.; Raaschou, H.O.; Olsen, T.S. Recovery of Walking Function in Stroke Patients: The Copenhagen Stroke Study. Arch. Phys. Med. Rehabil. 1995, 76, 27–32. [Google Scholar] [CrossRef]
- Kim, Y.H.; You, S.H.; Kwon, Y.H.; Hallett, M.; Kim, J.H.; Jang, S.H. Longitudinal FMRI Study for Locomotor Recovery in Patients with Stroke. Neurology 2006, 67, 330–333. [Google Scholar] [CrossRef]
- Binder, E.; Leimbach, M.; Pool, E.M.; Volz, L.J.; Eickhoff, S.B.; Fink, G.R.; Grefkes, C. Cortical Reorganization after Motor Stroke: A Pilot Study on Differences between the Upper and Lower Limbs. Hum. Brain Mapp. 2021, 42, 1013–1033. [Google Scholar] [CrossRef]
- Jayaram, G.; Stagg, C.J.; Esser, P.; Kischka, U.; Stinear, J.; Johansen-Berg, H. Relationships between Functional and Structural Corticospinal Tract Integrity and Walking Post Stroke. Clin. Neurophysiol. 2012, 123, 2422–2428. [Google Scholar] [CrossRef] [Green Version]
- Sivaramakrishnan, A.; Madhavan, S. Absence of a Transcranial Magnetic Stimulation–Induced Lower Limb Corticomotor Response Does Not Affect Walking Speed in Chronic Stroke Survivors. Stroke 2018, 49, 2004–2007. [Google Scholar] [CrossRef]
- Madhavan, S.; Rogers, L.M.; Stinear, J.W. A Paradox: After Stroke, the Non-Lesioned Lower Limb Motor Cortex May Be Maladaptive. Eur. J. Neurosci. 2010, 32, 1032–1039. [Google Scholar] [CrossRef] [Green Version]
- Chang, M.C.; Do, K.H.; Chun, M.H. Prediction of Lower Limb Motor Outcomes Based on Transcranial Magnetic Stimulation Findings in Patients with an Infarct of the Anterior Cerebral Artery. Somatosens. Mot. Res. 2015, 32, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Barthélemy, D.; Willerslev-Olsen, M.; Lundell, H.; Biering-Sørensen, F.; Nielsen, J.B. Assessment of Transmission in Specific Descending Pathways in Relation to Gait and Balance Following Spinal Cord Injury. Prog. Brain Res. 2015, 218, 79–101. [Google Scholar] [CrossRef] [PubMed]
- Barthélemy, D.; Willerslev-Olsen, M.; Lundell, H.; Conway, B.A.; Knudsen, H.; Biering-Sørensen, F.; Nielsen, J.B. Impaired Transmission in the Corticospinal Tract and Gait Disability in Spinal Cord Injured Persons. J. Neurophysiol. 2010, 104, 1167–1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, J.B.; Brittain, J.S.; Halliday, D.M.; Marchand-Pauvert, V.; Mazevet, D.; Conway, B.A. Reduction of Common Motoneuronal Drive on the Affected Side during Walking in Hemiplegic Stroke Patients. Clin. Neurophysiol. 2008, 119, 2813–2818. [Google Scholar] [CrossRef]
- Petersen, T.H.; Willerslev-Olsen, M.; Conway, B.A.; Nielsen, J.B. The Motor Cortex Drives the Muscles during Walking in Human Subjects. J. Physiol. 2012, 590, 2443–2452. [Google Scholar] [CrossRef]
- Halliday, D.M.; Rosenberg, J.R.; Amjad, A.M.; Breeze, P.; Conway, B.A.; Farmer, S.F. A Framework for the Analysis of Mixed Time Series/Point Process Datamtheory and Application to the Study of Physiological Tremor, Single Motor Unit Discharges and Electromyograms. Prog. Biophys. Mol. Biol. 1995, 64, 237–278. [Google Scholar] [CrossRef]
- Fisher, K.M.; Zaaimi, B.; Williams, T.L.; Baker, S.N.; Baker, M.R. Beta-Band Intermuscular Coherence: A Novel Biomarker of Upper Motor Neuron Dysfunction in Motor Neuron Disease. Brain 2012, 135, 2849–2864. [Google Scholar] [CrossRef] [Green Version]
- Chang, M.C.; Kim, D.Y.; Park, D.H. Enhancement of Cortical Excitability and Lower Limb Motor Function in Patients with Stroke by Transcranial Direct Current Stimulation. Brain Stimul. 2015, 8, 561–566. [Google Scholar] [CrossRef]
- Power, H.A.; Norton, J.A.; Porter, C.L.; Doyle, Z.; Hui, I.; Chan, K.M. Transcranial Direct Current Stimulation of the Primary Motor Cortex Affects Cortical Drive to Human Musculature as Assessed by Intermuscular Coherence. J. Physiol. 2006, 577, 795–803. [Google Scholar] [CrossRef]
