Changes in Hippocampus and Amygdala Volume with Hypoxic Stress Related to Cardiorespiratory Fitness under a High-Altitude Environment
Abstract
:1. Introduction
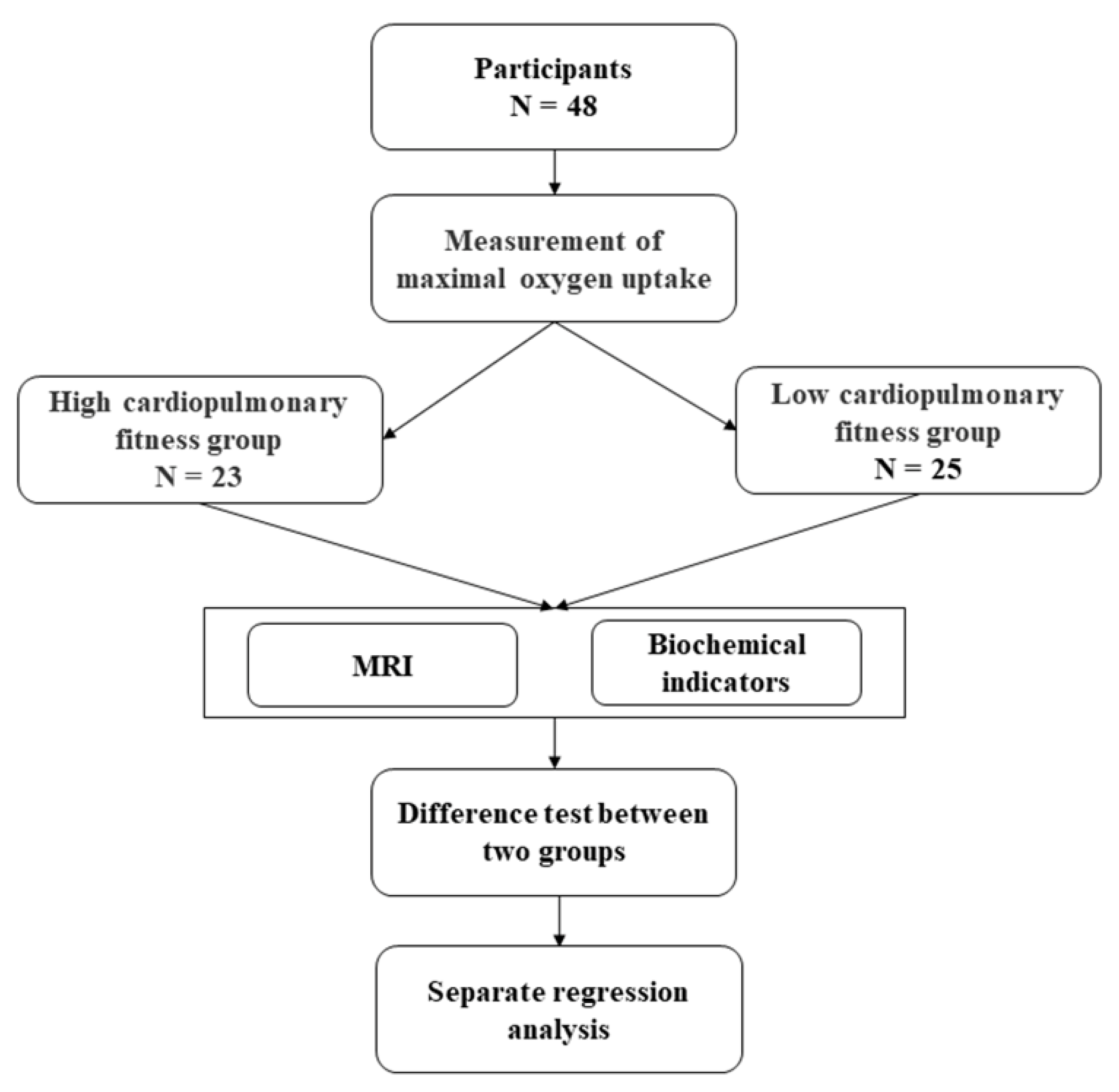
2. Materials and Methods
2.1. Participants
2.2. Experimental Design
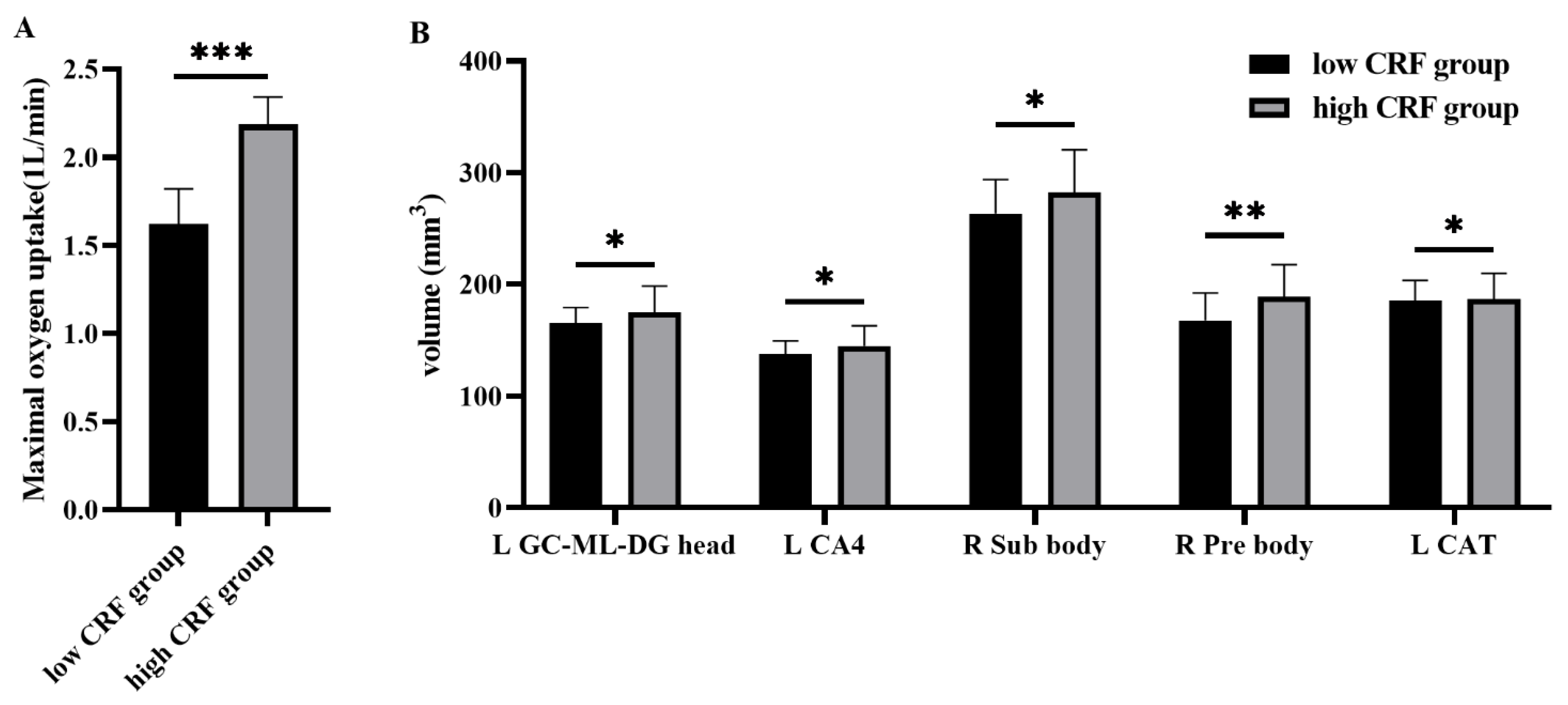
2.3. Maximum Oxygen Uptake
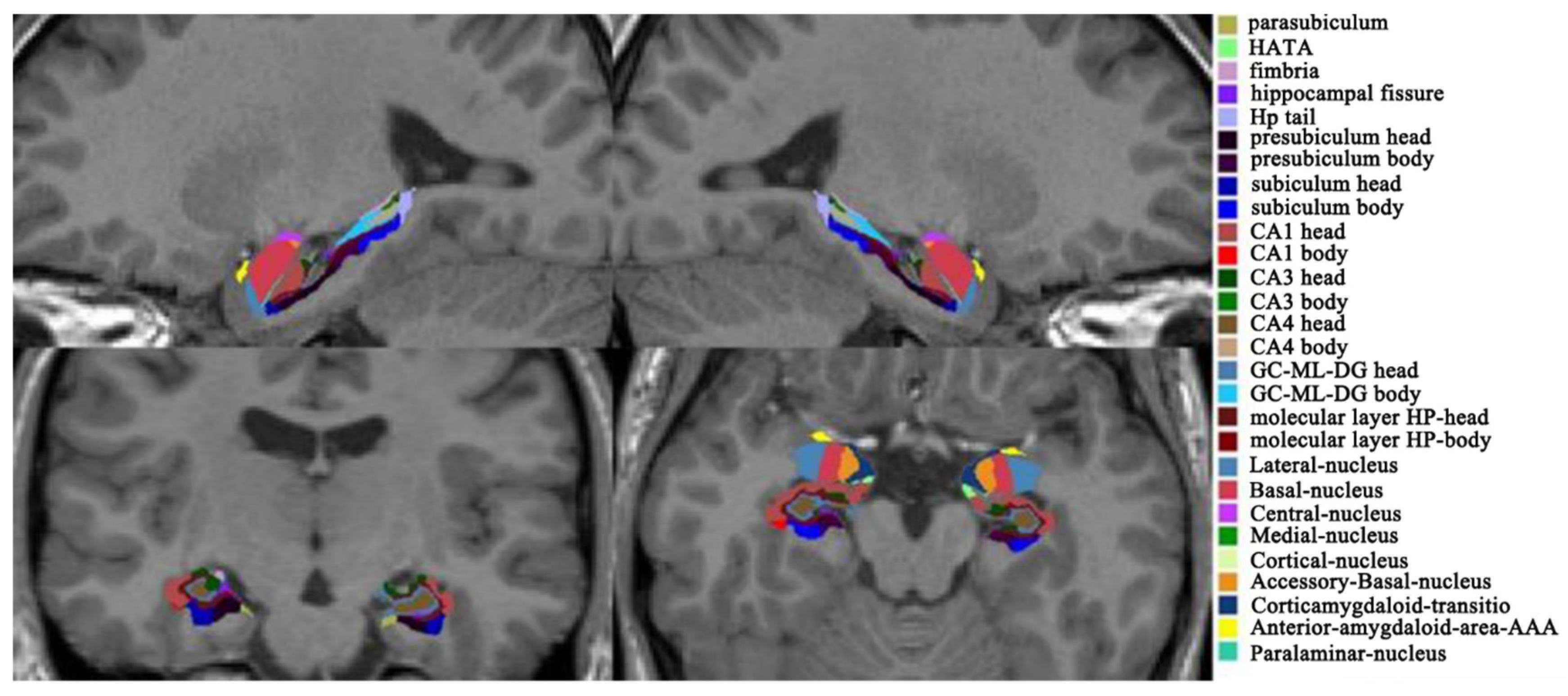
2.4. MRI Data Acquisition
2.5. Biochemical Indicators
2.6. Statistical Analysis
3. Results
3.1. Hippocampal and Amygdala Subfields
3.2. Biochemical Indicators
3.3. Multiple Regression Analysis
3.3.1. Hippocampus Subfields
3.3.2. Amygdala Subfields
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hossmann, K.-A. The Hypoxic Brain. In Hypoxia: Into the Next Millennium; Roach, R.C., Wagner, P.D., Hackett, P.H., Eds.; Springer: Boston, MA, USA, 1999; pp. 155–169. [Google Scholar]
- Kumari, P.; Kauser, H.; Wadhwa, M.; Roy, K.; Alam, S.; Sahu, S.; Kishore, K.; Ray, K.; Panjwani, U. Hypobaric hypoxia impairs cued and contextual fear memory in rats. Brain Res. 2018, 1692, 118–133. [Google Scholar] [CrossRef] [PubMed]
- Maiti, P.; Singh, S.B.; Mallick, B.; Muthuraju, S.; Ilavazhagan, G. High altitude memory impairment is due to neuronal apoptosis in hippocampus, cortex and striatum. J. Chem. Neuroanat. 2008, 36, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, H.; Li, J.; Chen, J.; Han, Q.; Lin, J.; Yang, T.; Fan, M. Adaptive Modulation of Adult Brain Gray and White Matter to High Altitude: Structural MRI Studies. PLoS ONE 2013, 8, e68621. [Google Scholar] [CrossRef]
- Ma, H.; Huang, X.; Liu, M.; Ma, H.; Zhang, D. Aging of stimulus-driven and goal-directed attentional processes in young immigrants with long-term high altitude exposure in Tibet: An ERP study. Sci. Rep. 2018, 8, 17417. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, X.; Ma, H.; Wang, Y.; Ma, H.; Liu, M. Competition among the attentional networks due to resource reduction in Tibetan indigenous residents: Evidence from event-related potentials. Sci. Rep. 2018, 8, 610. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Physiology and Neurobiology of Stress and Adaptation: Central Role of the Brain. Physiol. Rev. 2007, 87, 873–904. [Google Scholar] [CrossRef] [Green Version]
- Rybnikova, E.; Nalivaeva, N. Glucocorticoid-Dependent Mechanisms of Brain Tolerance to Hypoxia. Int. J. Mol. Sci. 2021, 22, 7982. [Google Scholar] [CrossRef]
- Zhao, F.; Yang, J.; Cui, R. Effect of Hypoxic Injury in Mood Disorder. Neural Plast. 2017, 2017, 6986983. [Google Scholar] [CrossRef] [Green Version]
- Gainey, S.J. Hypoxic immunomodulation results in increased disease risk and altered behavior via non-canonical pathways. Ph.D. Thesis, University of Illinois at Urbana-Champaign, Champaign, IL, USA, 28 February 2017. [Google Scholar]
- Yang, T.; Zhou, D.; Stefan, H. Why mesial temporal lobe epilepsy with hippocampal sclerosis is progressive: Uncontrolled inflammation drives disease progression? J. Neurol. Sci. 2010, 296, 1–6. [Google Scholar] [CrossRef]
