Differences in Odor Identification in Early-Onset and Late-Onset Depression
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Clinical Measurements
2.3. Neuropsychological Assessments
2.4. Olfactory Assessments
2.5. Statistical Analyses
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Comparison of Olfactory and Cognitive Functions between Different Groups
3.3. Correlation Analyses between OI Cognitive Function and Depression
3.4. Multiple Linear Regression Analysis
3.5. Mediation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saczynski, J.S.; Beiser, A.; Seshadri, S.; Auerbach, S.; Wolf, P.A.; Au, R. Depressive symptoms and risk of dementia: The Framingham Heart Study. Neurology 2010, 75, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Weyerer, S.; Eifflaender-Gorfer, S.; Wiese, B.; Luppa, M.; Pentzek, M.; Bickel, H.; Bachmann, C.; Scherer, M.; Maier, W.; Riedel-Heller, S.G. Incidence and predictors of depression in non-demented primary care attenders aged 75 years and older: Results from a 3-year follow-up study. Age Ageing 2013, 42, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.H.; Nasreldin, M.; Gomaa, M.A.; Khalaf, O.O. Late versus Early Onset Depression in Elderly Patients: Vascular Risk and Cognitive Impairment. Curr. Aging Sci. 2017, 10, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Korten, N.C.; Comijs, H.C.; Lamers, F.; Penninx, B.W. Early and late onset depression in young and middle aged adults: Differential symptomatology, characteristics and risk factors? J. Affect. Disord. 2012, 138, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Naismith, S.L.; Norrie, L.M.; Mowszowski, L.; Hickie, I.B. The neurobiology of depression in later-life: Clinical, neuropsychological, neuroimaging and pathophysiological features. Prog. Neurobiol. 2012, 98, 99–143. [Google Scholar] [CrossRef]
- Grayson, L.; Thomas, A. A systematic review comparing clinical features in early age at onset and late age at onset late-life depression. J. Affect. Disord. 2013, 150, 161–170. [Google Scholar] [CrossRef]
- Taylor, W.D.; Aizenstein, H.J.; Alexopoulos, G.S. The vascular depression hypothesis: Mechanisms linking vascular disease with depression. Mol. Psychiatry 2013, 18, 963–974. [Google Scholar] [CrossRef]
- Aizenstein, H.J.; Baskys, A.; Boldrini, M.; Butters, M.A.; Diniz, B.S.; Jaiswal, M.K.; Jellinger, K.A.; Kruglov, L.S.; Meshandin, I.A.; Mijajlovic, M.D.; et al. Vascular depression consensus report—A critical update. BMC Med. 2016, 14, 161. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, W.; Yuan, Y. Aberrant Default Mode Network Underlying the Cognitive Deficits in the Patients With Late-Onset Depression. Front. Aging Neurosci. 2018, 10, 310. [Google Scholar] [CrossRef]
- Laakso, M.P.; Soininen, H.; Partanen, K.; Lehtovirta, M.; Hallikainen, M.; Hänninen, T.; Helkala, E.L.; Vainio, P.; Riekkinen, P.J. MRI of the Hippocampus in Alzheimer’s Disease: Sensitivity, Specificity, and Analysis of the Incorrectly Classified Subjects. Neurobiol. Aging 1998, 19, 23–31. [Google Scholar] [CrossRef]
- Ballmaier, M.; Narr, K.L.; Toga, A.W.; Elderkin-Thompson, V.; Thompson, P.M.; Hamilton, L.; Haroon, E.; Pham, D.; Heinz, A.; Kumar, A. Hippocampal Morphology and Distinguishing Late-Onset From Early-Onset Elderly Depression. Am. J. Psychiatry 2008, 165, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Sorrells, S.F.; Sapolsky, R.M. An inflammatory review of glucocorticoid actions in the CNS. Brain Behav. Immun. 2007, 21, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.H.; Jung, W.S.; Um, Y.H.; Lee, C.U.; Park, Y.H.; Lim, H.K. Cerebral vascular burden on hippocampal subfields in first-onset drug-naive subjects with late-onset depression. J. Affect. Disord. 2017, 208, 47–53. [Google Scholar] [CrossRef]
- Caraci, F.; Copani, A.; Nicoletti, F.; Drago, F. Depression and Alzheimer’s disease: Neurobiological links and common pharmacological targets. Eur. J. Pharmacol. 2010, 626, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Rapp, M.A.; Schnaider-Beeri, M.; Purohit, D.P.; Perl, D.P.; Haroutunian, V.; Sano, M. Increased neurofibrillary tangles in patients with Alzheimer disease with comorbid depression. Am. J. Geriatr. Psychiatry 2008, 16, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Habes, M.; Pomponio, R.; Shou, H.; Doshi, J.; Mamourian, E.; Erus, G.; Nasrallah, I.; Launer, L.J.; Rashid, T.; Bilgel, M.; et al. The Brain Chart of Aging: Machine-learning analytics reveals links between brain aging, white matter disease, amyloid burden, and cognition in the iSTAGING consortium of 10,216 harmonized MR scans. Alzheimers Dement. 2021, 17, 89–102. [Google Scholar] [CrossRef]
