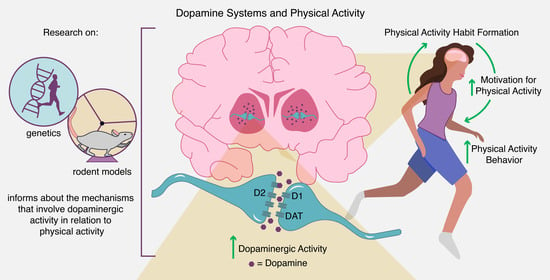
Regulation of Voluntary Physical Activity Behavior: A Review of Evidence Involving Dopaminergic Pathways in the Brain
Abstract
1. Introduction
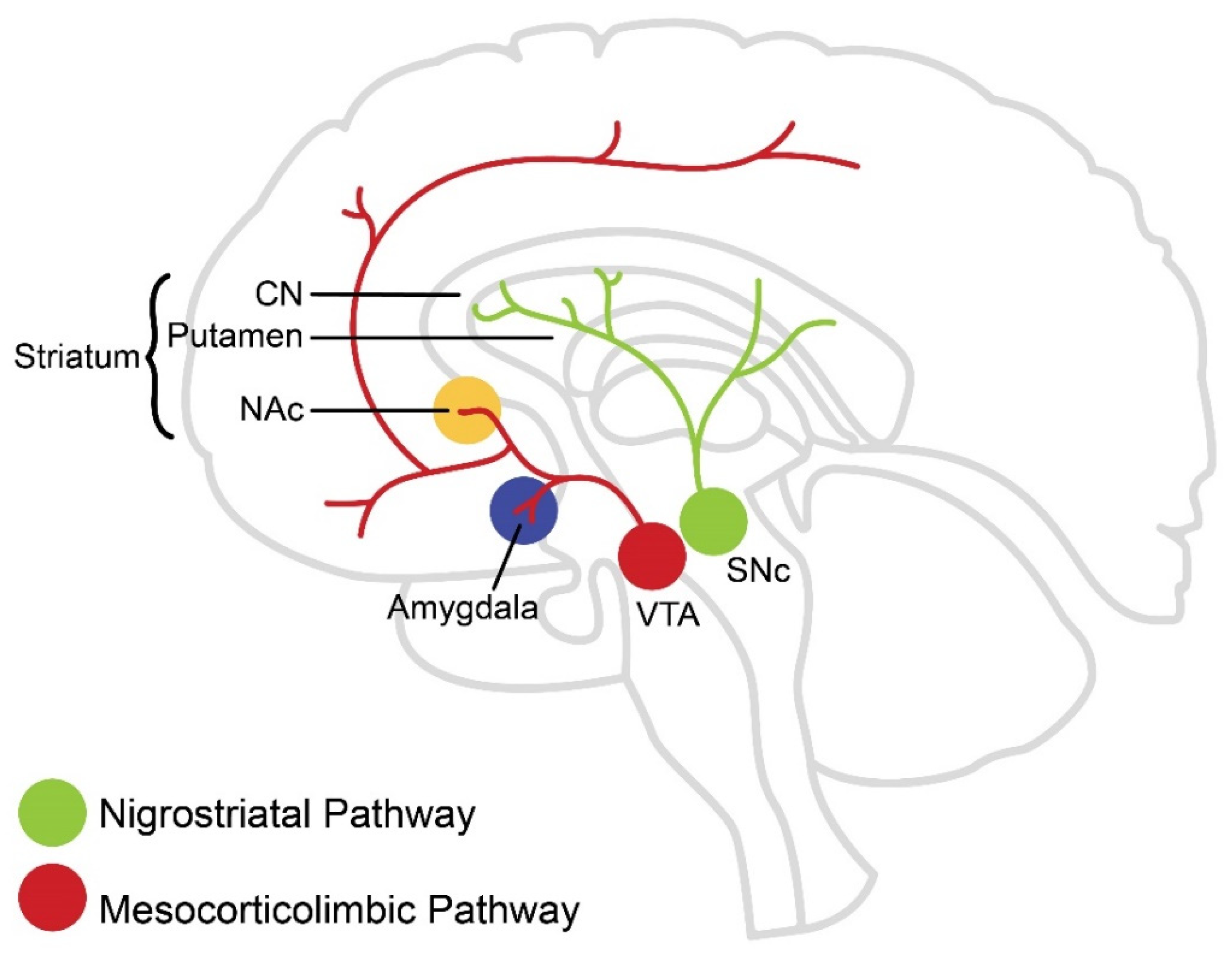
2. Dopaminergic Pathways in the Brain
3. Evidence for Regulation of Motivated Physical Activity Behavior through Dopaminergic Pathways
3.1. Selective Breeding and Pharmacological Studies in Rodents
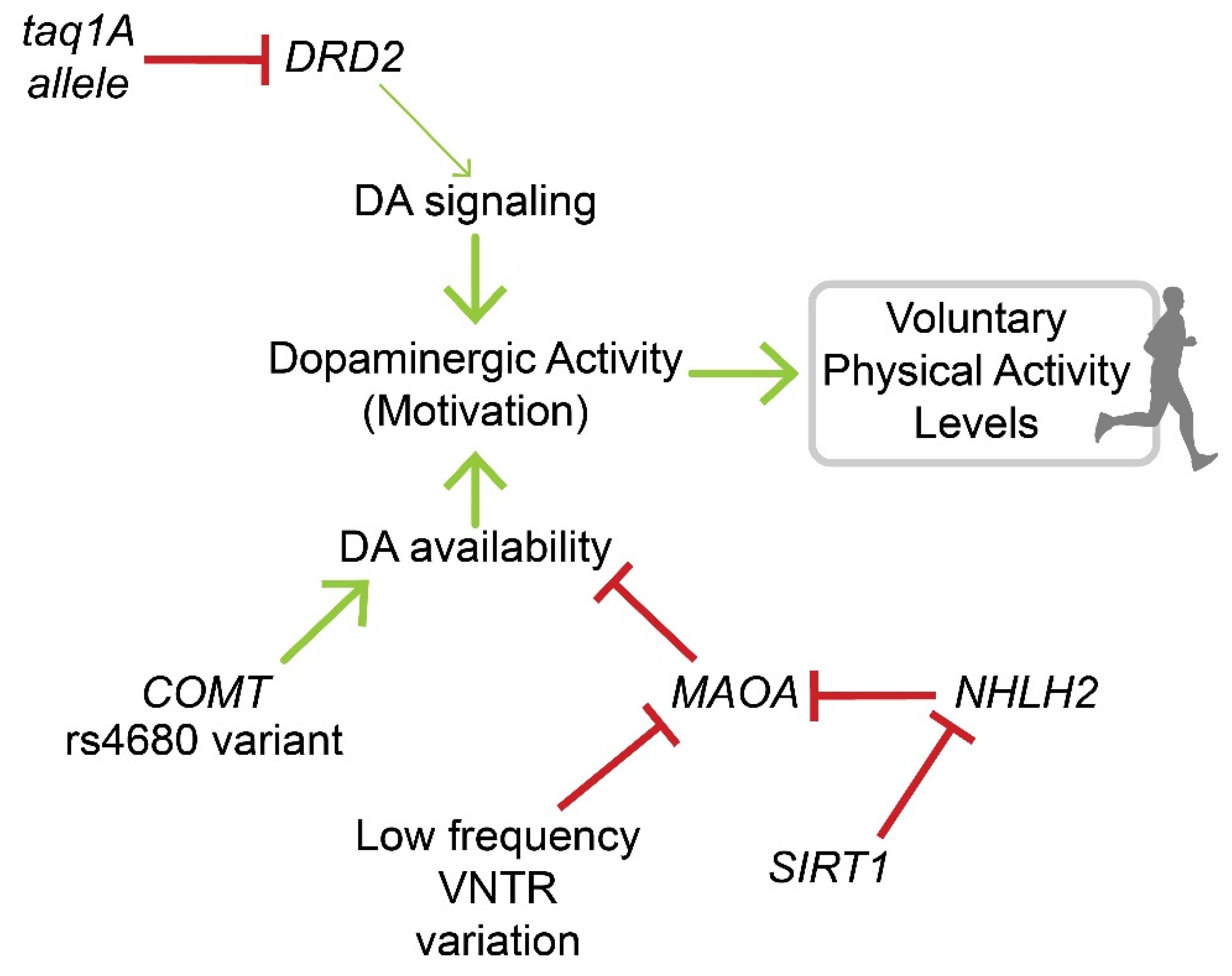
3.2. Genetic Studies in Rodents and Humans
4. Evidence for Regulation of Physical Activity Habit Formation through Dopaminergic Pathways
5. Biological Signals Supplementing Dopaminergic Regulation of Physical Activity Behavior
5.1. Serotonin
5.2. Leptin, Ghrelin, and Insulin
5.3. Estrogen
5.4. Endocannabinoids and Orexins
6. Applicability of the Findings on the Role of Dopamine in Regulating Physical Activity Behavior
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Lauzé, M.; Daneault, J.-F.; Duval, C. The Effects of Physical Activity in Parkinson’s Disease: A Review. J. Park. Dis. 2016, 6, 685–698. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Recommendations on Physical Activity for Health. Available online: http://www.ncbi.nlm.nih.gov/books/NBK305057/ (accessed on 16 June 2020).
- CDC. What Works: Strategies to Increase Physical Activity. Cent. Dis. Control Prev. 2020. Available online: https://www.cdc.gov/physicalactivity/activepeoplehealthynation/strategies-to-increase-physical-activity/index.html (accessed on 8 July 2020).
- U.S. Department of Health and Human Services. Physical Activity Guidelines for Americans, 2nd ed.; U.S. Department of Health and Human Services: Washington, DC, USA, 2018. Available online: https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf (accessed on 25 February 2022).
