Association of Alcohol Types, Coffee, and Tea Intake with Risk of Dementia: Prospective Cohort Study of UK Biobank Participants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study and Participants
2.2. Exposure Assessment
2.3. Outcome Assessment
2.4. Statistical Analyses
3. Results
3.1. Baseline Characteristics and Dementia Cases in UK Biobank Participants
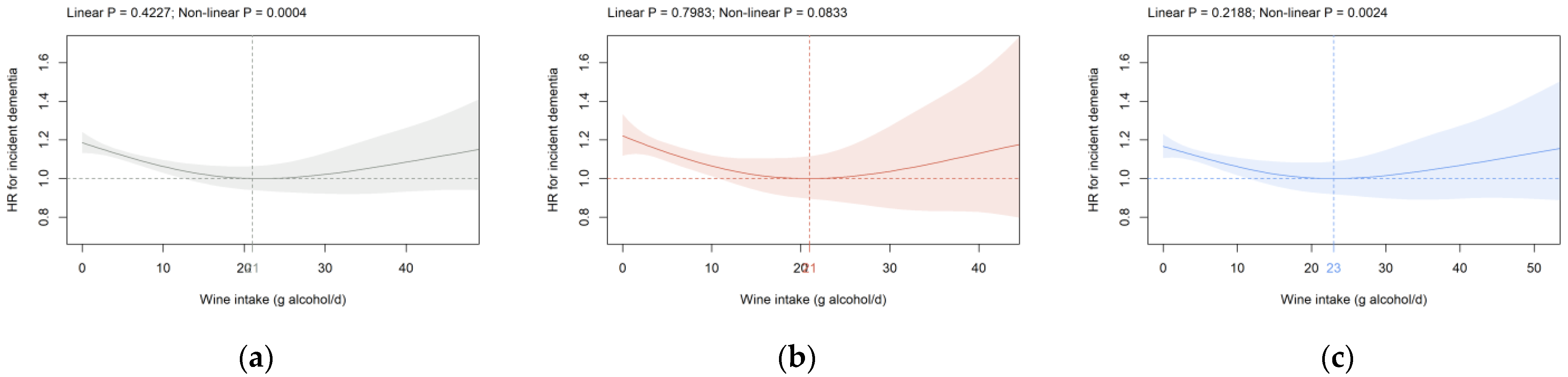
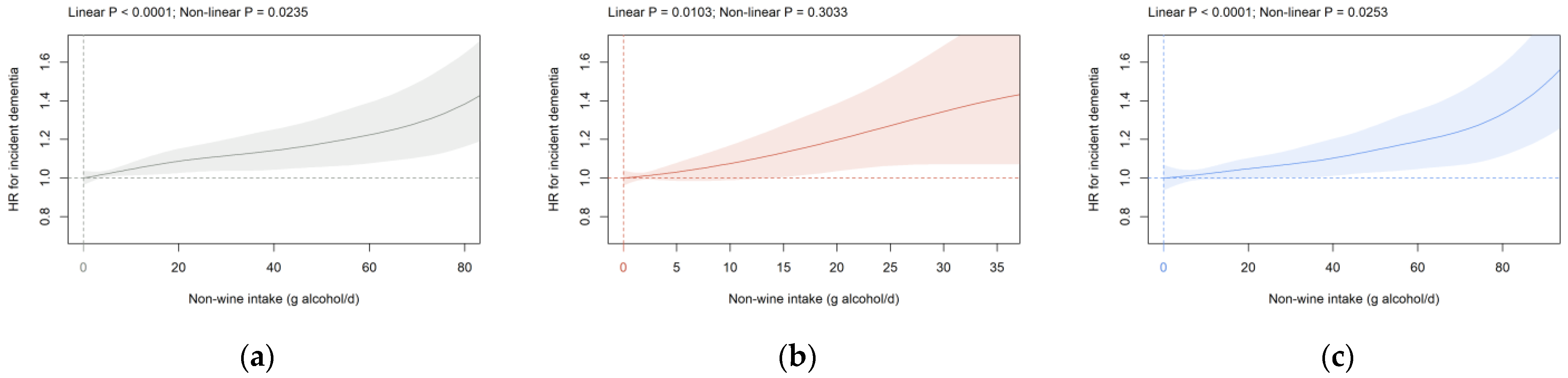
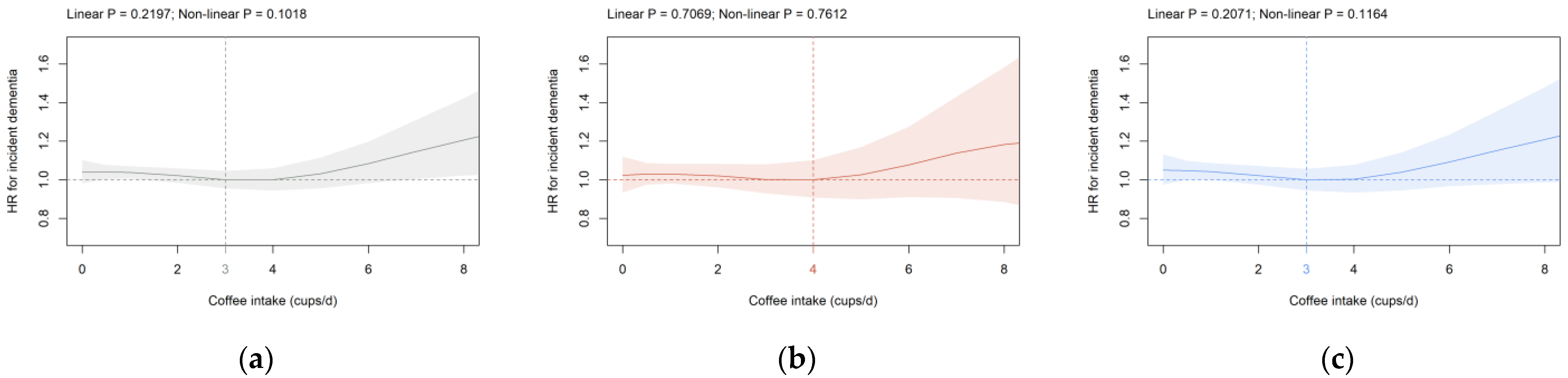
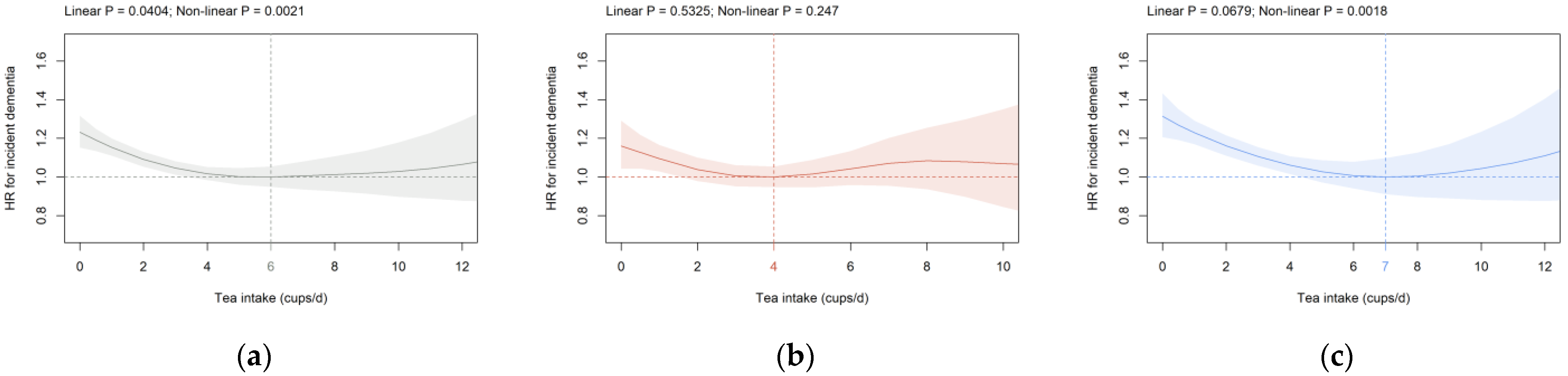
3.2. Beverage Intake and Dementia Risk
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Dementia. Key Facts. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 3 February 2022).