- Zhang, J.; Malik, A.; Choi, H.B.; Ko, R.W.; Dissing-Olesen, L.; MacVicar, B.A. Microglial CR3 Activation Triggers Long-Term Synaptic Depression in the Hippocampus via NADPH Oxidase. Neuron 2014, 82, 195–207. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.-F.; Zhao, X.; Gurkoff, G.G.; Van, K.C.; Shahlaie, K.; Lyeth, B.G. Post-Traumatic Hypoxia Exacerbates Neuronal Cell Death in the Hippocampus. J. Neurotrauma 2012, 29, 1167–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalak, Z.; Obari, D.; Ellis, M.; Thom, M.; Sisodiya, S.M. Neuropathology of SUDEP. Neurology 2017, 88, 551–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.-H.; Xie, H.; Shi, Z.-H.; Du, L.-D.; Wing, Y.-K.; Li, A.M.; Ke, Y.; Yung, W.-H. Critical Role of Endoplasmic Reticulum Stress in Chronic Intermittent Hypoxia-Induced Deficits in Synaptic Plasticity and Long-Term Memory. Antioxid. Redox Signal. 2015, 23, 695–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carty, M.L.; Wixey, J.A.; Kesby, J.; Reinebrant, H.E.; Colditz, P.B.; Gobe, G.; Buller, K.M. Long-term losses of amygdala corticotropin-releasing factor neurons are associated with behavioural outcomes following neonatal hypoxia-ischemia. Behav. Brain Res. 2010, 208, 609–618. [Google Scholar] [CrossRef]
- Suliga, E. Chapter 4—Lifestyle Factors Affecting Abdominal Obesity in Children and Adolescents: Risks and Benefits. In Nutrition in the Prevention and Treatment of Abdominal Obesity; Watson, R.R., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 39–56. [Google Scholar]
- Johnson, N.F.; Kim, C.; Clasey, J.L.; Bailey, A.; Gold, B.T. Cardiorespiratory fitness is positively correlated with cerebral white matter integrity in healthy seniors. NeuroImage 2012, 59, 1514–1523. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.T.; Chaloner, E.J.; Hollingsworth, S.J. The role of cardiopulmonary fitness and its genetic influences on surgical outcomes. Br. J. Surg. 2005, 93, 147–157. [Google Scholar] [CrossRef]
- Sapolsky, R.M. Glucocorticoids and Hippocampal Atrophy in Neuropsychiatric Disorders. Arch. Gen. Psychiatry 2000, 57, 925–935. [Google Scholar] [CrossRef]
- Lucassen, P.J.; Meerlo, P.; Naylor, A.S.; van Dam, A.M.; Dayer, A.G.; Fuchs, E.; Oomen, C.A.; Czéh, B. Regulation of adult neurogenesis by stress, sleep disruption, exercise and inflammation: Implications for depression and antidepressant action. Eur. Neuropsychopharmacol. 2010, 20, 1–17. [Google Scholar] [CrossRef]
- Boots, E.; Schultz, S.; Oh, J.M.; Larson, J.; Edwards, D.; Cook, D.; Koscik, R.L.; Dowling, M.N.; Gallagher, C.L.; Carlsson, C.M.; et al. Cardiorespiratory fitness is associated with brain structure, cognition, and mood in a middle-aged cohort at risk for Alzheimer’s disease. Brain Imaging Behav. 2014, 9, 639–649. [Google Scholar] [CrossRef]