- Jeffery, D.T.; Norton, J.A.; Roy, F.D.; Gorassini, M.A. Effects of Transcranial Direct Current Stimulation on the Excitability of the Leg Motor Cortex. Exp. Brain Res. 2007, 182, 281–287. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Excitability Changes Induced in the Human Motor Cortex by Weak Transcranial Direct Current Stimulation. J. Physiol. 2000, 527, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Takeda, K.; Otaka, Y.; Kita, K.; Osu, R.; Honda, M.; Sadato, N.; Hanakawa, T.; Watanabe, K. Single Session of Transcranial Direct Current Stimulation Transiently Increases Knee Extensor Force in Patients with Hemiparetic Stroke. Neurorehabil. Neural Repair 2011, 25, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Miyai, I.; Yagura, H.; Hatakenaka, M.; Oda, I.; Konishi, I.; Kubota, K. Longitudinal Optical Imaging Study for Locomotor Recovery after Stroke. Stroke 2003, 34, 2866–2870. [Google Scholar] [CrossRef] [PubMed]
- Norton, J.A.; Gorassini, M.A. Changes in Cortically Related Intermuscular Coherence Accompanying Improvements in Locomotor Skills in Incomplete Spinal Cord Injury. J. Neurophysiol. 2006, 95, 2580–2589. [Google Scholar] [CrossRef] [Green Version]
- Abe, H.; Kadowaki, K.; Tsujimoto, N.; Okanuka, T. A Narrative Review of Alternate Gait Training Using Knee-Ankle-Foot Orthosis in Stroke Patients with Severe Hemiparesis. Phys. Ther. Res. 2021, 24, 195–203. [Google Scholar] [CrossRef]
- Brunnstrom, S. Motor Testing Procedures in Hemiplegia: Based on Sequential Recovery Stages. Phys. Ther. 1966, 46, 357–375. [Google Scholar] [CrossRef] [Green Version]
- Gladstone, D.J.; Danells, C.J.; Black, S.E. The Fugl-Meyer Assessment of Motor Recovery after Stroke: A Critical Review of Its Measurement Properties. Neurorehabil. Neural Repair 2002, 16, 232–240. [Google Scholar] [CrossRef]
- Kautz, S.A.; Patten, C. Interlimb Influences on Paretic Leg Function in Poststroke Hemiparesis. J. Neurophysiol. 2005, 93, 2460–2473. [Google Scholar] [CrossRef] [Green Version]
- Bowden, M.G.; Clark, D.J.; Kautz, S.A. Evaluation of Abnormal Synergy Patterns Poststroke: Relationship of the Fugl-Meyer Assessment to Hemiparetic Locomotion. Neurorehabil. Neural Repair 2010, 24, 328–337. [Google Scholar] [CrossRef]
- Verheyden, G.; Nieuwboer, A.; Mertin, J.; Preger, R.; Kiekens, C.; de Weerdt, W. The Trunk Impairment Scale: A New Tool to Measure Motor Impairment of the Trunk after Stroke. Clin. Rehabil. 2004, 18, 326–334. [Google Scholar] [CrossRef]
- Kim, Y.K.; Shin, S.H. Comparison of Effects of Transcranial Magnetic Stimulation on Primary Motor Cortex and Supplementary Motor Area in Motor Skill Learning (Randomized, Cross over Study). Front. Hum. Neurosci. 2014, 8, 937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutta, A.; Chugh, S. Effect of Transcranial Direct Current Stimulation on Cortico-Muscular Coherence and Standing Postural Steadiness. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), San Diego, CA, USA, 28 August–1 September 2012; Volumes 7643–7646. [Google Scholar] [CrossRef]
- Bikson, M.; Datta, A.; Elwassif, M. Establishing Safety Limits for Transcranial Direct Current Stimulation. Clin. Neurophysiol. 2009, 120, 1033–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nitsche, M.A.; Liebetanz, D.; Antal, A.; Lang, N.; Tergau, F.; Paulus, W. Chapter 27—Modulation of Cortical Excitability by Weak Direct Current Stimulation—Technical, Safety and Functional Aspects. Suppl. Clin. Neurophysiol. 2003, 56, 255–276. [Google Scholar] [CrossRef]