- Danat, I.M.; Clifford, A.; Partridge, M.; Zhou, W.; Bakre, A.T.; Chen, A.; McFeeters, D.; Smith, T.; Wan, Y.; Copeland, J.; et al. Impacts of Overweight and Obesity in Older Age on the Risk of Dementia: A Systematic Literature Review and a Meta-Analysis. J. Alzheimer’s Dis. 2019, 70, S87–S99. [Google Scholar] [CrossRef] [PubMed]
- Lanni, C.; Fagiani, F.; Racchi, M.; Preda, S.; Pascale, A.; Grilli, M.; Allegri, N.; Govoni, S. Beta-amyloid short- and long-term synaptic entanglement. Pharmacol. Res. 2019, 139, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Lv, N.; Azar, K.M.; Rosas, L.G.; Wulfovich, S.; Xiao, L.; Ma, J. Behavioral lifestyle interventions for moderate and severe obesity: A systematic review. Prev. Med. 2017, 100, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Kivipelto, M.; Mangialasche, F.; Ngandu, T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat. Rev. Neurol. 2018, 14, 653–666. [Google Scholar] [CrossRef]
- Schlesinger, S.; Neuenschwander, M.; Schwedhelm, C.; Hoffmann, G.; Bechthold, A.; Boeing, H.; Schwingshackl, L. Food Groups and Risk of Overweight, Obesity, and Weight Gain: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Adv. Nutr. 2019, 10, 205–218. [Google Scholar] [CrossRef] [Green Version]
- Dominguez, L.J.; Veronese, N.; Vernuccio, L.; Catanese, G.; Inzerillo, F.; Salemi, G.; Barbagallo, M. Nutrition, Physical Activity, and Other Lifestyle Factors in the Prevention of Cognitive Decline and Dementia. Nutrients 2021, 13, 4080. [Google Scholar] [CrossRef]
- Barberger-Gateau, P.; Raffaitin, C.; Letenneur, L.; Berr, C.; Tzourio, C.; Dartigues, J.-F.; Alpérovitch, A. Dietary patterns and risk of dementia: The Three-City cohort study. Neurology 2007, 69, 1921–1930. [Google Scholar] [CrossRef]
- Shai, I.; Schwarzfuchs, D.; Henkin, Y.; Shahar, D.R.; Witkow, S.; Greenberg, I.; Golan, R.; Fraser, D.; Bolotin, A.; Vardi, H.; et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N. Engl. J. Med. 2008, 359, 229–241. [Google Scholar] [CrossRef] [Green Version]
- García-Casares, N.; Fuentes, P.G.; Barbancho, M.Á.; López-Gigosos, R.; García-Rodríguez, A.; Gutiérrez-Bedmar, M. Alzheimer’s Disease, Mild Cognitive Impairment and Mediterranean Diet. A Systematic Review and Dose-Response Meta-Analysis. J. Clin. Med. 2021, 10, 4642. [Google Scholar] [CrossRef]
- Petersson, S.D.; Philippou, E. Mediterranean Diet, Cognitive Function, and Dementia: A Systematic Review of the Evidence. Adv. Nutr. 2016, 7, 889–904. [Google Scholar] [CrossRef] [Green Version]
- Miao, H.; Chen, K.; Yan, X.; Chen, F. Sugar in Beverage and the Risk of Incident Dementia, Alzheimer’s Disease and Stroke: A Prospective Cohort Study. J. Prev. Alzheimer’s Dis. 2021, 8, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Morenga, L.T.; Mallard, S.; Mann, J. Dietary sugars and body weight: Systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ 2012, 346, e7492. [Google Scholar] [CrossRef] [Green Version]