- Prathap, S.; Nagel, B.J.; Herting, M.M. Understanding the role of aerobic fitness, spatial learning, and hippocampal subfields in adolescent males. Sci. Rep. 2021, 11, 9311. [Google Scholar] [CrossRef]
- Erickson, K.I.; Prakash, R.S.; Voss, M.W.; Chaddock, L.; Hu, L.; Morris, K.S.; White, S.M.; Wójcicki, T.R.; McAuley, E.; Kramer, A.F. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus 2009, 19, 1030–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herting, M.M.; Nagel, B.J. Aerobic fitness relates to learning on a virtual Morris Water Task and hippocampal volume in adolescents. Behav. Brain Res. 2012, 233, 517–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, T.-W.; Shih, Y.-H.; Chen, S.-J.; Lien, C.-H.; Chang, C.-Y.; Huang, T.-Y.; Chen, S.-H.; Jen, C.J.; Kuo, Y.-M. Running exercise delays neurodegeneration in amygdala and hippocampus of Alzheimer’s disease (APP/PS1) transgenic mice. Neurobiol. Learn. Mem. 2015, 118, 189–197. [Google Scholar] [CrossRef]
- Seo, B.; Kim, D.; Choi, D.; Kwon, C.; Shin, H. The Effect of Electrical Stimulation on Blood Lactate after Anaerobic Muscle Fatigue Induced in Taekwondo Athletes. J. Phys. Ther. Sci. 2011, 23, 271–275. [Google Scholar] [CrossRef] [Green Version]
- Heath, E.H. ACSM’s Guidelines for Exercise Testing and Prescription, 7th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005; p. 37. [Google Scholar]
- Grocott, M.; Montgomery, H.; Vercueil, A. High-altitude physiology and pathophysiology: Implications and relevance for intensive care medicine. Crit. Care 2007, 11, 203. [Google Scholar] [CrossRef]
- Ward, S.A.; Grocott, M.P.; Levett, D.Z. Exercise testing, supplemental oxygen, and hypoxia. Ann. Am. Thorac. Soc. 2017, 14, S140–S148. [Google Scholar] [CrossRef] [PubMed]
- Mairbäurl, H. Red blood cells in sports: Effects of exercise and training on oxygen supply by red blood cells. Front. Physiol. 2013, 4, 332. [Google Scholar] [CrossRef] [Green Version]
- Kleppe, S.; Bernhardt, C.; Wölfle, J.; Breuer, J. Red Blood Cell Function in Hypoxia at Altitude and Exercise. Int. J. Sports Med. 1994, 15, 51–63. [Google Scholar]
- Ogoh, S.; Ainslie, P.N. Regulatory Mechanisms of Cerebral Blood Flow during Exercise: New Concepts. Exerc. Sport Sci. Rev. 2009, 37, 123–129. [Google Scholar] [CrossRef]
- Smith, T.B.; Stonell, C.; Purkayastha, S.; Paraskevas, P. Cardiopulmonary exercise testing as a risk assessment method in non cardio-pulmonary surgery: A systematic review. Anaesthesia 2009, 64, 883–893. [Google Scholar] [CrossRef]
- Grover, R.F.; Weil, J.V.; Reeves, J.T. Cardiovascular adaptation to exercise at high altitude. Exerc. Sport Sci. Rev. 1986, 14, 269–302. [Google Scholar] [CrossRef] [PubMed]
- Haugen, A.H.; Melanson, E.L.; Tran, Z.V.; Kearney, J.T.; Hill, J.O. Variability of measured resting metabolic rate. Am. J. Clin. Nutr. 2003, 78, 1141–1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mala, L.; Maly, T.; Zahalka, F.; Heller, J.; Hrasky, P.; Vodicka, P. Differences in the morphological and physiological charac-teristics of senior and junior elite Czech judo athletes. Arch. Budo 2015, 11, 217–226. [Google Scholar]
