The Functional Interactions between Cortical Regions through Theta-Gamma Coupling during Resting-State and a Visual Working Memory Task
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Wender Utah Rating Scale
2.3. Neuropsycological Test
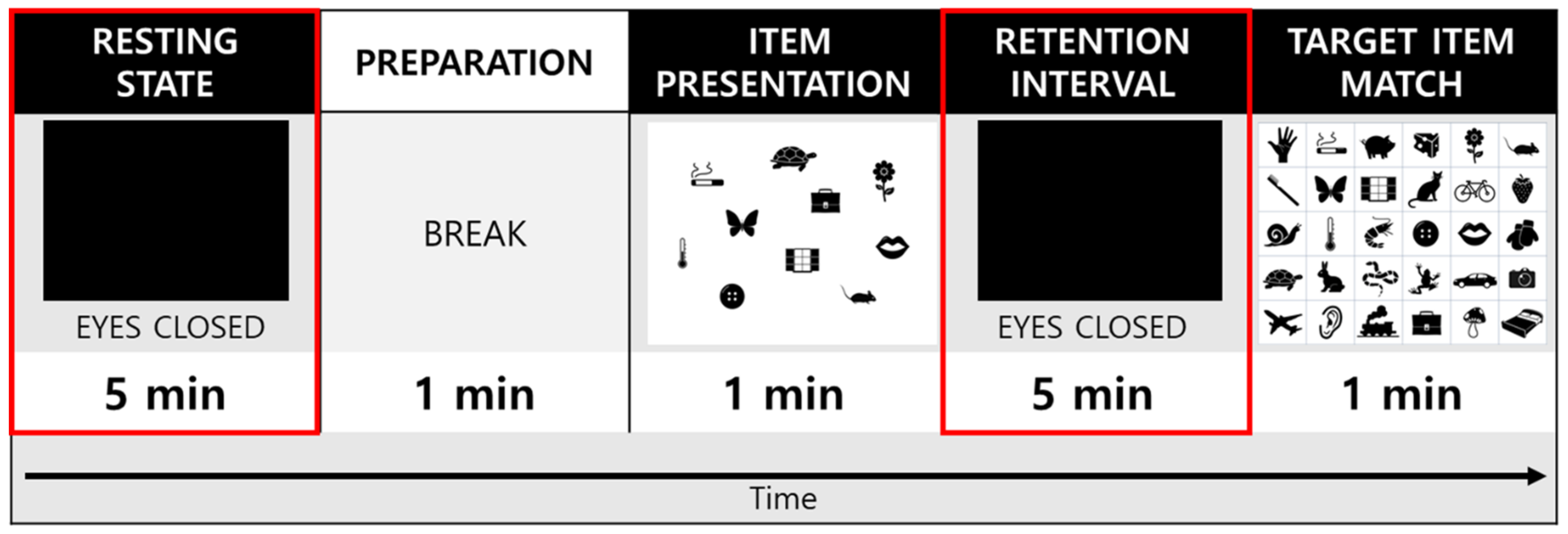
2.4. Experimental Procedures
2.5. Electroencephalogram Recording and Rreprocessing
2.6. Power Spectral Analysis
2.7. Modulation Index for Theta-Gamma Coupling
2.8. Statistical Analysis
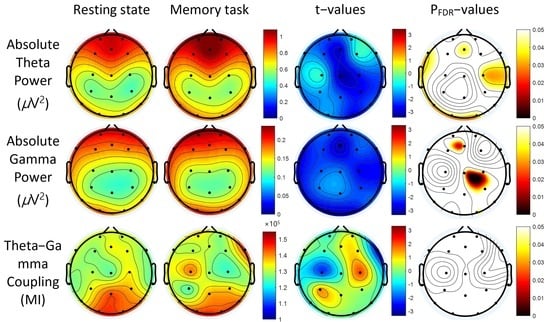
3. Results
3.1. Demograpic and Clinical Characteristics
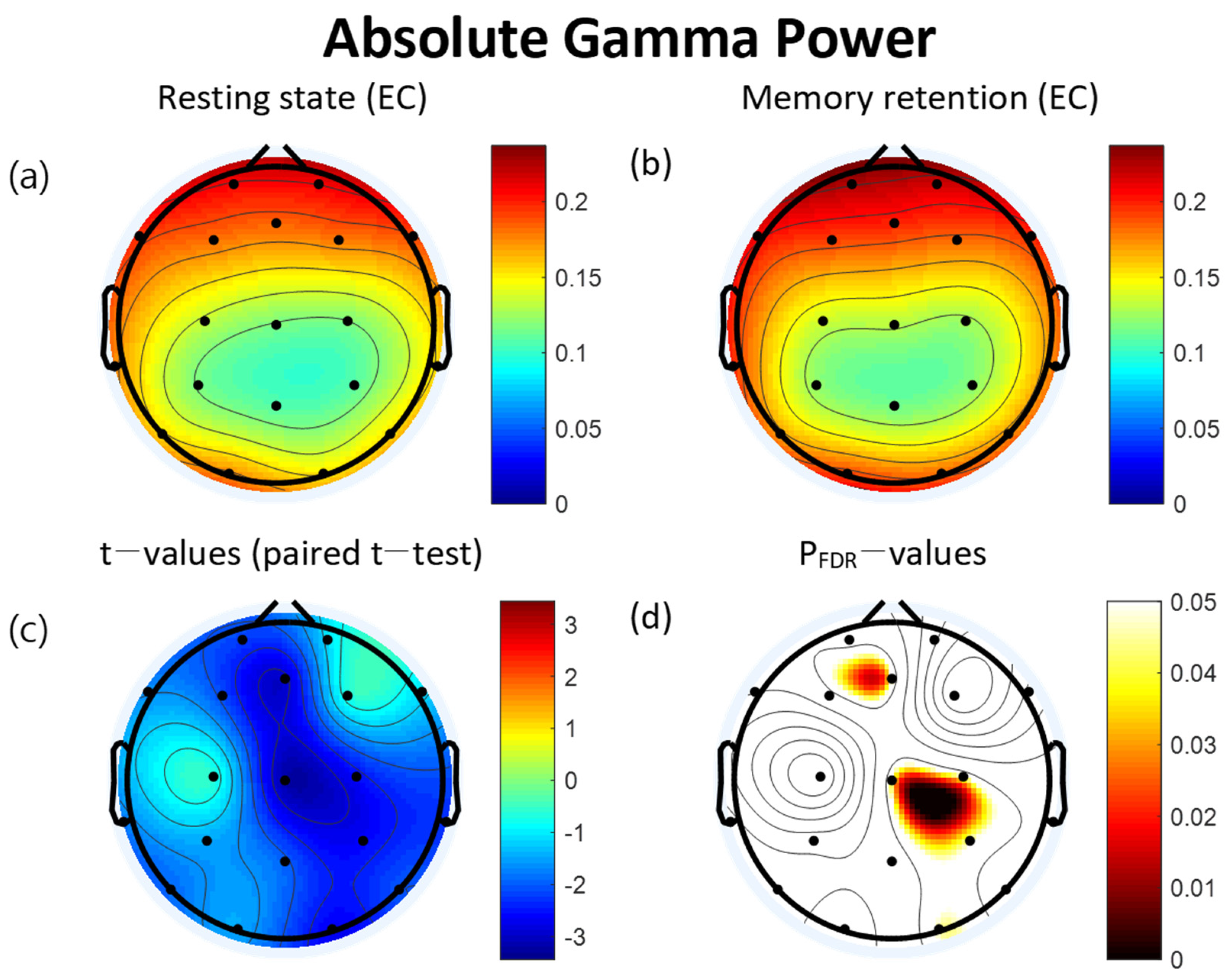
3.2. Power Spectra
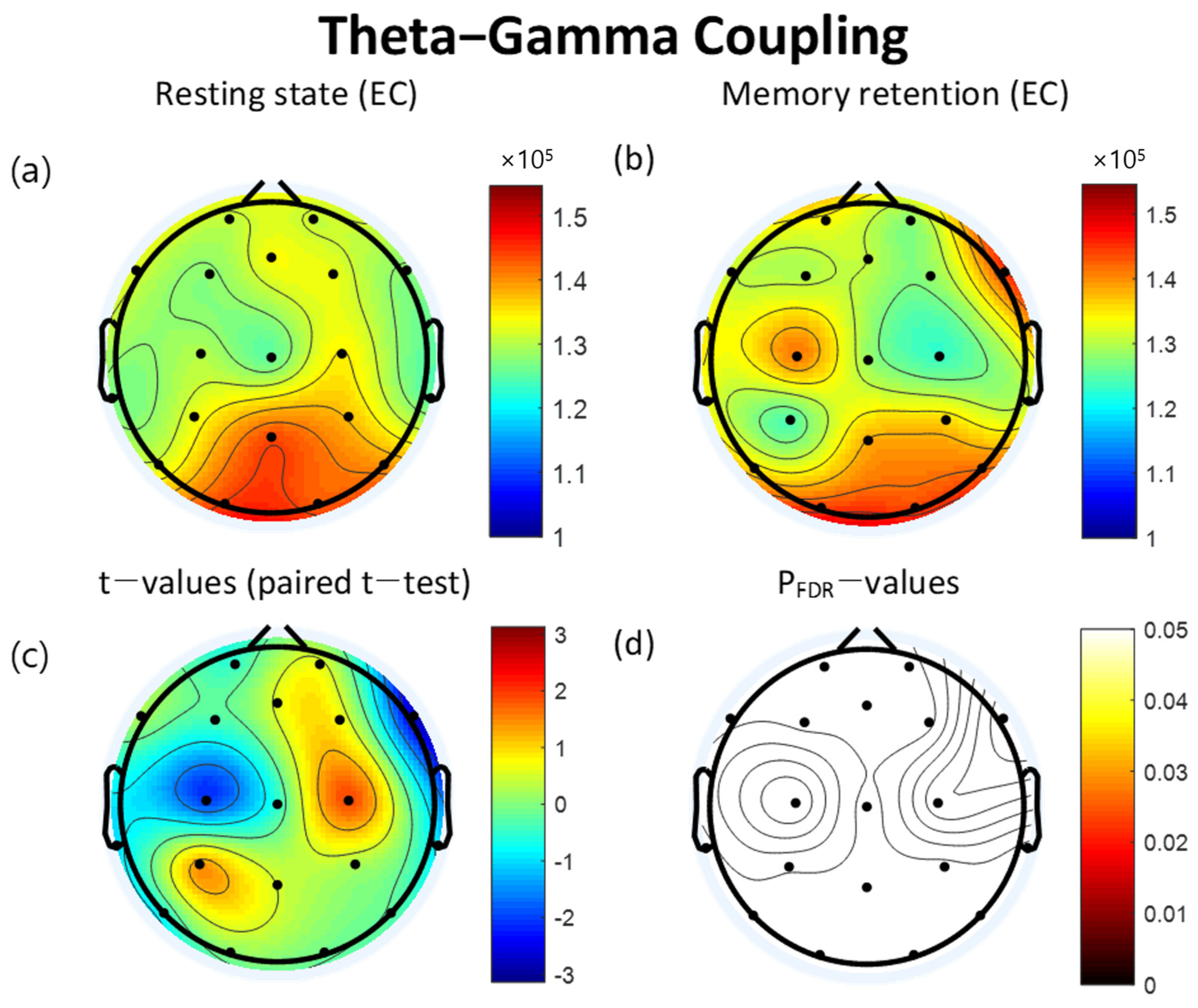
3.3. Theta-Gamma Coupling
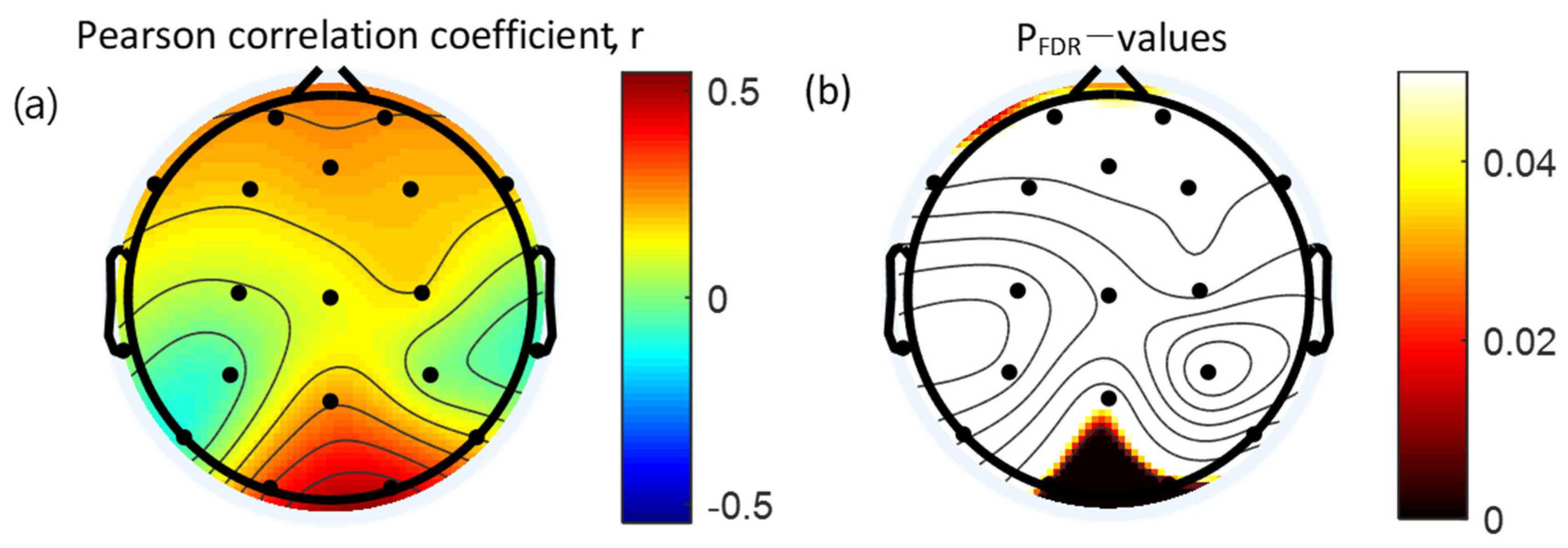
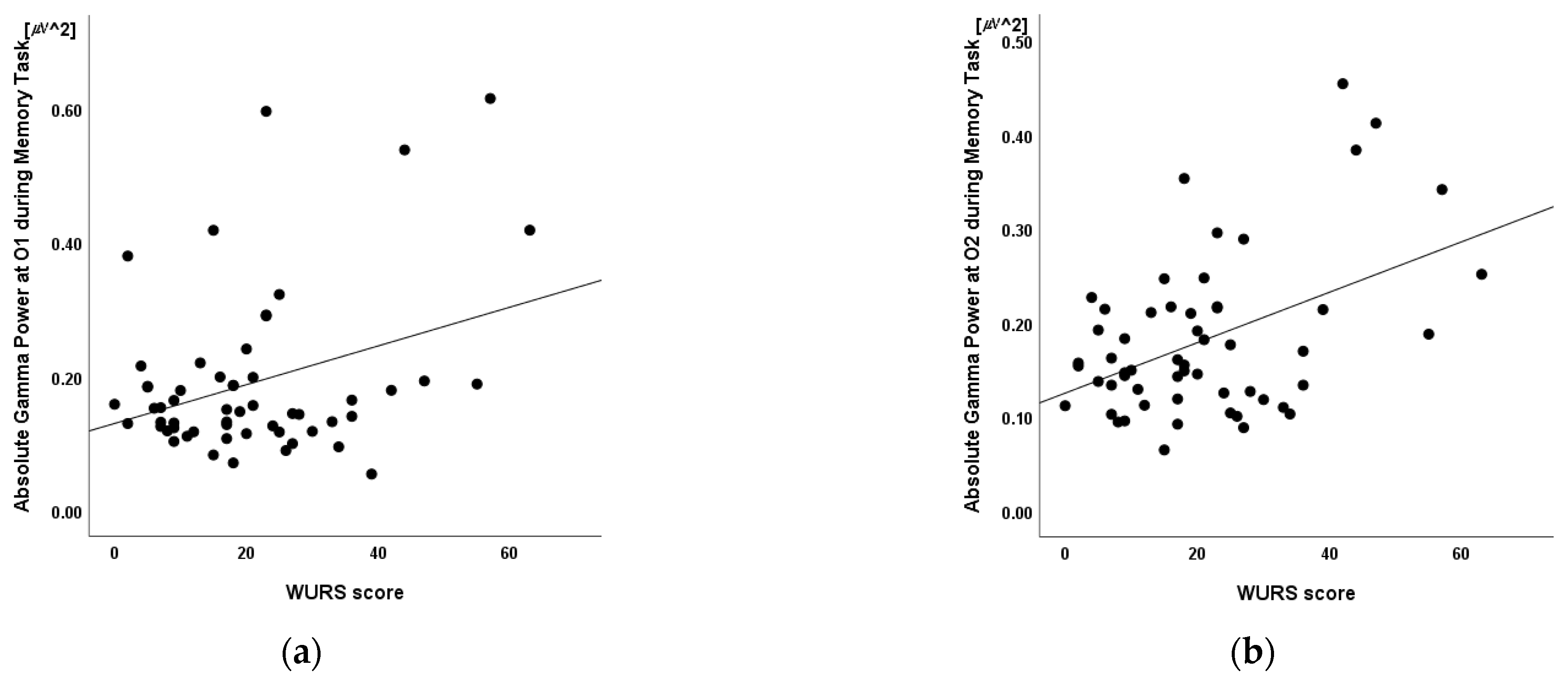
3.4. Correlation between Absolute Gamma Power and Clinical Measures
3.5. Linear Regression
4. Discussion
5. Limitations and Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paszkiel, S. Data acquisition methods for human brain activity. In Analysis and Classification of Eeg Signals for Brain–Computer Interfaces; Springer: Berlin, Germany, 2020; pp. 3–9. [Google Scholar] [CrossRef]
- Abubaker, M.; Al Qasem, W.; Kvašňák, E. Working memory and cross-frequency coupling of neuronal oscillations. Front. Psychol. 2021, 12, 756661. [Google Scholar] [CrossRef]
- Jensen, O.; Spaak, E.; Zumer, J.M. Human brain oscillations: From physiological mechanisms to analysis and cognition. In Magnetoencephalography: From Signals to Dynamic Cortical Networks; Springer: Berlin, Germany, 2019; pp. 471–517. [Google Scholar]
- Luo, W.; Guan, J.-S. Do brain oscillations orchestrate memory? Brain Sci. Adv. 2018, 4, 16–33. [Google Scholar] [CrossRef]
- Helfrich, R.F.; Knight, R.T. Oscillatory dynamics of prefrontal cognitive control. Trends Cogn. Sci. 2016, 20, 916–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroeder, C.E.; Lakatos, P. Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci. 2009, 32, 9–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fries, P.; Reynolds, J.H.; Rorie, A.E.; Desimone, R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science 2001, 291, 1560–1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friese, U.; Köster, M.; Hassler, U.; Martens, U.; Trujillo-Barreto, N.; Gruber, T. Successful memory encoding is associated with increased cross-frequency coupling between frontal theta and posterior gamma oscillations in human scalp-recorded eeg. NeuroImage 2013, 66, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Larios, J.; Alaerts, K. Tracking transient changes in the neural frequency architecture: Harmonic relationships between theta and alpha peaks facilitate cognitive performance. J. Neurosci. 2019, 39, 6291–6298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, L.M.; Jerbi, K.; Dalal, S.S. Can eeg and meg detect signals from the human cerebellum? NeuroImage 2020, 215, 116817. [Google Scholar] [CrossRef] [PubMed]
- Roux, F.; Uhlhaas, P.J. Working memory and neural oscillations: A-γ versus θ-γ codes for distinct wm information? Trends Cogn. Sci. 2014, 18, 16–25. [Google Scholar] [CrossRef]
- Fernández, A.; Pinal, D.; Díaz, F.; Zurrón, M. Working memory load modulates oscillatory activity and the distribution of fast frequencies across frontal theta phase during working memory maintenance. Neurobiol. Learn. Mem. 2021, 183, 107476. [Google Scholar] [CrossRef]
- Aben, B.; Stapert, S.; Blokland, A. About the distinction between working memory and short-term memory. Front. Psychol. 2012, 3, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cowan, N. Working memory underpins cognitive development, learning, and education. Educ. Psychol. Rev. 2014, 26, 197–223. [Google Scholar] [CrossRef] [PubMed]
- Cowan, N. The many faces of working memory and short-term storage. Psychon. Bull. Rev. 2017, 24, 1158–1170. [Google Scholar] [CrossRef] [Green Version]