- Venkataraman, A.; Kalk, N.; Sewell, G.; Ritchie, C.W.; Lingford-Hughes, A. Alcohol and Alzheimer’s Disease—Does Alcohol Dependence Contribute to Beta-Amyloid Deposition, Neuroinflammation and Neurodegeneration in Alzheimer’s Disease? Alcohol Alcohol. 2017, 52, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Arendash, G.W.; Mori, T.; Cao, C.; Mamcarz, M.; Runfeldt, M.; Dickson, A.; Rezai-Zadeh, K.; Tan, J.; Citron, B.A.; Lin, X.; et al. Caffeine Reverses Cognitive Impairment and Decreases Brain Amyloid-β Levels in Aged Alzheimer’s Disease Mice. J. Alzheimer’s Dis. 2009, 17, 661–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolahdouzan, M.; Hamadeh, M.J. The neuroprotective effects of caffeine in neurodegenerative diseases. CNS Neurosci. Ther. 2017, 23, 272–290. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, H.; Wan, Y.; Tan, C.; Li, J.; Tan, L.; Yu, J.-T. Alcohol consumption and dementia risk: A dose–response meta-analysis of prospective studies. Eur. J. Epidemiol. 2017, 32, 31–42. [Google Scholar] [CrossRef]
- Department of Health. UK Chief Medical Officers’ Alcohol Guidelines Review: Summary of the Proposed New Guidelines-January 2016. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/489795/summary.pdf (accessed on 3 February 2022).
- Inan-Eroglu, E.; Powell, L.; Hamer, M.; O’Donovan, G.; Duncan, M.J.; Stamatakis, E. Is There a Link between Different Types of Alcoholic Drinks and Obesity? An Analysis of 280,183 UK Biobank Participants. Int. J. Environ. Res. Public Health 2020, 17, 5178. [Google Scholar] [CrossRef]
- Huang, J.; Wang, X.; Zhang, Y. Specific types of alcoholic beverage consumption and risk of type 2 diabetes: A systematic review and meta-analysis. J. Diabetes Investig. 2017, 8, 56–68. [Google Scholar] [CrossRef] [Green Version]
- Luchsinger, J.A.; Tang, M.-X.; Siddiqui, M.; Shea, S.; Mayeux, R. Alcohol Intake and Risk of Dementia. J. Am. Geriatr. Soc. 2004, 52, 540–546. [Google Scholar] [CrossRef]
- Mukamal, K.J.; Kuller, L.H.; Fitzpatrick, A.L.; Longstreth, J.W.T.; Mittleman, M.; Siscovick, D.S. Prospective Study of Alcohol Consumption and Risk of Dementia in Older Adults. JAMA 2003, 289, 1405–1413. [Google Scholar] [CrossRef]
- Weyerer, S.; Schäufele, M.; Wiese, B.; Maier, W.; Tebarth, F.; van den Bussche, H.; Pentzek, M.; Bickel, H.; Luppa, M.; Riedel-Heller, S.G. Current alcohol consumption and its relationship to incident dementia: Results from a 3-year follow-up study among primary care attenders aged 75 years and older. Age Ageing 2011, 40, 456–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, K.; van Lent, D.M.; Wolfsgruber, S.; Weinhold, L.; Kleineidam, L.; Bickel, H.; Scherer, M.; Eisele, M.; van den Bussche, H.; Wiese, B.; et al. Prospective Associations between Single Foods, Alzheimer’s Dementia and Memory Decline in the Elderly. Nutrients 2018, 10, 852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ODPHP. Dietary Guidelines for Americans. Available online: https://health.gov/sites/default/files/2019-09/2015-2020_Dietary_Guidelines.pdf (accessed on 3 February 2022).