- Iglesias, J.E.; Augustinack, J.C.; Nguyen, K.; Player, C.M.; Player, A.; Wright, M.; Roy, N.; Frosch, M.P.; McKee, A.C.; Wald, L.; et al. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: Application to adaptive segmentation of in vivo MRI. NeuroImage 2015, 115, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Saygin, Z.; Kliemann, D.; Iglesias, J.E.; van der Kouwe, A.; Boyd, E.; Reuter, M.; Stevens, A.; Van Leemput, K.; McKee, A.; Frosch, M.; et al. High-resolution magnetic resonance imaging reveals nuclei of the human amygdala: Manual segmentation to automatic atlas. NeuroImage 2017, 155, 370–382. [Google Scholar] [CrossRef]
- Xue, X.-J.; Su, R.; Li, Z.-F.; Bu, X.-O.; Dang, P.; Yu, S.-F.; Wang, Z.-X.; Chen, D.-M.; Zeng, T.-A.; Liu, M.; et al. Oxygen Metabolism-induced Stress Response Underlies Heart–brain Interaction Governing Human Consciousness-breaking and Attention. Neurosci. Bull. 2021, 38, 166–180. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, Y.; Ren, Q.; Gong, G.; Zhang, D.; Shao, K.; Lin, P.; Yuan, Y.; Dai, T.; Zhang, Y.; et al. Research Square 2021. Available online: https://assets.researchsquare.com/files/rs-900442/v1/7c67881d-baba-4d40-b494-bd49d882bd54.pdf?c=1632165377 (accessed on 25 February 2022).
- Brown, S.S.G.; Rutland, J.W.; Verma, G.; Feldman, R.E.; Alper, J.; Schneider, M.; Delman, B.; Murrough, J.M.; Balchandani, P. Structural MRI at 7T reveals amygdala nuclei and hippocampal subfield volumetric association with Major Depressive Disorder symptom severity. Sci. Rep. 2019, 9, 10166. [Google Scholar] [CrossRef] [Green Version]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Haukvik, U.K.; McNeil, T.; Lange, E.H.; Melle, I.; Dale, A.M.; Andreassen, O.A.; Agartz, I. Pre- and perinatal hypoxia associated with hippocampus/amygdala volume in bipolar disorder. Psychol. Med. 2013, 44, 975–985. [Google Scholar] [CrossRef] [Green Version]
- Chaddock, L.; Erickson, K.I.; Prakash, R.S.; Kim, J.S.; Voss, M.W.; Vanpatter, M.; Pontifex, M.B.; Raine, L.B.; Konkel, A.; Hillman, C.H.; et al. A neuroimaging investigation of the association between aerobic fitness, hippocampal volume, and memory performance in preadolescent children. Brain Res. 2010, 1358, 172–183. [Google Scholar] [CrossRef] [Green Version]
- Chaddock, L.; Hillman, C.; Pontifex, M.; Johnson, C.R.; Raine, L.B.; Kramer, A. Childhood aerobic fitness predicts cognitive performance one year later. J. Sports Sci. 2012, 30, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Engeroff, T.; Füzéki, E.; Vogt, L.; Fleckenstein, J.; Schwarz, S.; Matura, S.; Pilatus, U.; Deichmann, R.; Hellweg, R.; Pantel, J.; et al. Is Objectively Assessed Sedentary Behavior, Physical Activity and Cardiorespiratory Fitness Linked to Brain Plasticity Outcomes in Old Age? Neuroscience 2018, 388, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Daniele, T.M.D.C.; de Bruin, P.F.C.; de Matos, R.S.; de Bruin, G.S.; Chaves, C.M.; de Bruin, V.M.S. Exercise effects on brain and behavior in healthy mice, Alzheimer’s disease and Parkinson’s disease model—A systematic review and meta-analysis. Behav. Brain Res. 2020, 383, 112488. [Google Scholar] [CrossRef]