- Persuh, M.; LaRock, E.; Berger, J. Working memory and consciousness: The current state of play. Front. Hum. Neurosci. 2018, 12, 78. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Guo, L.; Liu, D.; Xu, G. The dynamic properties of a brain network during working memory based on the algorithm of cross-frequency coupling. Cogn. Neurodyn. 2020, 14, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Baddeley, A. Working memory: Theories, models, and controversies. Annu. Rev. Psychol. 2012, 63, 2–5. [Google Scholar] [CrossRef] [Green Version]
- Albouy, P.; Weiss, A.; Baillet, S.; Zatorre, R.J. Selective entrainment of theta oscillations in the dorsal stream causally enhances auditory working memory performance. Neuron 2017, 94, 193–206.e5. [Google Scholar] [CrossRef] [Green Version]
- Lundqvist, M.; Herman, P.; Warden, M.R.; Brincat, S.L.; Miller, E.K. Gamma and beta bursts during working memory readout suggest roles in its volitional control. Nat. Commun. 2018, 9, 394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esmaeili, V.; Diamond, M.E. Neuronal correlates of tactile working memory in prefrontal and vibrissal somatosensory cortex. Cell Rep. 2019, 27, 3167–3181.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeCoteau, W.E.; Thorn, C.; Gibson, D.J.; Courtemanche, R.; Mitra, P.; Kubota, Y.; Graybiel, A.M. Oscillations of local field potentials in the rat dorsal striatum during spontaneous and instructed behaviors. J. Neurophysiol. 2007, 97, 3800–3805. [Google Scholar] [CrossRef] [Green Version]
- Liebe, S.; Hoerzer, G.M.; Logothetis, N.K.; Rainer, G. Theta coupling between v4 and prefrontal cortex predicts visual short-term memory performance. Nat. Neurosci. 2012, 15, 456–462. [Google Scholar] [CrossRef] [Green Version]
- Missonnier, P.; Herrmann, F.R.; Michon, A.; Fazio-Costa, L.; Gold, G.; Giannakopoulos, P. Early disturbances of gamma band dynamics in mild cognitive impairment. J. Neural Transm. 2010, 117, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Wianda, E.; Ross, B. The roles of alpha oscillation in working memory retention. Brain Behav. 2019, 9, e01263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, W.K.; Tseng, P.; Yeh, J.R.; Huang, N.E.; Juan, C.H. Frontoparietal beta amplitude modulation and its interareal cross-frequency coupling in visual working memory. Neuroscience 2021, 460, 69–87. [Google Scholar] [CrossRef]
- Nunez, P.L.; Srinivasan, R. Electric Fields of the Brain: The Neurophysics of Eeg; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Buzsáki, G.; Watson, B.O. Brain rhythms and neural syntax: Implications for efficient coding of cognitive content and neuropsychiatric disease. Dialogues Clin. Neurosci. 2012, 14, 345. [Google Scholar] [CrossRef] [PubMed]
- Sauseng, P.; Klimesch, W. What does phase information of oscillatory brain activity tell us about cognitive processes? Neurosci. Biobehav. Rev. 2008, 32, 1001–1013. [Google Scholar] [CrossRef]
- Morillas-Romero, A.; Tortella-Feliu, M.; Bornas, X.; Putman, P. Spontaneous eeg theta/beta ratio and delta-beta coupling in relation to attentional network functioning and self-reported attentional control. Cogn. Affect. Behav. Neurosci. 2015, 15, 598–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voytek, B.; Kayser, A.S.; Badre, D.; Fegen, D.; Chang, E.F.; Crone, N.E.; Parvizi, J.; Knight, R.T.; D’Esposito, M. Oscillatory dynamics coordinating human frontal networks in support of goal maintenance. Nat. Neurosci. 2015, 18, 1318–1324. [Google Scholar] [CrossRef]
- Lisman, J.E.; Jensen, O. The θ-γ neural code. Neuron 2013, 77, 1002–1016. [Google Scholar] [CrossRef] [Green Version]
- Riddle, J.; Vogelsang, D.A.; Hwang, K.; Cellier, D.; D’Esposito, M. Distinct oscillatory dynamics underlie different components of hierarchical cognitive control. J. Neurosci. 2020, 40, 4945–4953. [Google Scholar] [CrossRef]