- Troiano, R.P.; Berrigan, D.; Dodd, K.W.; Mâsse, L.C.; Tilert, T.; Mcdowell, M. Physical Activity in the United States Measured by Accelerometer. Med. Sci. Sports Exerc. 2008, 40, 181–188. [Google Scholar] [CrossRef]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The Physical Activity Guidelines for Americans. JAMA J. Am. Med Assoc. 2018, 320, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Willinger, N.; Steele, J.; Atkinson, L.; Liguori, G.; Jimenez, A.; Mann, S.; Horton, E. Effectiveness of Structured Physical Activity Interventions Through the Evaluation of Physical Activity Levels, Adoption, Retention, Maintenance, and Adherence Rates: A Systematic Review and Meta-Analysis. J. Phys. Act. Health 2021, 18, 116–129. [Google Scholar] [CrossRef]
- Luong, M.-L.N.; Hall, M.; Bennell, K.L.; Kasza, J.; Harris, A.; Hinman, R.S. The Impact of Financial Incentives on Physical Activity: A Systematic Review and Meta-Analysis. Am. J. Health Promot. 2021, 35, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, J.S.; Garland, T.; Gammie, S.C. Patterns of Brain Activity Associated With Variation in Voluntary Wheel-Running Behavior. Behav. Neurosci. 2003, 117, 1243–1256. [Google Scholar] [CrossRef]
- Roberts, M.D.; Ruegsegger, G.; Brown, J.D.; Booth, F.W. Mechanisms Associated with Physical Activity Behavior: Insights from Rodent Experiments. Exerc. Sport Sci. Rev. 2017, 45, 217–222. [Google Scholar] [CrossRef]
- Lightfoot, J.T.; de Geus, E.; Booth, F.W.; Bray, M.S.; Hoed, M.D.; Kaprio, J.; Kelly, S.A.; Pomp, D.; Saul, M.; Thomis, M.; et al. Biological/Genetic Regulation of Physical Activity Level: Consensus from GenBioPAC. Med. Sci. Sports Exerc. 2018, 50, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Garland, T.; Schutz, H.; Chappell, M.A.; Keeney, B.K.; Meek, T.H.; Copes, L.E.; Acosta, W.; Drenowatz, C.; Maciel, R.C.; van Dijk, G.; et al. The biological control of voluntary exercise, spontaneous physical activity and daily energy expenditure in relation to obesity: Human and rodent perspectives. J. Exp. Biol. 2011, 214, 206–229. [Google Scholar] [CrossRef]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef]
- Berridge, K.C. The debate over dopamine’s role in reward: The case for incentive salience. Psychopharmacology 2007, 191, 391–431. [Google Scholar] [CrossRef] [PubMed]
- Cools, R. Role of Dopamine in the Motivational and Cognitive Control of Behavior. Neuroscientist 2008, 14, 381–395. [Google Scholar] [CrossRef]
- Groenewegen, H.J. The Basal Ganglia and Motor Control. Neural Plast. 2003, 10, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Haber, S.N.; Knutson, B. The Reward Circuit: Linking Primate Anatomy and Human Imaging. Neuropsychopharmacology 2009, 35, 4–26. [Google Scholar] [CrossRef]
- Walton, M.E.; Gan, J.O.; Phillips, P.E.M. The Influence of Dopamine in Generating Action from Motivation. In Neural Basis of Motivational and Cognitive Control; MIT Press: Cambridge, MA, USA, 2011; pp. 163–166. [Google Scholar]
- Kravitz, A.; Kreitzer, A.C. Striatal Mechanisms Underlying Movement, Reinforcement, and Punishment. Physiology 2012, 27, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Seger, C.A.; Spiering, B.J. A Critical Review of Habit Learning and the Basal Ganglia. Front. Syst. Neurosci. 2011, 5, 66. [Google Scholar] [CrossRef]
- Boekhoudt, L.; Omrani, A.; Luijendijk, M.C.; Wolterink-Donselaar, I.G.; Wijbrans, E.C.; van der Plasse, G.; Adan, R.A. Chemogenetic activation of dopamine neurons in the ventral tegmental area, but not substantia nigra, induces hyperactivity in rats. Eur. Neuropsychopharmacol. 2016, 26, 1784–1793. [Google Scholar] [CrossRef]
- Sherwin, C. Voluntary wheel running: A review and novel interpretation. Anim. Behav. 1998, 56, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Novak, C.M.; Burghardt, P.R.; Levine, J.A. The use of a running wheel to measure activity in rodents: Relationship to energy balance, general activity, and reward. Neurosci. Biobehav. Rev. 2012, 36, 1001–1014. [Google Scholar] [CrossRef]
- Rhodes, J.S.; Garland, T. Differential sensitivity to acute administration of Ritalin, apormorphine, SCH 23390, but not raclopride in mice selectively bred for hyperactive wheel-running behavior. Psychopharmacology 2003, 167, 242–250. [Google Scholar] [CrossRef]