- Segerstrom, S.C.; Miller, G.E. Psychological Stress and the Human Immune System: A Meta-Analytic Study of 30 Years of Inquiry. Psychol. Bull. 2004, 130, 601–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majidi, J.; Kosari-Nasab, M.; Salari, A.-A. Developmental minocycline treatment reverses the effects of neonatal immune activation on anxiety- and depression-like behaviors, hippocampal inflammation, and HPA axis activity in adult mice. Brain Res. Bull. 2016, 120, 1–13. [Google Scholar] [CrossRef]
- Dickens, A.M.; Tovar-Y-Romo, L.B.; Yoo, S.-W.; Trout, A.L.; Bae, M.; Kanmogne, M.; Megra, B.; Williams, D.W.; Witwer, K.W.; Gacias, M.; et al. Astrocyte-shed extracellular vesicles regulate the peripheral leukocyte response to inflammatory brain lesions. Sci. Signal. 2017, 10, eaai7696. [Google Scholar] [CrossRef] [Green Version]
- Collombet, J.-M.; Piérard, C.; Béracochéa, D.; Coubard, S.; Burckhart, M.-F.; Four, E.; Masqueliez, C.; Baubichon, D.; Lallement, G. Long-term consequences of soman poisoning in mice: Part 1. Neuropathology and neuronal regeneration in the amygdala. Behav. Brain Res. 2008, 191, 88–94. [Google Scholar] [CrossRef]
- Men, S.; Lee, D.H.; Barron, J.R.; Muñoz, D.G. Selective Neuronal Necrosis Associated with Status Epilepticus: MR Findings. Am. J. Neuroradiol. 2000, 21, 1837–1840. [Google Scholar]
- Loss, C.M.; Córdova, S.D.; de Oliveira, D.L. Ketamine reduces neuronal degeneration and anxiety levels when administered during early life-induced status epilepticus in rats. Brain Res. 2012, 1474, 110–117. [Google Scholar] [CrossRef] [Green Version]
- Handayani, R.N.; Yunus, F.; Ibrahim, E.I.; Rengganis, I. Correlation between Improve Lung Function with Decrease of Eosinophil Levels in Atopic Asthma Persistent After Asthma Exercise. Ann. Trop. Med. Public Heal. 2019, 22, 338–346. [Google Scholar] [CrossRef]
- Bae, G.H.; Lee, H.Y.; Jung, Y.S.; Shim, J.W.; Kim, S.D.; Baek, S.-H.; Kwon, J.Y.; Park, J.S.; Bae, Y.-S. Identification of novel peptides that stimulate human neutrophils. Exp. Mol. Med. 2012, 44, 130–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pillay, J.; Hietbrink, F.; Koenderman, L.; Leenen, L. The systemic inflammatory response induced by trauma is reflected by multiple phenotypes of blood neutrophils. Injury 2007, 38, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Stahel, P.F.; Morganti-Kossmann, C.; Kossmann, T. The role of the complement system in traumatic brain injury. Brain Res. Rev. 1998, 27, 243–256. [Google Scholar] [CrossRef]