- Pham, K.; Mulugeta, A.; Zhou, A.; O’Brien, J.T.; Llewellyn, D.J.; Hyppönen, E. Coffee, Brain Volume and Risk of Dementia and Stroke. Int. J. Epidemiol. 2021, 50, 1–12. [Google Scholar] [CrossRef]
- Mirza, S.S.; Tiemeier, H.; de Bruijn, R.F.A.G.; Hofman, A.; Franco, O.H.; Jong, J.K.-D.; Koudstaal, P.J.; Ikram, M.A. Coffee consumption and incident dementia. Eur. J. Epidemiol. 2014, 29, 735–741. [Google Scholar] [CrossRef]
- Sugiyama, K.; Tomata, Y.; Kaiho, Y.; Honkura, K.; Sugawara, Y.; Tsuji, I. Association between Coffee Consumption and Incident Risk of Disabling Dementia in Elderly Japanese: The Ohsaki Cohort 2006 Study. J. Alzheimer’s Dis. 2016, 50, 491–500. [Google Scholar] [CrossRef]
- Ran, L.S.; Liu, W.H.; Fang, Y.Y.; Xu, S.B.; Li, J.; Luo, X.; Pan, D.J.; Wang, M.H.; Wang, W. Alcohol, coffee and tea intake and the risk of cognitive deficits: A dose–response meta-analysis. Epidemiol. Psychiatr. Sci. 2021, 30, e13. [Google Scholar] [CrossRef]
- Johnson-Kozlow, M.; Kritz-Silverstein, D.; Barrett-Connor, E.; Morton, D. Coffee Consumption and Cognitive Function among Older Adults. Am. J. Epidemiol. 2002, 156, 842–850. [Google Scholar] [CrossRef] [Green Version]
- Tomata, Y.; Sugiyama, K.; Kaiho, Y.; Honkura, K.; Watanabe, T.; Zhang, S.; Sugawara, Y.; Tsuji, I. Green Tea Consumption and the Risk of Incident Dementia in Elderly Japanese: The Ohsaki Cohort 2006 Study. Am. J. Geriatr. Psychiatry 2016, 24, 881–889. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.-P.; Huang, C.; Cui, Q.-Y.; Yang, D.-J.; Sun, K.; Chen, X.; Li, X.-H. Meta-Analysis of the Association between Tea Intake and the Risk of Cognitive Disorders. PLoS ONE 2016, 11, e0165861. [Google Scholar] [CrossRef]
- Kakutani, S.; Watanabe, H.; Murayama, N. Green Tea Intake and Risks for Dementia, Alzheimer’s Disease, Mild Cognitive Impairment, and Cognitive Impairment: A Systematic Review. Nutrients 2019, 11, 1165. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Yang, H.; Li, S.; Li, W.-D.; Wang, Y. Consumption of coffee and tea and risk of developing stroke, dementia, and poststroke dementia: A cohort study in the UK Biobank. PLoS Med. 2021, 18, e1003830. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, N.; Nakanishi, Y.; Watanabe, Y.; Kitamura, K.; Kabasawa, K.; Takahashi, A.; Saito, T.; Kobayashi, R.; Takachi, R.; Oshiki, R.; et al. Association of coffee, green tea, and caffeine with the risk of dementia in older Japanese people. J. Am. Geriatr. Soc. 2021, 69, 3529–3544. [Google Scholar] [CrossRef] [PubMed]
- Roizen, R.; Fillmore, K.; Chikritzhs, T.; Stockwell, T. Light-to-moderate drinking and dementia risk: The former drinkers problem re-visited. Addict. Res. Theory 2013, 21, 181–193. [Google Scholar] [CrossRef]
- Richard, T.; Pawlus, A.D.; Iglésias, M.-L.; Pedrot, E.; Waffo-Teguo, P.; Mérillon, J.-M.; Monti, J.-P. Neuroprotective properties of resveratrol and derivatives. Ann. N. Y. Acad. Sci. 2011, 1215, 103–108. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Wang, Y.; Wang, G.; Mao, L.; Zhang, D.; Wang, J. Effects of resveratrol on learning and memory in rats with vascular dementia. Mol. Med. Rep. 2019, 20, 4587–4593. [Google Scholar] [CrossRef] [Green Version]
- UK Biobank. UK Biobank: Protocol for a Large-Scale Prospective Epidemiological Resource. Available online: https://www.ukbiobank.ac.uk/media/gnkeyh2q/study-rationale.pdf (accessed on 2 February 2022).