- Barr, M.S.; Rajji, T.K.; Zomorrodi, R.; Radhu, N.; George, T.P.; Blumberger, D.M.; Daskalakis, Z.J. Impaired theta-gamma coupling during working memory performance in schizophrenia. Schizophr. Res. 2017, 189, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Heusser, A.C.; Poeppel, D.; Ezzyat, Y.; Davachi, L. Episodic sequence memory is supported by a theta-gamma phase code. Nat. Neurosci. 2016, 19, 1374–1380. [Google Scholar] [CrossRef] [PubMed]
- Sauseng, P.; Peylo, C.; Biel, A.L.; Friedrich, E.V.C.; Romberg-Taylor, C. Does cross-frequency phase coupling of oscillatory brain activity contribute to a better understanding of visual working memory? Br. J. Psychol. 2019, 110, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Spellman, T.J.; Rosen, A.M.; Gogos, J.A.; Gordon, J.A. Hippocampal-prefrontal theta-gamma coupling during performance of a spatial working memory task. Nat. Commun. 2017, 8, 2182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nir, Y.; Andrillon, T.; Marmelshtein, A.; Suthana, N.; Cirelli, C.; Tononi, G.; Fried, I. Selective neuronal lapses precede human cognitive lapses following sleep deprivation. Nat. Med. 2017, 23, 1474–1480. [Google Scholar] [CrossRef]
- Zutshi, I.; Brandon, M.P.; Fu, M.L.; Donegan, M.L.; Leutgeb, J.K.; Leutgeb, S. Hippocampal neural circuits respond to optogenetic pacing of theta frequencies by generating accelerated oscillation frequencies. Curr. Biol. 2018, 28, 1179–1188.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wutz, A.; Loonis, R.; Roy, J.E.; Donoghue, J.A.; Miller, E.K. Different levels of category abstraction by different dynamics in different prefrontal areas. Neuron 2018, 97, 716–726.e8. [Google Scholar] [CrossRef] [PubMed]
- Axmacher, N.; Henseler, M.M.; Jensen, O.; Weinreich, I.; Elger, C.E.; Fell, J. Cross-frequency coupling supports multi-item working memory in the human hippocampus. Proc. Natl. Acad. Sci. USA 2010, 107, 3228–3233. [Google Scholar] [CrossRef] [Green Version]
- Goodman, M.S.; Kumar, S.; Zomorrodi, R.; Ghazala, Z.; Cheam, A.S.M.; Barr, M.S.; Daskalakis, Z.J.; Blumberger, D.M.; Fischer, C.; Flint, A.; et al. Theta-gamma coupling and working memory in alzheimer’s dementia and mild cognitive impairment. Front. Aging Neurosci. 2018, 10, 101. [Google Scholar] [CrossRef] [Green Version]
- Fougnie, D.; Zughni, S.; Godwin, D.; Marois, R. Working memory storage is intrinsically domain specific. J. Exp. Psychol. Gen. 2015, 144, 30–47. [Google Scholar] [CrossRef] [Green Version]
- Botvinick, M.; Watanabe, T. From numerosity to ordinal rank: A gain-field model of serial order representation in cortical working memory. J. Neurosci. 2007, 27, 8636–8642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macoveanu, J.; Klingberg, T.; Tegnér, J. Neuronal firing rates account for distractor effects on mnemonic accuracy in a visuo-spatial working memory task. Biol. Cybern. 2007, 96, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Edin, F.; Klingberg, T.; Johansson, P.; McNab, F.; Tegnér, J.; Compte, A. Mechanism for top-down control of working memory capacity. Proc. Natl. Acad. Sci. USA 2009, 106, 6802–6807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klingberg, T.; Forssberg, H.; Westerberg, H. Training of working memory in children with adhd. J. Clin. Exp. Neuropsychol. 2002, 24, 781–791. [Google Scholar] [CrossRef] [Green Version]
- Jaeggi, S.M.; Buschkuehl, M.; Jonides, J.; Perrig, W.J. Improving fluid intelligence with training on working memory. Proc. Natl. Acad. Sci. USA 2008, 105, 6829–6833. [Google Scholar] [CrossRef] [Green Version]
- Siegel, M.; Donner, T.H.; Engel, A.K. Spectral fingerprints of large-scale neuronal interactions. Nature Rev. Neurosci. 2012, 13, 121–134. [Google Scholar] [CrossRef]
- Constantinidis, C.; Klingberg, T. The neuroscience of working memory capacity and training. Nat. Rev. Neurosci. 2016, 17, 438–449. [Google Scholar] [CrossRef]