- Keeling, K.; Hicks, R.; Mahesh, J.; Billings, B.; Kotwal, G. Local neutrophil influx following lateral fluid-percussion brain injury in rats is associated with accumulation of complement activation fragments of the third component (C3) of the complement system. J. Neuroimmunol. 2000, 105, 20–30. [Google Scholar] [CrossRef]
- Craige, S.M.; Kant, S.; Jr, J.F.K. Reactive Oxygen Species in Endothelial Function: From Disease to Adaptation. Circ. J. 2015, 79, 1145–1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mooren, F.C.; Blöming, D.; Lechtermann, A.; Lerch, M.M.; Völker, K. Lymphocyte apoptosis after exhaustive and moderate exercise. J. Appl. Physiol. 2002, 93, 147–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, K.; Sato, H.; Kikuchi, T.; Abe, T.; Nakaji, S.; Sugawara, K.; Totsuka, M.; Sato, K.; Yamaya, K. Capacity of circulating neutrophils to produce reactive oxygen species after exhaustive exercise. J. Appl. Physiol. 1996, 81, 1213–1222. [Google Scholar] [CrossRef]
- Ferrer, M.D.; Tauler, P.; Sureda, A.; Tur, J.A.; Pons, A. Antioxidant regulatory mechanisms in neutrophils and lymphocytes after intense exercise. J. Sports Sci. 2009, 27, 49–58. [Google Scholar] [CrossRef]
- Stults-Kolehmainen, M.A.; Sinha, R. The Effects of Stress on Physical Activity and Exercise. Sports Med. 2013, 44, 81–121. [Google Scholar] [CrossRef]
- Wärnberg, J.; Cunningham, K.; Romeo, J.; Marcos, A. Physical activity, exercise and low-grade systemic inflammation. Proc. Nutr. Soc. 2010, 69, 400–406. [Google Scholar] [CrossRef] [Green Version]
- D’Alessandro, A.; Nemkov, T.; Sun, K.; Liu, H.; Song, A.; Monte, A.A.; Subudhi, A.W.; Lovering, A.T.; Dvorkin, D.; Julian, C.G.; et al. AltitudeOmics: Red Blood Cell Metabolic Adaptation to High Altitude Hypoxia. J. Proteome Res. 2016, 15, 3883–3895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Risso, A.; Turello, M.; Biffoni, F.; Antonutto, G. Red blood cell senescence and neocytolysis in humans after high altitude acclimatization. Blood Cells Mol. Dis. 2007, 38, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Celik, A.; Aydin, N.; Ozcirpici, B.; Saricicek, E.; Sezen, H.; Okumus, M.; Bozkurt, S.; Kilinc, M. Elevated red blood cell distri-bution width and inflammation in printing workers. Med. Sci. Monit. 2013, 19, 1001–1005. [Google Scholar] [PubMed] [Green Version]
- Wojsiat, J.; Laskowska-Kaszub, K.; Mietelska-Porowska, A.; Wojda, U. Search for Alzheimer’s disease biomarkers in blood cells: Hypotheses-driven approach. Biomarkers Med. 2017, 11, 917–931. [Google Scholar] [CrossRef] [Green Version]
- Ferrer, I.; Gómez, A.; Carmona, M.; Huesa, G.; Porta, S.; Riera-Codina, M.; Biagioli, M.; Gustincich, S.; Aso, E. Neuronal Hemoglobin is Reduced in Alzheimer’s Disease, Argyrophilic Grain Disease, Parkinson’s Disease, and Dementia with Lewy Bodies. J. Alzheimers Dis. 2011, 23, 537–550. [Google Scholar] [CrossRef] [Green Version]