- Knab, A.M.; Bowen, R.S.; Hamilton, A.T.; Lightfoot, J.T. Pharmacological manipulation of the dopaminergic system affects wheel-running activity in differentially active mice. J. Biol. Regul. Homeost. Agents 2012, 26, 119–129. [Google Scholar] [PubMed]
- Roberts, M.D.; Gilpin, L.; Parker, K.E.; Childs, T.E.; Will, M.; Booth, F.W. Dopamine D1 receptor modulation in nucleus accumbens lowers voluntary wheel running in rats bred to run high distances. Physiol. Behav. 2012, 105, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Alleweireldt, A.T.; Weber, S.M.; Kirschner, K.F.; Bullock, B.L.; Neisewander, J.L. Blockade or stimulation of D1 dopamine receptors attenuates cue reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology 2002, 159, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Alleweireldt, A.T.; Kirschner, K.F.; Blake, C.B.; Neisewander, J.L. D1-receptor drugs and cocaine-seeking behavior: Investigation of receptor mediation and behavioral disruption in rats. Psychopharmacology 2003, 168, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Leasure, J.; Jones, M. Forced and voluntary exercise differentially affect brain and behavior. Neuroscience 2008, 156, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Toval, A.; Garrigos, D.; Kutsenko, Y.; Popović, M.; Do-Couto, B.R.; Morales-Delgado, N.; Tseng, K.Y.; Ferran, J.L. Dopaminergic Modulation of Forced Running Performance in Adolescent Rats: Role of Striatal D1 and Extra-striatal D2 Dopamine Receptors. Mol. Neurobiol. 2021, 58, 1782–1791. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Ottenheimer, D.; DiLeone, R.J. Activity of D1/2 Receptor Expressing Neurons in the Nucleus Accumbens Regulates Running, Locomotion, and Food Intake. Front. Behav. Neurosci. 2016, 10, 66. [Google Scholar] [CrossRef]
- Armbruster, B.N.; Li, X.; Pausch, M.H.; Herlitze, S.; Roth, B.L. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl. Acad. Sci. USA 2007, 104, 5163–5168. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Aryal, D.K.; Olsen, R.H.; Urban, D.J.; Swearingen, A.; Forbes, S.; Roth, B.L.; Hochgeschwender, U. Cre-dependent DREADD (Designer Receptors Exclusively Activated by Designer Drugs) mice. Genesis 2016, 54, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Simonen, R.L.; Russe, L.P.; Rankinen, T.; Rice, T.; Rao, D.C.; Bouchard, C. Familial aggregation of physical activity levels in the Québec family study. Med. Sci. Sports Exerc. 2002, 34, 1137–1142. [Google Scholar] [CrossRef]
- Lightfoot, J.T. Current Understanding of the Genetic Basis for Physical Activity. J. Nutr. 2010, 141, 526–530. [Google Scholar] [CrossRef]
- Knab, A.M.; Bowen, R.; Hamilton, A.T.; Gulledge, A.A.; Lightfoot, J.T. Altered dopaminergic profiles: Implications for the regulation of voluntary physical activity. Behav. Brain Res. 2009, 204, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.S.; Shimomura, K.; Vitaterna, M.H.; Turek, F.W. High-resolution mapping of a novel genetic locus regulating voluntary physical activity in mice. Genes Brain Behav. 2012, 11, 113–124. [Google Scholar] [CrossRef]
- Ferguson, D.; Dangott, L.J.; Vellers, H.L.; Schmitt, E.; Lightfoot, J.T. Differential protein expression in the nucleus accumbens of high and low active mice. Behav. Brain Res. 2015, 291, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Rosso, A.L.; Metti, A.L.; Glynn, N.W.; Boudreau, R.M.; Rejeski, W.J.; Bohnen, N.; Chen, H.; Johannsen, N.M.; King, A.C.; Manini, T.M.; et al. Dopamine-Related Genotypes and Physical Activity Change During an Intervention: The Lifestyle Interventions and Independence for Elders Study. J. Am. Geriatr. Soc. 2018, 66, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, E.G.; Nöthen, M.; Grünhage, F.; Farde, L.; Nakashima, Y.; Propping, P.; Sedvall, G.C. Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Mol. Psychiatry 1999, 4, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Flack, K.; Pankey, C.; Ufholz, K.; Johnson, L.; Roemmich, J.N. Genetic variations in the dopamine reward system influence exercise reinforcement and tolerance for exercise intensity. Behav. Brain Res. 2019, 375, 112148. [Google Scholar] [CrossRef] [PubMed]
- Neville, M.J.; Johnstone, E.C.; Walton, R. Identification and characterization of ANKK1: A novel kinase gene closely linked to DRD2 on chromosome band 11q23.1. Hum. Mutat. 2004, 23, 540–545. [Google Scholar] [CrossRef]