- Thair, H.; Holloway, A.L.; Newport, R.; Smith, A.D. Transcranial Direct Current Stimulation (TDCS): A Beginner’s Guide for Design and Implementation. Front. Neurosci. 2017, 11, 641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Artoni, F.; Fanciullacci, C.; Bertolucci, F.; Panarese, A.; Makeig, S.; Micera, S.; Chisari, C. Unidirectional Brain to Muscle Connectivity Reveals Motor Cortex Control of Leg Muscles during Stereotyped Walking. NeuroImage 2017, 159, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Wilken, J.M.; Rodriguez, K.M.; Brawner, M.; Darter, B.J. Reliability and Minimal Detectible Change Values for Gait Kinematics and Kinetics in Healthy Adults. Gait Posture 2012, 35, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Spedden, M.E.; Nielsen, J.B.; Geertsen, S.S. Oscillatory Corticospinal Activity during Static Contraction of Ankle Muscles Is Reduced in Healthy Old versus Young Adults. Neural Plast. 2018, 2018, 3432649. [Google Scholar] [CrossRef]
- Roeder, L.; Boonstra, T.W.; Smith, S.S.; Kerr, G.K. Dynamics of Corticospinal Motor Control during Overground and Treadmill Walking in Humans. J. Neurophysiol. 2018, 120, 1017–1031. [Google Scholar] [CrossRef]
- Sousa, A.S.; Tavares, R.S. Effect of Gait Speed on Muscle Activity Patterns and Magnitude during Stance. Mot. Control 2012, 16, 480–492. [Google Scholar] [CrossRef] [Green Version]
- Hesse, S.; Werner, C.; Paul, T.; Bardeleben, A.; Chaler, J. Influence of Walking Speed on Lower Limb Muscle Activity and Energy Consumption during Treadmill Walking of Hemiparetic Patients. Arch. Phys. Med. Rehabil. 2001, 82, 1547–1550. [Google Scholar] [CrossRef]
- Lim, Y.P.; Lin, Y.C.; Pandy, M.G. Effects of Step Length and Step Frequency on Lower-Limb Muscle Function in Human Gait. J. Biomech. 2017, 57, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Geiger, M.; Supiot, A.; Pradon, D.; Do, M.C.; Zory, R.; Roche, N. Minimal Detectable Change of Kinematic and Spatiotemporal Parameters in Patients with Chronic Stroke across Three Sessions of Gait Analysis. Hum. Mov. Sci. 2019, 64, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.L.; Wang, R.Y.; Liao, K.K.; Huang, C.C.; Yang, Y.R. Gait Training-Induced Change in Corticomotor Excitability in Patients with Chronic Stroke. Neurorehabil. Neural Repair 2008, 22, 22–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enzinger, C.; Johansen-Berg, H.; Dawes, H.; Bogdanovic, M.; Collett, J.; Guy, C.; Ropele, S.; Kischka, U.; Wade, D.; Fazekas, F.; et al. Functional MRI Correlates of Lower Limb Function in Stroke Victims with Gait Impairment. Stroke 2008, 39, 1507–1513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Period A Start Time | Period A End Time | Period B Start Time | Period B End Time | |
|---|---|---|---|---|
| BRS (lower limb): max = 6 | Ⅰ | Ⅰ | Ⅰ | Ⅰ |
| FMS (lower limb): max = 22 | 0 | 0 | 0 | 0 |
| TIS: max = 23 | 0 | 0 | 0 | 2 |
| FIM for transfer: max = 7 | 1 | 2 | 2 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohnishi, S.; Mizuta, N.; Hasui, N.; Taguchi, J.; Nakatani, T.; Morioka, S. Effects of Transcranial Direct Current Stimulation of Bilateral Supplementary Motor Area on the Lower Limb Motor Function in a Stroke Patient with Severe Motor Paralysis: A Case Study. Brain Sci. 2022, 12, 452. https://doi.org/10.3390/brainsci12040452
Ohnishi S, Mizuta N, Hasui N, Taguchi J, Nakatani T, Morioka S. Effects of Transcranial Direct Current Stimulation of Bilateral Supplementary Motor Area on the Lower Limb Motor Function in a Stroke Patient with Severe Motor Paralysis: A Case Study. Brain Sciences. 2022; 12(4):452. https://doi.org/10.3390/brainsci12040452
Chicago/Turabian StyleOhnishi, Sora, Naomichi Mizuta, Naruhito Hasui, Junji Taguchi, Tomoki Nakatani, and Shu Morioka. 2022. "Effects of Transcranial Direct Current Stimulation of Bilateral Supplementary Motor Area on the Lower Limb Motor Function in a Stroke Patient with Severe Motor Paralysis: A Case Study" Brain Sciences 12, no. 4: 452. https://doi.org/10.3390/brainsci12040452
APA StyleOhnishi, S., Mizuta, N., Hasui, N., Taguchi, J., Nakatani, T., & Morioka, S. (2022). Effects of Transcranial Direct Current Stimulation of Bilateral Supplementary Motor Area on the Lower Limb Motor Function in a Stroke Patient with Severe Motor Paralysis: A Case Study. Brain Sciences, 12(4), 452. https://doi.org/10.3390/brainsci12040452