- Jones, K.T.; Johnson, E.L.; Berryhill, M.E. Frontoparietal theta-gamma interactions track working memory enhancement with training and tdcs. NeuroImage 2020, 211, 116615. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, W.; Brankačk, J.; Weyer, S.W.; Müller, U.C.; Tort, A.B.; Draguhn, A. Impaired theta-gamma coupling in app-deficient mice. Sci. Rep. 2016, 6, 21948. [Google Scholar] [CrossRef] [Green Version]
- Bähner, F.; Demanuele, C.; Schweiger, J.; Gerchen, M.F.; Zamoscik, V.; Ueltzhöffer, K.; Hahn, T.; Meyer, P.; Flor, H.; Durstewitz, D.; et al. Hippocampal-dorsolateral prefrontal coupling as a species-conserved cognitive mechanism: A human translational imaging study. Neuropsychopharmacology 2015, 40, 1674–1681. [Google Scholar] [CrossRef] [Green Version]
- Gruart, A.; Leal-Campanario, R.; López-Ramos, J.C.; Delgado-García, J.M. Functional basis of associative learning and its relationships with long-term potentiation evoked in the involved neural circuits: Lessons from studies in behaving mammals. Neurobiol. Learn. Mem. 2015, 124, 3–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marmpena, M.; Dimitriadis, S.I.; Thakor, N.; Bezerianos, A. Phase to amplitude coupling as a potential biomarker for creative ideation: An eeg study. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Orlando, FL, USA, 16–20 August 2016; pp. 383–386. [Google Scholar] [CrossRef]
- Scheffer-Teixeira, R.; Tort, A.B. On cross-frequency phase-phase coupling between theta and gamma oscillations in the hippocampus. eLife 2016, 5, e20515. [Google Scholar] [CrossRef] [PubMed]
- Colgin, L.L. Theta-gamma coupling in the entorhinal-hippocampal system. Curr. Opin. Neurobiol. 2015, 31, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Frank, C.; Land, W.M.; Schack, T. Perceptual-cognitive changes during motor learning: The influence of mental and physical practice on mental representation, gaze behavior, and performance of a complex action. Front. Psychol. 2015, 6, 1981. [Google Scholar] [CrossRef] [Green Version]
- Garofalo, S.; Battaglia, S.; Starita, F.; di Pellegrino, G. Modulation of cue-guided choices by transcranial direct current stimulation. Cortex 2021, 137, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Harrison, B.J.; Fullana, M.A. Does the human ventromedial prefrontal cortex support fear learning, fear extinction or both? A commentary on subregional contributions. Mol. Psychiatry 2021, 137, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Tashjian, S.M.; Zbozinek, T.D.; Mobbs, D. A decision architecture for safety computations. Trends Cogn. Sci. 2021, 25, 342–354. [Google Scholar] [CrossRef]
- Garofalo, S.; Battaglia, S.; di Pellegrino, G. Individual differences in working memory capacity and cue-guided behavior in humans. Sci. Rep. 2019, 9, 7327. [Google Scholar] [CrossRef]
- Jarrold, C.; Towse, J.N. Individual differences in working memory. Neuroscience 2006, 139, 39–50. [Google Scholar] [CrossRef]
- Li, F.; Liu, T.; Wang, F.; Li, H.; Gong, D.; Zhang, R.; Jiang, Y.; Tian, Y.; Guo, D.; Yao, D.; et al. Relationships between the resting-state network and the p3: Evidence from a scalp eeg study. Sci. Rep. 2015, 5, 15129. [Google Scholar] [CrossRef] [Green Version]
- Vlahou, E.L.; Thurm, F.; Kolassa, I.T.; Schlee, W. Resting-state slow wave power, healthy aging and cognitive performance. Sci. Rep. 2014, 4, 5101. [Google Scholar] [CrossRef] [PubMed]
- Carhart-Harris, R.L.; Friston, K.J. The default-mode, ego-functions and free-energy: A neurobiological account of freudian ideas. Brain J. Neurol. 2010, 133, 1265–1283. [Google Scholar] [CrossRef] [PubMed]