- Ponce, G.; Pérez-González, R.; Aragüés, M.; Palomo, T.; Rodríguez-Jiménez, R.; Jiménez-Arriero, M.A.; Hoenicka, J. The ANKK1 Kinase Gene and Psychiatric Disorders. Neurotox. Res. 2009, 16, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Männistö, P.T.; Kaakkola, S. Catechol-O-methyltransferase (COMT): Biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol. Rev. 1999, 51, 593–628. [Google Scholar] [PubMed]
- Shih, J.; Thompson, R. Monoamine Oxidase in Neuropsychiatry and Behavior. Am. J. Hum. Genet. 1999, 65, 593–598. [Google Scholar] [CrossRef]
- Sabol, S.Z.; Hu, S.; Hamer, D. A functional polymorphism in the monoamine oxidase A gene promoter. Qual. Life Res. 1998, 103, 273–279. [Google Scholar] [CrossRef]
- Good, D.J.; Li, M.; Deater-Deckard, K. A Genetic Basis for Motivated Exercise. Exerc. Sport Sci. Rev. 2015, 43, 231–237. [Google Scholar] [CrossRef]
- Goleva-Fjellet, S.; Bjurholt, A.M.; Kure, E.H.; Larsen, I.K.; Støren, Ø.; Sæbø, M. Distribution of allele frequencies for genes associated with physical activity and/or physical capacity in a homogenous Norwegian cohort—A cross-sectional study. BMC Genet. 2020, 21, 8. [Google Scholar] [CrossRef]
- Fowler, J.S.; Alia-Klein, N.; Kriplani, A.; Logan, J.; Williams, B.; Zhu, W.; Craig, I.W.; Telang, F.; Goldstein, R.; Volkow, N.D.; et al. Evidence That Brain MAO A Activity Does Not Correspond to MAO A Genotype in Healthy Male Subjects. Biol. Psychiatry 2007, 62, 355–358. [Google Scholar] [CrossRef]
- Libert, S.; Pointer, K.; Bell, E.L.; Das, A.; Cohen, D.E.; Asara, J.M.; Kapur, K.; Bergmann, S.; Preisig, M.; Otowa, T.; et al. SIRT1 Activates MAO-A in the Brain to Mediate Anxiety and Exploratory Drive. Cell 2011, 147, 1459–1472. [Google Scholar] [CrossRef]
- Dickinson, A.; Balleine, B. Motivational control of goal-directed action. Anim. Learn. Behav. 1994, 22, 1–18. [Google Scholar] [CrossRef]
- Amaya, K.A.; Smith, K.S. Neurobiology of habit formation. Curr. Opin. Behav. Sci. 2018, 20, 145–152. [Google Scholar] [CrossRef]
- Graybiel, A.M. Habits, Rituals, and the Evaluative Brain. Annu. Rev. Neurosci. 2008, 31, 359–387. [Google Scholar] [CrossRef]
- Aarts, H.; Paulussen, T.; Schaalma, H. Physical exercise habit: On the conceptualization and formation of habitual health behaviours. Health Educ. Res. 1997, 12, 363–374. [Google Scholar] [CrossRef]
- Gardner, B.; Lally, P. Does intrinsic motivation strengthen physical activity habit? Modeling relationships between self-determination, past behaviour, and habit strength. J. Behav. Med. 2013, 36, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.S.; Graybiel, A.M. Habit formation coincides with shifts in reinforcement representations in the sensorimotor striatum. J. Neurophysiol. 2016, 115, 1487–1498. [Google Scholar] [CrossRef]
- Yin, H.H.; Knowlton, B.J. The role of the basal ganglia in habit formation. Nat. Rev. Neurosci. 2006, 7, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Faure, A.; Haberland, U.; Condé, F.; El Massioui, N. Lesion to the Nigrostriatal Dopamine System Disrupts Stimulus-Response Habit Formation. J. Neurosci. 2005, 25, 2771–2780. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.H.; Ostlund, S.B.; Knowlton, B.J.; Balleine, B.W. The role of the dorsomedial striatum in instrumental conditioning: Striatum and instrumental conditioning. Eur. J. Neurosci. 2005, 22, 513–523. [Google Scholar] [CrossRef]
- Yin, H.H.; Knowlton, B.J.; Balleine, B. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur. J. Neurosci. 2004, 19, 181–189. [Google Scholar] [CrossRef]
- Calipari, E.S.; Ferris, M.J. Amphetamine Mechanisms and Actions at the Dopamine Terminal Revisited. J. Neurosci. 2013, 33, 8923–8925. [Google Scholar] [CrossRef]
- Nelson, A. Amphetamine Exposure Enhances Habit Formation. J. Neurosci. 2006, 26, 3805–3812. [Google Scholar] [CrossRef]
- Vaughan, R.A.; Foster, J.D. Mechanisms of dopamine transporter regulation in normal and disease states. Trends Pharmacol. Sci. 2013, 34, 489–496. [Google Scholar] [CrossRef]
- Nelson, A.J.D.; Ekillcross, S. Accelerated habit formation following amphetamine exposure is reversed by D1, but enhanced by D2, receptor antagonists. Front. Neurosci. 2013, 7, 76. [Google Scholar] [CrossRef]
- Shan, Q.; Christie, M.; Balleine, B.W. Plasticity in striatopallidal projection neurons mediates the acquisition of habitual actions. Eur. J. Neurosci. 2015, 42, 2097–2104. [Google Scholar] [CrossRef]
- Di Giovanni, G.; Esposito, E.; Di Matteo, V. Role of Serotonin in Central Dopamine Dysfunction. CNS Neurosci. Ther. 2010, 16, 179–194. [Google Scholar] [CrossRef]