- Schelshorn, D.W.; Schneider, A.; Kuschinsky, W.; Weber, D.; Krüger, C.; Dittgen, T.; Bürgers, H.F.; Sabouri, F.; Gassler, N.; Bach, A.; et al. Expression of Hemoglobin in Rodent Neurons. J. Cereb. Blood Flow Metab. 2008, 29, 585–595. [Google Scholar] [CrossRef] [Green Version]
- Buttari, B.; Profumo, E.; Riganò, R. Crosstalk between Red Blood Cells and the Immune System and Its Impact on Atherosclerosis. BioMed. Res. Int. 2015, 2015, 616834. [Google Scholar] [CrossRef] [Green Version]
- Devi, S.A.; Subramanyam, M.; Vani, R.; Jeevaratnam, K. Adaptations of the antioxidant system in erythrocytes of trained adult rats: Impact of intermittent hypobaric-hypoxia at two altitudes. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2005, 140, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Otero Regino, W.; Velasco, H.; Sandoval, H. Papel protector de la bilirrubina en el ser humano. Rev. Colomb. Gastroenterología 2009, 24, 293–301. (In Spanish) [Google Scholar]
- Altland, P.D.; Parker, M.G. Bilirubinemia and intravascular hemolysis during acclimatization to high altitude. Int. J. Biometeorol. 1977, 21, 165–170. [Google Scholar] [CrossRef]
- Dani, C.; Poggi, C.; Fancelli, C.; Pratesi, S. Changes in bilirubin in infants with hypoxic–ischemic encephalopathy. Eur. J. Pediatr. 2018, 177, 1795–1801. [Google Scholar] [CrossRef] [PubMed]
- Vaz, A.R.; Silva, S.L.; Barateiro, A.; Falcão, A.S.; Fernandes, A.; Brito, M.A.; Brites, D. Selective vulnerability of rat brain regions to unconjugated bilirubin. Mol. Cell. Neurosci. 2011, 48, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Thong, Y.; Ness, D.; Ferrante, A. Effect of bilirubin on the fungicidal capacity of human neutrophils. Med. Mycol. 1979, 17, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Zelenka, J.; Dvořák, A.; Alán, L.; Zadinová, M.; Haluzik, M.; Vítek, L. Hyperbilirubinemia Protects against Aging-Associated Inflammation and Metabolic Deterioration. Oxidative Med. Cell. Longev. 2016, 2016, 6190609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedlander, A.H.; Boström, K.I.; Tran, H.-A.; Chang, T.I.; Polanco, J.C.; Lee, U.K. Severe Sleep Apnea Associated with Increased Systemic Inflammation and Decreased Serum Bilirubin. J. Oral Maxillofac. Surg. 2019, 77, 2318–2323. [Google Scholar] [CrossRef]
- Zelenka, J.; Muchova, L.; Zelenkova, M.; Vanova, K.; Vreman, H.J.; Wong, R.J.; Vitek, L. Intracellular accumulation of bilirubin as a defense mechanism against increased oxidative stress. Biochimie 2012, 94, 1821–1827. [Google Scholar] [CrossRef]
- Julian, C.G. Epigenomics and human adaptation to high altitude. J. Appl. Physiol. 2017, 123, 1362–1370. [Google Scholar] [CrossRef]
| Low | High | t | p | |
|---|---|---|---|---|
| Age | 21.00 ± 1.00 (years) | 20.83 ± 1.07 (years) | 0.58 | 0.56 |
| BMI | 20.64 ± 3.34 | 21.65 ± 1.95 | −1.26 | 0.20 |
| Education | 14.08 ± 0.28 (years) | 14.17 ± 0.39 (years) | −0.97 | 0.34 |
| Multimedia | 6.04 ± 2.62 (hours) | 5.57 ± 1.59 (hours) | 0.75 | 0.46 |
| Low | High | t | p | |