- Behrendt, I.; Fasshauer, M.; Eichner, G. Gluten intake and metabolic health: Conflicting findings from the UK Biobank. Eur. J. Nutr. 2021, 60, 1547–1559. [Google Scholar] [CrossRef]
- Stockwell, T.; Zhao, J.; Panwar, S.; Roemer, A.; Naimi, T.; Chikritzhs, T. Do “Moderate” Drinkers Have Reduced Mortality Risk? A Systematic Review and Meta-Analysis of Alcohol Consumption and All-Cause Mortality. J. Stud. Alcohol Drugs 2016, 77, 185–198. [Google Scholar] [CrossRef] [Green Version]
- Naimi, T.S.; Stockwell, T.; Zhao, J.; Xuan, Z.; Dangardt, F.; Saitz, R.; Liang, W.; Chikritzhs, T. Selection biases in observational studies affect associations between ‘moderate’ alcohol consumption and mortality. Addiction 2017, 112, 207–214. [Google Scholar] [CrossRef]
- Schaefer, S.M.; Kaiser, A.; Behrendt, I.; Eichner, G.; Fasshauer, M. Association of alcohol types, coffee and tea intake with mortality: Prospective cohort study of UK Biobank participants. Br. J. Nutr. 2022; (published online before print). [Google Scholar] [CrossRef]
- UK Biobank. First Occurrence of Health Outcomes Defined by 3-Character ICD10 Code. Available online: https://biobank.ndph.ox.ac.uk/showcase/showcase/docs/first_occurrences_outcomes.pdf (accessed on 3 February 2022).
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org (accessed on 2 February 2022).
- Wickham, H.; Bryan, J. readxl: Read Excel Files. R Package Version 1.3.1. Available online: https://CRAN.R-project.org/package=readxl (accessed on 2 February 2022).
- Wickham, H. tidyverse: Easily Install and Load the ‘Tidyverse’. R Package Version 1.3.1. Available online: https://CRAN.R-project.org/package=tidyverse (accessed on 2 February 2022).
- Dusa, A. venn: Draw Venn Diagrams. R Package Version 1.10. Available online: https://CRAN.R-project.org/package=venn (accessed on 3 February 2022).
- Waring, E.; Quinn, M.; McNamara, A.; de la Rubia, E.A.; Zhu, H.; Lowndes, J.; Ellis, S.; McLeod, H.; Wickham, H.; Müller, K.; et al. skimr: Compact and Flexible Summaries of Data. R Package Version 2.1.3. Available online: https://CRAN.R-project.org/package=skimr (accessed on 4 February 2022).
- Therneau, T.M. A Package for Survival Analysis in S. R Package Version 3.2-11. Available online: https://CRAN.R-project.org/package=survival (accessed on 4 February 2022).
- Therneau, T.M. Spline Terms in a Cox Model. Available online: https://cran.r-project.org/web/packages/survival/vignettes/splines.pdf (accessed on 4 February 2022).