- Greicius, M.D.; Krasnow, B.; Reiss, A.L.; Menon, V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. USA 2003, 100, 253–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sweeney-Reed, C.M.; Zaehle, T.; Voges, J.; Schmitt, F.C.; Buentjen, L.; Borchardt, V.; Walter, M.; Hinrichs, H.; Heinze, H.J.; Rugg, M.D.; et al. Anterior thalamic high frequency band activity is coupled with theta oscillations at rest. Front. Hum. Neurosci. 2017, 11, 358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arieli, A.; Sterkin, A.; Grinvald, A.; Aertsen, A. Dynamics of ongoing activity: Explanation of the large variability in evoked cortical responses. Science 1996, 273, 1868–1871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabral, J.; Kringelbach, M.L.; Deco, G. Exploring the network dynamics underlying brain activity during rest. Prog. Neurobiol. 2014, 114, 102–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Northoff, G.; Qin, P.; Nakao, T. Rest-stimulus interaction in the brain: A review. Trends Neurosci. 2010, 33, 277–284. [Google Scholar] [CrossRef]
- Barry, R.J.; Clarke, A.R.; Hajos, M.; McCarthy, R.; Selikowitz, M.; Dupuy, F.E. Resting-state eeg gamma activity in children with attention-deficit/hyperactivity disorder. Clin. Neurophysiol. 2010, 121, 1871–1877. [Google Scholar] [CrossRef]
- Berman, J.I.; Liu, S.; Bloy, L.; Blaskey, L.; Roberts, T.P.; Edgar, J.C. Alpha-to-gamma phase-amplitude coupling methods and application to autism spectrum disorder. Brain Connect. 2015, 5, 80–90. [Google Scholar] [CrossRef]
- Raichle, M.E. The brain’s default mode network. Annu. Rev. Neurosci. 2015, 38, 433–447. [Google Scholar] [CrossRef] [Green Version]
- Shulman, G.L.; Corbetta, M.; Buckner, R.L.; Fiez, J.A.; Miezin, F.M.; Raichle, M.E.; Petersen, S.E. Common blood flow changes across visual tasks: I. Increases in subcortical structures and cerebellum but not in nonvisual cortex. J. Cogn. Neurosci. 1997, 9, 624–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Platas-Neri, D.; Hidalgo-Tobón, S.; Chico-Ponce de León, F.; Muñoz-Delgado, J.; Phillips, K.A.; Téllez-Alanís, B.; Villanueva-Valle, J.; de Celis Alonso, B. Brain connectivity in ateles geoffroyi: Resting-state functional magnetic resonance imaging of working memory and executive control. Brain Behav. Evol. 2019, 93, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Tsolaki, A.; Kazis, D.; Kompatsiaris, I.; Kosmidou, V.; Tsolaki, M. Electroencephalogram and alzheimer’s disease: Clinical and research approaches. Int. J. Alzheimer’s Dis. 2014, 2014, 349249. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jeong, J. Correlation of risk-taking propensity with cross-frequency phase-amplitude coupling in the resting eeg. Clin. Neurophysiol. 2013, 124, 2172–2180. [Google Scholar] [CrossRef]
- Hsieh, L.T.; Ranganath, C. Frontal midline theta oscillations during working memory maintenance and episodic encoding and retrieval. NeuroImage 2014, 85 Pt 2, 721–729. [Google Scholar] [CrossRef] [Green Version]
- Singer, W. Synchronization of cortical activity and its putative role in information processing and learning. Annu. Rev. Physiol. 1993, 55, 349–374. [Google Scholar] [CrossRef]
- Sheehan, D.V.; Lecrubier, Y.; Sheehan, K.H.; Amorim, P.; Janavs, J.; Weiller, E.; Hergueta, T.; Baker, R.; Dunbar, G.C. The mini-international neuropsychiatric interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for dsm-iv and icd-10. J. Clin. Psychiatry 1998, 59 (Suppl. S20), 22–57. [Google Scholar]
- Oldfield, R.C. The assessment and analysis of handedness: The edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Ward, M.F.; Wender, P.H.; Reimherr, F.W. The wender utah rating scale: An aid in the retrospective diagnosis of childhood attention deficit hyperactivity disorder. Am. J. Psychiatry 1993, 150, 885–890. [Google Scholar] [CrossRef]
- Wechsler, D. Wechsler Memory Scale; American Psychological Association: Washington, DC, USA, 1945. [Google Scholar]
- Wechsler, D. Wechsler Adult Intelligence Scale; American Psychological Association: Washington, DC, USA, 1955. [Google Scholar]
- Delorme, A.; Makeig, S. Eeglab: An open source toolbox for analysis of single-trial eeg dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Gabard-Durnam, L.J.; Mendez Leal, A.S.; Wilkinson, C.L.; Levin, A.R. The harvard automated processing pipeline for electroencephalography (happe): Standardized processing software for developmental and high-artifact data. Front. Neurosci. 2018, 12, 97. [Google Scholar] [CrossRef] [PubMed]