- Dremencov, E.; Newman, M.E.; Kinor, N.; Blatman-Jan, G.; Schindler, C.J.; Overstreet, D.H.; Yadid, G. Hyperfunctionality of serotonin-2C receptor-mediated inhibition of accumbal dopamine release in an animal model of depression is reversed by antidepressant treatment. Neuropharmacology 2005, 48, 34–42. [Google Scholar] [CrossRef]
- Waters, R.; Pringle, R.; Forster, G.; Renner, K.; Malisch, J.; Garland, T., Jr.; Swallow, J. Selection for increased voluntary wheel-running affects behavior and brain monoamines in mice. Brain Res. 2013, 1508, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Claghorn, G.C.; Fonseca, I.A.; Thompson, Z.; Barber, C.; Garland, T. Serotonin-mediated central fatigue underlies increased endurance capacity in mice from lines selectively bred for high voluntary wheel running. Physiol. Behav. 2016, 161, 145–154. [Google Scholar] [CrossRef]
- Tops, M.; Russo, S.; Boksem, M.A.; Tucker, D.M. Serotonin: Modulator of a drive to withdraw. Brain Cogn. 2009, 71, 427–436. [Google Scholar] [CrossRef]
- Fulton, S.; Pissios, P.; Manchon, R.P.; Stiles, L.; Frank, L.; Pothos, E.N.; Maratos-Flier, E.; Flier, J.S. Leptin Regulation of the Mesoaccumbens Dopamine Pathway. Neuron 2006, 51, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Hommel, J.; Trinko, R.; Sears, R.M.; Georgescu, D.; Liu, Z.-W.; Gao, X.-B.; Thurmon, J.J.; Marinelli, M.; DiLeone, R. Leptin Receptor Signaling in Midbrain Dopamine Neurons Regulates Feeding. Neuron 2006, 51, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Ruegsegger, G.N.; Booth, F.W. Running from Disease: Molecular Mechanisms Associating Dopamine and Leptin Signaling in the Brain with Physical Inactivity, Obesity, and Type 2 Diabetes. Front. Endocrinol. 2017, 8, 109. [Google Scholar] [CrossRef]
- Zigman, J.M.; Jones, J.E.; Lee, C.E.; Saper, C.B.; Elmquist, J.K. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J. Comp. Neurol. 2006, 494, 528–548. [Google Scholar] [CrossRef]
- Menzies, J.R.W.; Skibicka, K.P.; Leng, G.; Dickson, S.L. Ghrelin, Reward and Motivation. Ghrelin Syst. 2013, 25, 101–111. [Google Scholar] [CrossRef]
- Tajiri, Y. Ghrelin and exercise: A possible virtuous circle. Diabetol. Int. 2017, 8, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Brüning, J.C.; Gautam, D.; Burks, D.J.; Gillette, J.; Schubert, M.; Orban, P.C.; Klein, R.; Krone, W.; Müller-Wieland, D.; Kahn, C.R. Role of Brain Insulin Receptor in Control of Body Weight and Reproduction. Science 2000, 289, 2122–2125. [Google Scholar] [CrossRef] [PubMed]
- Tiedemann, L.J.; Schmid, S.; Hettel, J.; Giesen, K.; Francke, P.; Büchel, C.; Brassen, S. Central insulin modulates food valuation via mesolimbic pathways. Nat. Commun. 2017, 8, 16052. [Google Scholar] [CrossRef] [PubMed]
- Sallam, N.A.; Borgland, S.L. Insulin and endocannabinoids in the mesolimbic system. J. Neuroendocr. 2021, 33, e12965. [Google Scholar] [CrossRef]
- Van Hartesveldt, C.; Joyce, J.N. Effects of estrogen on the basal ganglia. Neurosci. Biobehav. Rev. 1986, 10, 1–14. [Google Scholar] [CrossRef]
- Lightfoot, J.T. Sex Hormones’ Regulation of Rodent Physical Activity: A Review. Int. J. Biol. Sci. 2008, 4, 126–132. [Google Scholar] [CrossRef]
- Krentzel, A.A.; Proaño, S.; Patisaul, H.B.; Meitzen, J. Temporal and bidirectional influences of estradiol on voluntary wheel running in adult female and male rats. Horm. Behav. 2020, 120, 104694. [Google Scholar] [CrossRef] [PubMed]
- Berrendero, F.; Flores, Á.; Robledo, P. When orexins meet cannabinoids: Bidirectional functional interactions. Biochem. Pharmacol. 2018, 157, 43–50. [Google Scholar] [CrossRef]
- Busquets-Garcia, A.; Desprez, T.; Metna-Laurent, M.; Bellocchio, L.; Marsicano, G.; Soria-Gomez, E. Dissecting the cannabinergic control of behavior: Thewherematters. BioEssays News Rev. Mol. Cell Dev. Biol. 2015, 37, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- Melis, M.; Pistis, M. Endocannabinoid Signaling in Midbrain Dopamine Neurons: More than Physiology? Curr. Neuropharmacol. 2007, 5, 268–277. [Google Scholar] [CrossRef][Green Version]
- Hilário, M.R.; Clouse, E.; Yin, H.H.; Costa, R.M. Endocannabinoid signaling is critical for habit formation. Front. Integr. Neurosci. 2007, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Matias, I.; Bisogno, T.; Di Marzo, V. Endogenous cannabinoids in the brain and peripheral tissues: Regulation of their levels and control of food intake. Int. J. Obes. 2006, 30 (Suppl. 1), S7–S12. [Google Scholar] [CrossRef] [PubMed]