- Therneau, T.M. Modeling Survival Data; Springer: New York, NY, USA, 2000. [Google Scholar]
- Orgogozo, J.M.; Dartigues, J.F.; Lafont, S.; Letenneur, L.; Commenges, D.; Salamon, R.; Renaud, S.; Breteler, M.B. Wine consumption and dementia in the elderly: A prospective community study in the Bordeaux area. Rev. Neurol. 1997, 153, 185–192. [Google Scholar]
- Truelsen, T.; Thudium, D.; Grønbaek, M. Amount and type of alcohol and risk of dementia: The Copenhagen City Heart Study. Neurology 2002, 59, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Mehlig, K.; Skoog, I.; Gustafson, D.; Waern, M.; Östling, S.; Guo, X.; Schütze, M.; Björkelund, C.; Lissner, L. Alcoholic Beverages and Incidence of Dementia: 34-Year Follow-up of the Prospective Population Study of Women in Goteborg. Am. J. Epidemiol. 2008, 167, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, J.; Laurin, D.; Verreault, R.; Hébert, R.; Helliwell, B.; Hill, G.B.; McDowell, I. Risk Factors for Alzheimer’s Disease: A Prospective Analysis from the Canadian Study of Health and Aging. Am. J. Epidemiol. 2002, 156, 445–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nooyens, A.C.J.; Bueno-De-Mesquita, H.B.; van Gelder, B.M.; van Boxtel, M.P.J.; Verschuren, W.M.M. Consumption of alcoholic beverages and cognitive decline at middle age: The Doetinchem Cohort Study. Br. J. Nutr. 2014, 111, 715–723. [Google Scholar] [CrossRef] [Green Version]
- Hassing, L.B. Light Alcohol Consumption Does Not Protect Cognitive Function: A Longitudinal Prospective Study. Front. Aging Neurosci. 2018, 10, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsson, S.C.; Orsini, N. Coffee Consumption and Risk of Dementia and Alzheimer’s Disease: A Dose-Response Meta-Analysis of Prospective Studies. Nutrients 2018, 10, 1501. [Google Scholar] [CrossRef] [Green Version]
- Shirai, Y.; Kuriki, K.; Otsuka, R.; Kato, Y.; Nishita, Y.; Tange, C.; Tomida, M.; Imai, T.; Ando, F.; Shimokata, H. Green tea and coffee intake and risk of cognitive decline in older adults: The National Institute for Longevity Sciences, Longitudinal Study of Aging. Public Health Nutr. 2020, 23, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Noguchi-Shinohara, M.; Yuki, S.; Dohmoto, C.; Ikeda, Y.; Samuraki, M.; Iwasa, K.; Yokogawa, M.; Asai, K.; Komai, K.; Nakamura, H.; et al. Consumption of Green Tea, but Not Black Tea or Coffee, Is Associated with Reduced Risk of Cognitive Decline. PLoS ONE 2014, 9, e96013. [Google Scholar] [CrossRef] [Green Version]
- Beresniak, A.; Duru, G.; Berger, G.; Bremond-Gignac, D. Relationships between black tea consumption and key health indicators in the world: An ecological study. BMJ Open 2012, 2, e000648. [Google Scholar] [CrossRef] [Green Version]
- Fry, A.; Littlejohns, T.J.; Sudlow, C.; Doherty, N.; Adamska, L.; Sprosen, T.; Collins, R.; Allen, N.E. Comparison of Sociodemographic and Health-Related Characteristics of UK Biobank Participants with Those of the General Population. Am. J. Epidemiol. 2017, 186, 1026–1034. [Google Scholar] [CrossRef] [Green Version]
| Parameter | All (n = 351,436) | Female (n = 178,389) | Male # (n = 173,047) |
|---|---|---|---|