- Winkler, I.; Brandl, S.; Horn, F.; Waldburger, E.; Allefeld, C.; Tangermann, M. Robust artifactual independent component classification for bci practitioners. J. Neural Eng. 2014, 11, 035013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tort, A.B.; Komorowski, R.; Eichenbaum, H.; Kopell, N. Measuring phase-amplitude coupling between neuronal oscillations of different frequencies. J. Neurophysiol. 2010, 104, 1195–1210. [Google Scholar] [CrossRef] [PubMed]
- Tort, A.B.; Komorowski, R.W.; Manns, J.R.; Kopell, N.J.; Eichenbaum, H. Theta-gamma coupling increases during the learning of item-context associations. Proc. Natl. Acad. Sci. USA 2009, 106, 20942–20947. [Google Scholar] [CrossRef] [Green Version]
- Bowman, D. A List of Analytic Expressions for Instantaneous Frequency Determined via the Hilbert Transform; The University of North Carolina at Chapel Hill: Chapel Hill, NC, USA, 2013. [Google Scholar]
- Santner, T.J.; Duffy, D.E. The Statistical Analysis of Discrete Data; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Sauseng, P.; Griesmayr, B.; Freunberger, R.; Klimesch, W. Control mechanisms in working memory: A possible function of eeg theta oscillations. Neurosci. Biobehav. Rev. 2010, 34, 1015–1022. [Google Scholar] [CrossRef]
- Park, J.Y.; Jhung, K.; Lee, J.; An, S.K. Theta-gamma coupling during a working memory task as compared to a simple vigilance task. Neurosci. Lett. 2013, 532, 39–43. [Google Scholar] [CrossRef]
- Dürschmid, S.; Zaehle, T.; Kopitzki, K.; Voges, J.; Schmitt, F.C.; Heinze, H.J.; Knight, R.T.; Hinrichs, H. Phase-amplitude cross-frequency coupling in the human nucleus accumbens tracks action monitoring during cognitive control. Front. Hum. Neurosci. 2013, 7, 635. [Google Scholar] [CrossRef] [Green Version]
- Broyd, S.J.; Demanuele, C.; Debener, S.; Helps, S.K.; James, C.J.; Sonuga-Barke, E.J. Default-mode brain dysfunction in mental disorders: A systematic review. Neurosci. Biobehav. Rev. 2009, 33, 279–296. [Google Scholar] [CrossRef]
- Fox, M.D.; Snyder, A.Z.; Vincent, J.L.; Corbetta, M.; Van Essen, D.C.; Raichle, M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. USA 2005, 102, 9673–9678. [Google Scholar] [CrossRef] [Green Version]
- Ferrer, M.; Andión, O.; Matalí, J.; Valero, S.; Navarro, J.A.; Ramos-Quiroga, J.A.; Torrubia, R.; Casas, M. Comorbid attention-deficit/hyperactivity disorder in borderline patients defines an impulsive subtype of borderline personality disorder. J. Personal. Disord. 2010, 24, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Z.; Kaplan, A.S.; Zai, C.C.; Levitan, R.D.; Kennedy, J.L. Comt val158met variant and functional haplotypes associated with childhood adhd history in women with bulimia nervosa. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Lee, K.S.; An, S.K.; Lee, J.; Kim, J.-J.; Kim, K.H.; Namkoong, K. Gamma oscillatory activity in relation to memory ability in older adults. Int. J. Psychophysiol. 2012, 86, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Prehn-Kristensen, A.; Wiesner, C.D.; Baving, L. Early gamma-band activity during interference predicts working memory distractibility in adhd. J. Atten. Disord. 2015, 19, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Gazzaley, A.; Nobre, A.C. Top-down modulation: Bridging selective attention and working memory. Trends Cogn. Sci. 2012, 16, 129–135. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, B.K.; Vogel, E.K.; Woodman, G.F.; Luck, S.J. Voluntazy and automatic attentional control of visual working memory. Percept. Psychophys. 2002, 64, 754–763. [Google Scholar] [CrossRef] [Green Version]
- Vogel, E.K.; Woodman, G.F.; Luck, S.J. Pushing around the locus of selection: Evidence for the flexible-selection hypothesis. J. Cogn. Neurosci. 2005, 17, 1907–1922. [Google Scholar] [CrossRef]