|---|---|---|---|---|
| DBIL | 4.9 ± 2.53 (umol/L) | 6.43 ± 2.3 (umol/L) | −2.18 * | 0.035 |
| TBIL | 16.56 ± 8.14 (umol/L) | 21.64 ± 8.73 (umol/L) | −2.09 * | 0.042 |
| NEUT | 3.19 ± 1.34 (10^9/L) | 3.55 ± 1.16 (10^9/L) | −1.00 | 0.322 |
| LYMPH | 2.46 ± 0.56 (10^9/L) | 2.56 ± 0.51 (10^9/L) | −0.64 | 0.528 |
| EO | 0.10 ± 0.07 (10^9/L) | 0.08 ± 0.07 (10^9/L) | 0.95 | 0.346 |
| RBC | 5.31 ± 0.53 (10^9/L) | 5.87 ± 0.42 (10^9/L) | −3.97 *** | <0.001 |
| HGB | 163.20 ± 19.53 (g/L) | 180.17 ± 13.41 (g/L) | −3.48 *** | <0.001 |
| RDW-SD | 42.70 ± 3.11 (%) | 40.22 ± 2.56 (%) | 3.00 ** | 0.004 |
| Dependent Variable | Predictors | B | Ser | Beta | t |
|---|---|---|---|---|---|
| R Sub body | Constant | 152.33 | 44.04 | 3.459 | |
| HGB | 0.70 | 2.56 | 0.38 | 2.75 | |
| R Pre body | Constant | 83.47 | 35.86 | 2.33 | |
| HGB | 0.55 | 0.21 | 0.36 | 2.65 |
| Dependent Variable | Predictors | B | Ser | Beta | t | |
|---|---|---|---|---|---|---|
| Low CRF | L GC_ML_DG head | Constant | 80.18 | 22.97 | 3.49 | |
| EO | −101.75 | 26.48 | −0.49 | −3.84 | ||
| L CA4 head | Constant | 65.92 | 19.07 | 3.43 | ||
| EO | −91.35 | 26.54 | −0.53 | −3.44 | ||
| R Sub body | Constant | 228.79 | 14.37 | 15.92 | ||
| NEUT | 10.86 | 4.17 | 0.48 | 2.61 | ||
| R Pre body | Constant | 125.16 | 9.10 | 13.75 | ||
| NEUT | 13.32 | 2.64 | 0.73 | 5.05 | ||
| L CAT | Constant | 93.703 | 46.48 | 2.029 | ||
| EO | −744.84 | 302.94 | −0.63 | −3.35 | ||
| High CRF | L GC_ML_DG head | Constant | 208.01 | 13.17 | 15.80 | |
| DBIL | −4.61 | −1.74 | −0.45 | −2.65 | ||
| L CA4 head | Constant | 60.99 | 41.39 | 1.47 | ||
| TBIL | −3.40 | 1.31 | −0.44 | −2.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.-X.; Su, R.; Li, H.; Dang, P.; Zeng, T.-A.; Chen, D.-M.; Wu, J.-G.; Zhang, D.-L.; Ma, H.-L. Changes in Hippocampus and Amygdala Volume with Hypoxic Stress Related to Cardiorespiratory Fitness under a High-Altitude Environment. Brain Sci. 2022, 12, 359. https://doi.org/10.3390/brainsci12030359
Wang Z-X, Su R, Li H, Dang P, Zeng T-A, Chen D-M, Wu J-G, Zhang D-L, Ma H-L. Changes in Hippocampus and Amygdala Volume with Hypoxic Stress Related to Cardiorespiratory Fitness under a High-Altitude Environment. Brain Sciences. 2022; 12(3):359. https://doi.org/10.3390/brainsci12030359
Chicago/Turabian StyleWang, Zhi-Xin, Rui Su, Hao Li, Peng Dang, Tong-Ao Zeng, Dong-Mei Chen, Jian-Guo Wu, De-Long Zhang, and Hai-Lin Ma. 2022. "Changes in Hippocampus and Amygdala Volume with Hypoxic Stress Related to Cardiorespiratory Fitness under a High-Altitude Environment" Brain Sciences 12, no. 3: 359. https://doi.org/10.3390/brainsci12030359
APA StyleWang, Z.-X., Su, R., Li, H., Dang, P., Zeng, T.-A., Chen, D.-M., Wu, J.-G., Zhang, D.-L., & Ma, H.-L. (2022). Changes in Hippocampus and Amygdala Volume with Hypoxic Stress Related to Cardiorespiratory Fitness under a High-Altitude Environment. Brain Sciences, 12(3), 359. https://doi.org/10.3390/brainsci12030359