- Fuss, J.; Gass, P. Endocannabinoids and voluntary activity in mice: Runner’s high and long-term consequences in emotional behaviors. Exp. Neurol. 2010, 224, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Hilário, M.R.; Costa, R.M. High on habits. Front. Neurosci. 2008, 2, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Parsons, L.H.; Hurd, Y.L. Endocannabinoid signalling in reward and addiction. Nat. Rev. Neurosci. 2015, 16, 579–594. [Google Scholar] [CrossRef] [PubMed]
- Corbillé, A.-G.; Valjent, E.; Marsicano, G.; Ledent, C.; Lutz, B.; Herve, D.; Girault, J.-A. Role of Cannabinoid Type 1 Receptors in Locomotor Activity and Striatal Signaling in Response to Psychostimulants. J. Neurosci. 2007, 27, 6937–6947. [Google Scholar] [CrossRef]
- Keeney, B.K.; Raichlen, D.A.; Meek, T.H.; Wijeratne, R.S.; Middleton, K.M.; Gerdeman, G.L.; Garland, T. Differential response to a selective cannabinoid receptor antagonist (SR141716: Rimonabant) in female mice from lines selectively bred for high voluntary wheel-running behaviour. Behav. Pharmacol. 2008, 19, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Amemiya, A.; Ishii, M.; Matsuzaki, I.; Chemelli, R.M.; Tanaka, H.; Williams, S.C.; Richardson, J.A.; Kozlowski, G.P.; Wilson, S.; et al. Orexins and Orexin Receptors: A Family of Hypothalamic Neuropeptides and G Protein-Coupled Receptors that Regulate Feeding Behavior. Cell 1998, 92, 573–585. [Google Scholar] [CrossRef]
- Sakurai, T. The role of orexin in motivated behaviours. Nat. Rev. Neurosci. 2014, 15, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Kotz, C.M. Integration of feeding and spontaneous physical activity: Role for orexin. Physiol. Behav. 2006, 88, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Ibero-Baraibar, I.; Perez-Cornago, A.; Ramirez, M.J.; Martínez, J.A.; Zulet, M.A. An Increase in Plasma Homovanillic Acid with Cocoa Extract Consumption Is Associated with the Alleviation of Depressive Symptoms in Overweight or Obese Adults on an Energy Restricted Diet in a Randomized Controlled Trial. J. Nutr. 2015, 146, 897S–904S. [Google Scholar] [CrossRef] [PubMed]
- Kendler, K.S.; Mohs, R.C.; Davis, K.L. The effects of diet and physical activity on plasma homovanillic acid in normal human subjects. Psychiatry Res. 1983, 8, 215–223. [Google Scholar] [CrossRef]
- Vellers, H.L.; Letsinger, A.C.; Walker, N.R.; Granados, J.Z.; Lightfoot, J.T. High Fat High Sugar Diet Reduces Voluntary Wheel Running in Mice Independent of Sex Hormone Involvement. Front. Physiol. 2017, 8, 628. [Google Scholar] [CrossRef] [PubMed]
- Kravitz, A.V.; O’Neal, T.; Friend, D. Do Dopaminergic Impairments Underlie Physical Inactivity in People with Obesity? Front. Hum. Neurosci. 2016, 10, 514. [Google Scholar] [CrossRef]
- Hryhorczuk, C.; Florea, M.; Rodaros, D.; Poirier, I.; Daneault, C.; Rosiers, C.D.; Arvanitogiannis, A.; Alquier, T.; Fulton, S. Dampened Mesolimbic Dopamine Function and Signaling by Saturated but not Monounsaturated Dietary Lipids. Neuropsychopharmacology 2015, 41, 811–821. [Google Scholar] [CrossRef]
- Kien, C.L.; Bunn, J.Y.; Tompkins, C.L.; Dumas, J.A.; Crain, K.I.; Ebenstein, D.B.; Koves, T.; Muoio, D.M. Substituting dietary monounsaturated fat for saturated fat is associated with increased daily physical activity and resting energy expenditure and with changes in mood. Am. J. Clin. Nutr. 2013, 97, 689–697. [Google Scholar] [CrossRef]
- Ferre, S.; Fuxe, K.; Fredholm, B.B.; Morelli, M.; Popoli, P. Adenosine–dopamine receptor–receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci. 1997, 20, 482–487. [Google Scholar] [CrossRef]
- Solinas, M.; Ferré, S.; You, Z.-B.; Karcz-Kubicha, M.; Popoli, P.; Goldberg, S.R. Caffeine Induces Dopamine and Glutamate Release in the Shell of the Nucleus Accumbens. J. Neurosci. 2002, 22, 6321–6324. [Google Scholar] [CrossRef]
- Franco, R.; Lluis, C.; Canela, E.I.; Mallol, J.; Agnati, L.; Casadó, V.; Ciruela, F.; Ferre, S.; Fuxe, K. Receptor–receptor interactions involving adenosine A1 or dopamine D1 receptors and accessory proteins. J. Neural Transm. 2006, 114, 93–104. [Google Scholar] [CrossRef]
- Claghorn, G.C.; Thompson, Z.; Wi, K.; Van, L.; Garland, T. Caffeine stimulates voluntary wheel running in mice without increasing aerobic capacity. Physiol. Behav. 2017, 170, 133–140. [Google Scholar] [CrossRef]