- Garofalo, S.; Timmermann, C.; Battaglia, S.; Maier, M.E.; Di Pellegrino, G. Mediofrontal negativity signals unexpected timing of salient outcomes. J. Cogn. Neurosci. 2017, 29, 718–727. [Google Scholar] [CrossRef]
- Tang, V.M.; Le Foll, B.; Blumberger, D.M.; Voineskos, D. Repetitive transcranial magnetic stimulation for comorbid major depressive disorder and alcohol use disorder. Brain Sci. 2022, 12, 48. [Google Scholar] [CrossRef]
- Garofalo, S.; Sagliano, L.; Starita, F.; Trojano, L.; di Pellegrino, G. Subliminal determinants of cue-guided choice. Sci. Rep. 2020, 10, 11926. [Google Scholar] [CrossRef]
- Das, A.; de Los Angeles, C.; Menon, V. Electrophysiological foundations of the human default-mode network revealed by intracranial-eeg recordings during resting-state and cognition. NeuroImage 2022, 21, 118927. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Kim, B.N.; Lee, J.; Na, C.; Kee, B.S.; Min, K.J.; Han, D.H.; Kim, J.I.; Lee, Y.S. Desynchronization of theta-phase gamma-amplitude coupling during a mental arithmetic task in children with attention deficit/hyperactivity disorder. PLoS ONE 2016, 11, e0145288. [Google Scholar] [CrossRef]
- Jensen, O.; Colgin, L.L. Cross-frequency coupling between neuronal oscillations. Trends Cogn. Sci. 2007, 11, 267–269. [Google Scholar] [CrossRef]
- Canolty, R.T.; Knight, R.T. The functional role of cross-frequency coupling. Trends Cogn. Sci. 2010, 14, 506–515. [Google Scholar] [CrossRef] [Green Version]
- Lenartowicz, A.; Truong, H.; Enriquez, K.D.; Webster, J.; Pochon, J.B.; Rissman, J.; Bearden, C.E.; Loo, S.K.; Bilder, R.M. Neurocognitive subprocesses of working memory performance. Cogn. Affect. Behav. Neurosci. 2021, 21, 1130–1152. [Google Scholar] [CrossRef] [PubMed]
- Hanslmayr, S.; Staudigl, T.; Fellner, M.-C. Oscillatory power decreases and long-term memory: The information via desynchronization hypothesis. Front. Hum. Neurosci. 2012, 6, 74. [Google Scholar] [CrossRef] [Green Version]
- Klimesch, W. EEG-alpha rhythms and memory processes. Int. J. Psychophysiol. 1997, 26, 319–340. [Google Scholar] [CrossRef]

| Variables | Mean | SD | Min | Max |
|---|---|---|---|---|
| Age (year) | 23.9 | 4.0 | 18 | 41 |
| Sex | ||||
| Male, N (%) | 31 (55.4) | |||
| Female, N (%) | 25 (44.6) | |||
| Handedness, N (%) | ||||
| Right, N (%) | 53 (94.6) | |||
| Left, N (%) | 3 (5.4) | |||
| Education (year) | 13.2 | 1.7 | 12 | 16 |
| WURS | 20.9 | 14.3 | 0 | 63 |
| LNS | 11.8 | 2.6 | 7 | 18 |
| Spatial Span | 20.5 | 3.8 | 14 | 29 |
| Lead (μV2) | Resting State | VWM Task | t | pFDR | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| FP2 | 0.97 | 0.33 | 1.06 | 0.40 | −2.07 | 0.066 |
| Fz | 0.99 | 0.38 | 1.13 | 0.44 | −3.05 * | 0.036 |
| FP1 | 0.98 | 0.37 | 1.07 | 0.40 | −2.01 | 0.066 |
| F3 | 0.88 | 0.32 | 0.96 | 0.36 | −2.19 | 0.058 |
| F7 | 0.84 | 0.33 | 0.94 | 0.37 | −2.56 * | 0.036 |
| C3 | 0.60 | 0.25 | 0.65 | 0.26 | −1.94 | 0.072 |
| T3 | 0.71 | 0.33 | 0.82 | 0.36 | −2.70 * | 0.036 |
| P3 | 0.52 | 0.24 | 0.57 | 0.25 | −1.69 | 0.102 |
| T5 | 0.74 | 0.36 | 0.82 | 0.38 | −2.59 * | 0.036 |
| Pz | 0.55 | 0.24 | 0.58 | 0.23 | −1.63 | 0.108 |
| O1 | 0.72 | 0.33 | 0.79 | 0.35 | −2.90 * | 0.036 |
| O2 | 0.69 | 0.32 | 0.76 | 0.33 | −2.74 * | 0.036 |
| P4 | 0.49 | 0.25 | 0.55 | 0.25 | −2.18 | 0.058 |
| T6 | 0.73 | 0.40 | 0.81 | 0.43 | −1.87 | 0.079 |
| C4 | 0.56 | 0.25 | 0.62 | 0.26 | −2.59 * | 0.036 |
| T4 | 0.67 | 0.35 | 0.76 | 0.38 | −2.46 * | 0.040 |
| F8 | 0.81 | 0.33 | 0.89 | 0.40 | −2.19 | 0.058 |
| F4 | 0.87 | 0.33 | 0.96 | 0.39 | −2.03 | 0.066 |
| Cz | 0.71 | 0.31 | 0.77 | 0.33 | −1.71 | 0.102 |
| Lead (μV2) | Resting State | VWM Task | t | pFDR | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| FP2 | 0.21 | 0.04 | 0.22 | 0.06 | −1.15 | 0.286 |
| Fz | 0.18 | 0.04 | 0.20 | 0.05 | −2.95 * | 0.044 |
| FP1 | 0.21 | 0.05 | 0.22 | 0.06 | −2.22 | 0.083 |