| Wine intake (g/d) | 5.7 (1.4, 11.4) | 5.7 (2.9, 11.4) | 5.7 (0.7, 11.4) |
| Non-wine intake (g/d) | 4.3 (0.0, 12.9) | 1.4 (0.0, 4.3) | 11.4 (4.3, 22.9) |
| Coffee intake (cups/d) | 2.0 (0.5, 3.0) | 2.0 (0.5, 3.0) | 2.0 (1.0, 3.0) |
| Tea intake (cups/d) | 3.0 (1.0, 5.0) | 3.0 (1.0, 5.0) | 3.0 (1.0, 5.0) |
| Age (years) | 58 (50, 63) | 57 (50, 63) | 58 (50, 63) |
| AHI (k£) | |||
| <18 | 55,960 (15.9) | 29,328 (16.4) | 26,632 (15.4) |
| - 18 to <31 | 76,591 (21.8) | 39,078 (21.9) | 37,513 (21.7) |
| - 31 to <52 | 85,290 (24.3) | 41,302 (23.2) | 43,988 (25.4) |
| - 52 to <100 | 71,810 (20.4) | 33,190 (18.6) | 38,620 (22.3) |
| - ≥100 - Unknown | 20,161 (5.7) 41,624 (11.8) | 9121 (5.1) 26,370 (14.8) | 11,040 (6.4) 15,254 (8.8) |
| Ethnicity | |||
| - White | 340,001 (96.7) | 172,819 (96.9) | 167,182 (96.6) |
| - Mixed, Asian, Black, Chinese, Other | 11,435 (3.3) | 5570 (3.1) | 5865 (3.4) |
| Highest Qualification | |||
| - None of the below | 50,416 (14.6) | 24,189 (13.6) | 26,227 (15.2) |
| - National exams at age 16 years | 58,119 (16.5) | 34,787 (19.5) | 23,332 (13.5) |
| - Vocational qualifications | 38,492 (11.0) | 14,660 (8.2) | 23,832 (13.8) |
| - Optional national exams at ages 17–18 years | 26,619 (7.6) | 14,469 (8.1) | 12,150 (7.0) |
| - Professional | 52,545 (15.0) | 27,559 (15.4) | 24,986 (14.4) |
| - College or University | 125,245 (35.6) | 62,725 (35.2) | 62,520 (36.1) |
| OHR | |||
| - Poor | 10,478 (3.0) | 4229 (2.4) | 6249 (3.8) |
| - Fair | 66,120 (18.8) | 29,189 (16.4) | 36,931 (21.3) |
| - Good | 211,398 (60.2) | 110,514 (62.0) | 100,884 (58.3) |
| - Excellent | 63,440 (18.1) | 34,457 (19.3) | 28,983 (16.7) |
| PA (MET-min/week) | 1800 (845, 3546) | 1764 (838, 3375) | 1857 (848, 3714) |
| Percentage body fat | 30.2 (24.7, 36.7) | 36.2 (31.6, 40.7) | 25.3 (21.5, 28.9) |
| Smoking status | |||
| - Never | 185,929 (52.9) | 102,189 (57.3) | 83,740 (48.4) |
| - Previous | 130,726 (37.2) | 61,360 (34.4) | 69,366 (40.1) |
| - Current | 34,781 (9.9) | 14,840 (8.3) | 29,941 (11.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schaefer, S.M.; Kaiser, A.; Behrendt, I.; Eichner, G.; Fasshauer, M. Association of Alcohol Types, Coffee, and Tea Intake with Risk of Dementia: Prospective Cohort Study of UK Biobank Participants. Brain Sci. 2022, 12, 360. https://doi.org/10.3390/brainsci12030360
Schaefer SM, Kaiser A, Behrendt I, Eichner G, Fasshauer M. Association of Alcohol Types, Coffee, and Tea Intake with Risk of Dementia: Prospective Cohort Study of UK Biobank Participants. Brain Sciences. 2022; 12(3):360. https://doi.org/10.3390/brainsci12030360
Chicago/Turabian StyleSchaefer, Sylva Mareike, Anna Kaiser, Inken Behrendt, Gerrit Eichner, and Mathias Fasshauer. 2022. "Association of Alcohol Types, Coffee, and Tea Intake with Risk of Dementia: Prospective Cohort Study of UK Biobank Participants" Brain Sciences 12, no. 3: 360. https://doi.org/10.3390/brainsci12030360
APA StyleSchaefer, S. M., Kaiser, A., Behrendt, I., Eichner, G., & Fasshauer, M. (2022). Association of Alcohol Types, Coffee, and Tea Intake with Risk of Dementia: Prospective Cohort Study of UK Biobank Participants. Brain Sciences, 12(3), 360. https://doi.org/10.3390/brainsci12030360