- Schrader, P.; Panek, L.M.; Temple, J.L. Acute and chronic caffeine administration increases physical activity in sedentary adults. Nutr. Res. 2013, 33, 457–463. [Google Scholar] [CrossRef]
- Greenwood, B.N.; Foley, T.E.; Le, T.V.; Strong, P.V.; Loughridge, A.B.; Day, H.; Fleshner, M. Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway. Behav. Brain Res. 2011, 217, 354–362. [Google Scholar] [CrossRef]
- Msc, M.A.S.; Neva, J.L.; Lakhani, B.; Msc, D.K.M.; Vafai, N.; Shahinfard, E.; English, C.; McCormick, S.; Dinelle, K.; Rn, N.N.; et al. Exercise increases caudate dopamine release and ventral striatal activation in Parkinson’s disease. Mov. Disord. 2019, 34, 1891–1900. [Google Scholar] [CrossRef]
- Dendy, R.; Stinson, E.J.; Guerithault, N.; Gluck, M.E. Brain Stimulation to Modulate Food Intake and Eating Behavior. Curr. Diabetes Rep. 2019, 19, 152. [Google Scholar] [CrossRef] [PubMed]
- Okano, A.H.; Fontes, E.B.; Montenegro, R.; Farinatti, P.; Cyrino, E.; Li, L.; Bikson, M.; Noakes, T.D. Brain stimulation modulates the autonomic nervous system, rating of perceived exertion and performance during maximal exercise. Br. J. Sports Med. 2015, 49, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Fonteneau, C.; Redoute, J.; Haesebaert, F.; Le Bars, D.; Costes, N.; Suaud-Chagny, M.-F.; Brunelin, J. Frontal Transcranial Direct Current Stimulation Induces Dopamine Release in the Ventral Striatum in Human. Cereb. Cortex 2018, 28, 2636–2646. [Google Scholar] [CrossRef]
| Reference | Rodent Model | Pharmacological Agent (Mode of Action) | Route of Administration | High Activity | Low Activity/Control Strain |
|---|---|---|---|---|---|
| Strain | |||||
| Rhodes et al., 2001 | Mouse | Cocaine (non-selective DAT antagonist) | Systemic | ↓ WR (speed) | no overall effect |
| GBR 12909 (DAT antagonist) | ↓ WR (speed) | no overall effect | |||
| Fluoxetine (Prozac) (non-selective DAT antagonist) | ↓ WR (speed and duration) | ↓ WR (speed and duration) | |||
| Rhodes and Garland, 2003 | Mouse | Methylphenidate (Ritalin) (non-selective DAT antagonist) | Systemic | ↓ WR (distance) | ↑ WR (distance) |
| Apomorphine (non-selective DA agonist) | ↓ WR (distance) | ↓ WR (distance) | |||
| SCH 23390 (D1-like DA antagonist) | ↓ WR | ↓ WR | |||
| Raclopride (D2-like DA antagonist) | ↓ WR | ↓ WR | |||
| Knab et al., 2012 | Mouse | SKF 81297 (D1-like DA agonist) | Systemic | ↓ WR | no overall effect |
| SCH 23390 (D1-like DA antagonist) | ↓ duration | no overall effect | |||
| GBR 12909 (DAT antagonist) | no overall effect | ↑ WR | |||
| AMPT (tyrosine hydroxilase inhibitor) | ↓ duration | no overall effect | |||
| Roberts et al., 2012 | Rat | SKF 81297 (D1-like DA agonist) | Bilateral injection to NAc | ↓ WR (distance) | no overall effect |
| SCH 23390 (D1-like DA antagonist) | ↓ WR (distance) | no overall effect | |||
| Toval et al., 2021 | Rat | Raclopride (D2-like DA antagonist) SCH 23390 (D1-like DA antagonist) Raclopride (D2-like DA antagonist) SCH 23390 (D1-like DA antagonist) | Effect on Forced Running | Effect on Open Field Test | |
| Systemic | ↓ duration | ↓ locomotor behavior | |||
| ↓ duration | ↓ locomotor behavior | ||||
| Bilateral injection to DS | no overall effect | no overall effect | |||
| ↓ duration | no overall effect |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Tejada, A.; Neisewander, J.; Katsanos, C.S. Regulation of Voluntary Physical Activity Behavior: A Review of Evidence Involving Dopaminergic Pathways in the Brain. Brain Sci. 2022, 12, 333. https://doi.org/10.3390/brainsci12030333
Ruiz-Tejada A, Neisewander J, Katsanos CS. Regulation of Voluntary Physical Activity Behavior: A Review of Evidence Involving Dopaminergic Pathways in the Brain. Brain Sciences. 2022; 12(3):333. https://doi.org/10.3390/brainsci12030333
Chicago/Turabian StyleRuiz-Tejada, Anaissa, Janet Neisewander, and Christos S. Katsanos. 2022. "Regulation of Voluntary Physical Activity Behavior: A Review of Evidence Involving Dopaminergic Pathways in the Brain" Brain Sciences 12, no. 3: 333. https://doi.org/10.3390/brainsci12030333
APA StyleRuiz-Tejada, A., Neisewander, J., & Katsanos, C. S. (2022). Regulation of Voluntary Physical Activity Behavior: A Review of Evidence Involving Dopaminergic Pathways in the Brain. Brain Sciences, 12(3), 333. https://doi.org/10.3390/brainsci12030333