| F3 | 0.18 | 0.03 | 0.19 | 0.04 | −2.15 | 0.085 |
| F7 | 0.20 | 0.05 | 0.21 | 0.05 | −1.69 | 0.143 |
| C3 | 0.13 | 0.03 | 0.14 | 0.03 | −0.64 | 0.523 |
| T3 | 0.18 | 0.07 | 0.20 | 0.07 | −1.81 | 0.119 |
| P3 | 0.12 | 0.03 | 0.12 | 0.03 | −1.49 | 0.181 |
| T5 | 0.16 | 0.05 | 0.17 | 0.05 | −1.93 | 0.102 |
| Pz | 0.11 | 0.03 | 0.11 | 0.04 | −1.99 | 0.097 |
| O1 | 0.17 | 0.10 | 0.19 | 0.12 | −1.51 | 0.181 |
| O2 | 0.15 | 0.07 | 0.18 | 0.08 | −2.70 * | 0.046 |
| P4 | 0.11 | 0.03 | 0.12 | 0.03 | −2.66 * | 0.046 |
| T6 | 0.16 | 0.05 | 0.17 | 0.06 | −2.08 | 0.089 |
| C4 | 0.12 | 0.03 | 0.13 | 0.03 | −2.60 * | 0.046 |
| T4 | 0.16 | 0.05 | 0.18 | 0.07 | −2.31 | 0.078 |
| F8 | 0.19 | 0.05 | 0.20 | 0.05 | −1.15 | 0.286 |
| F4 | 0.18 | 0.04 | 0.18 | 0.04 | −0.69 | 0.520 |
| Cz | 0.12 | 0.03 | 0.13 | 0.04 | −3.20 * | 0.043 |
| Lead (×10−5) | Resting State | VWM Task | t | pFDR | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| FP2 | 1.31 | 0.31 | 1.28 | 0.29 | 0.58 | 0.942 |
| Fz | 1.33 | 0.28 | 1.30 | 0.34 | 0.57 | 0.942 |
| FP1 | 1.30 | 0.31 | 1.33 | 0.32 | −0.49 | 0.942 |
| F3 | 1.27 | 0.33 | 1.30 | 0.28 | −0.35 | 0.946 |
| F7 | 1.32 | 0.30 | 1.31 | 0.31 | 0.18 | 0.946 |
| C3 | 1.30 | 0.32 | 1.41 | 0.32 | −2.07 | 0.413 |
| T3 | 1.27 | 0.34 | 1.33 | 0.29 | −1.03 | 0.942 |
| P3 | 1.33 | 0.31 | 1.25 | 0.32 | 1.34 | 0.877 |
| T5 | 1.32 | 0.28 | 1.36 | 0.34 | −0.63 | 0.942 |
| Pz | 1.43 | 0.78 | 1.38 | 0.44 | 0.46 | 0.942 |
| O1 | 1.44 | 0.45 | 1.44 | 0.55 | 0.04 | 0.969 |
| O2 | 1.43 | 0.57 | 1.44 | 0.38 | −0.13 | 0.946 |
| P4 | 1.37 | 0.40 | 1.36 | 0.44 | 0.22 | 0.946 |
| T6 | 1.40 | 0.43 | 1.41 | 0.37 | −0.26 | 0.946 |
| C4 | 1.34 | 0.36 | 1.23 | 0.23 | 1.86 | 0.434 |
| T4 | 1.25 | 0.32 | 1.31 | 0.32 | −1.01 | 0.942 |
| F8 | 1.27 | 0.32 | 1.44 | 0.32 | −2.71 | 0.174 |
| F4 | 1.33 | 0.31 | 1.28 | 0.31 | 0.74 | 0.942 |
| Cz | 1.25 | 0.29 | 1.28 | 0.34 | −0.53 | 0.942 |
| Lead | WURS | |
|---|---|---|
| r | pFDR | |
| FP2 | 0.25 | 0.067 |
| Fz | 0.23 | 0.089 |
| FP1 | 0.24 | 0.071 |
| F3 | 0.18 | 0.189 |
| F7 | 0.20 | 0.133 |
| C3 | 0.06 | 0.686 |
| T3 | 0.03 | 0.806 |
| P3 | −0.06 | 0.677 |
| T5 | −0.10 | 0.487 |
| Pz | 0.24 | 0.080 |
| O1 | 0.34 * | 0.010 |
| O2 | 0.46 † | <0.001 |
| P4 | 0.02 | 0.901 |
| T6 | 0.16 | 0.252 |
| C4 | 0.15 | 0.257 |
| T4 | −0.09 | 0.535 |
| F8 | 0.19 | 0.168 |
| F4 | 0.20 | 0.149 |
| Cz | 0.11 | 0.424 |
| β | B (SE) | t | p | |
|---|---|---|---|---|
| Model | 0.010 | |||
| (Constant) | 12.46 (13.90) | 0.90 | 0.374 | |
| Age | −0.06 | −0.21 (0.46) | −0.45 | 0.657 |
| Sex | −0.03 | −0.95 (3.98) | −0.24 | 0.813 |
| Absolute Gamma Power at O1 | 0.14 | 16.38 (19.24) | 0.85 | 0.399 |
| Absolute Gamma Power at O2 | 0.38 | 64.46 (24.88) | 2.59 * | 0.012 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, J.S.; Heo, J.; Oh, J.; Lee, D.; Jhung, K.; Kim, J.-J.; Park, J.Y. The Functional Interactions between Cortical Regions through Theta-Gamma Coupling during Resting-State and a Visual Working Memory Task. Brain Sci. 2022, 12, 274. https://doi.org/10.3390/brainsci12020274
Ahn JS, Heo J, Oh J, Lee D, Jhung K, Kim J-J, Park JY. The Functional Interactions between Cortical Regions through Theta-Gamma Coupling during Resting-State and a Visual Working Memory Task. Brain Sciences. 2022; 12(2):274. https://doi.org/10.3390/brainsci12020274
Chicago/Turabian StyleAhn, Ji Seon, Jaeseok Heo, Jooyoung Oh, Deokjong Lee, Kyungun Jhung, Jae-Jin Kim, and Jin Young Park. 2022. "The Functional Interactions between Cortical Regions through Theta-Gamma Coupling during Resting-State and a Visual Working Memory Task" Brain Sciences 12, no. 2: 274. https://doi.org/10.3390/brainsci12020274
APA StyleAhn, J. S., Heo, J., Oh, J., Lee, D., Jhung, K., Kim, J.-J., & Park, J. Y. (2022). The Functional Interactions between Cortical Regions through Theta-Gamma Coupling during Resting-State and a Visual Working Memory Task. Brain Sciences, 12(2), 274. https://doi.org/10.3390/brainsci12020274