- Leonard, B.E. Inflammation, depression and dementia: Are they connected? Neurochem. Res. 2007, 32, 1749–1756. [Google Scholar] [CrossRef]
- Rojo, L.E.; Fernandez, J.A.; Maccioni, A.A.; Jimenez, J.M.; Maccioni, R.B. Neuroinflammation: Implications for the pathogenesis and molecular diagnosis of Alzheimer’s disease. Arch. Med. Res. 2008, 39, 1–16. [Google Scholar] [CrossRef]
- Maes, M.; Yirmyia, R.; Noraberg, J.; Brene, S.; Hibbeln, J.; Perini, G.; Kubera, M.; Bob, P.; Lerer, B.; Maj, M. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: Leads for future research and new drug developments in depression. Metab. Brain Dis. 2009, 24, 27–53. [Google Scholar] [CrossRef]
- Marine, N.; Boriana, A. Olfactory markers of depression and Alzheimer’s disease. Neurosci. Biobehav. Rev. 2014, 45, 262–270. [Google Scholar] [CrossRef]
- Lafaille-Magnan, M.E.; Poirier, J.; Etienne, P.; Tremblay-Mercier, J.; Frenette, J.; Rosa-Neto, P.; Breitner, J.C.S.; Group, P.-A.R. Odor identification as a biomarker of preclinical AD in older adults at risk. Neurology 2017, 89, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Shin, I.S.; Lee, J.E. Olfactory function in mild cognitive impairment and Alzheimer’s disease: A meta-analysis. Laryngoscope 2019, 129, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Dintica, C.S.; Marseglia, A.; Rizzuto, D.; Wang, R.; Seubert, J.; Arfanakis, K.; Bennett, D.A.; Xu, W. Impaired olfaction is associated with cognitive decline and neurodegeneration in the brain. Neurology 2019, 92, e700–e709. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhong, X.; Mai, N.; Peng, Q.; Zhang, M.; Chen, X.; Wu, Z.; Zou, L.; Liang, W.; Ouyang, C.; et al. Interactive Effect of Depression and Cognitive Impairment on Olfactory Identification in Elderly People. J. Alzheimers Dis. 2018, 66, 1645–1655. [Google Scholar] [CrossRef]
- Chen, B.; Zhong, X.; Mai, N.; Peng, Q.; Wu, Z.; Ouyang, C.; Zhang, W.; Liang, W.; Wu, Y.; Liu, S.; et al. Cognitive Impairment and Structural Abnormalities in Late Life Depression with Olfactory Identification Impairment: An Alzheimer’s Disease-Like Pattern. Int. J. Neuropsychopharmacol. 2018, 21, 640–648. [Google Scholar] [CrossRef]
- Chen, B.; Zhong, X.; Zhang, M.; Mai, N.; Wu, Z.; Chen, X.; Peng, Q.; Zhou, H.; Wang, Q.; Yang, M.; et al. The additive effect of late-life depression and olfactory dysfunction on the risk of dementia was mediated by hypersynchronization of the hippocampus/fusiform gyrus. Transl. Psychiatry 2021, 11, 172. [Google Scholar] [CrossRef]
- Alexopoulos, G.S. Mechanisms and treatment of late-life depression. Transl. Psychiatry 2019, 9, 188. [Google Scholar] [CrossRef]
- Kohler, S.; Thomas, A.J.; Lloyd, A.; Barber, R.; Almeida, O.P.; O’Brien, J.T. White matter hyperintensities, cortisol levels, brain atrophy and continuing cognitive deficits in late-life depression. Br. J. Psychiatry 2010, 196, 143–149. [Google Scholar] [CrossRef]
- Zald, D.H.; Pardo, J.V. Functional neuroimaging of the olfactory system in humans. Int. J. Psychophysiol. 2000, 36, 165–181. [Google Scholar] [CrossRef]
- Zhou, G.; Olofsson, J.K.; Koubeissi, M.Z.; Menelaou, G.; Rosenow, J.; Schuele, S.U.; Xu, P.; Voss, J.L.; Lane, G.; Zelano, C. Human hippocampal connectivity is stronger in olfaction than other sensory systems. Prog. Neurobiol. 2021, 201, 102027. [Google Scholar] [CrossRef]
- Rasch, B.; Buchel, C.; Gais, S.; Born, J. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science 2007, 315, 1426–1429. [Google Scholar] [CrossRef] [PubMed]
- Hummel, T.; Sekinger, B.; Wolf, S.R.; Pauli, E.; Kobal, G. ‘Sniffin’ sticks’: Olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem. Senses 1997, 22, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Abu-Bader, S. Statistical Mediation Analysis Using the Sobel Test and Hayes SPSS Process Macro. Int. J. Quant. Qual. Res. Methods 2021, 9, 42–61. [Google Scholar]
- Preacher, K.J.; Hayes, A.F. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav. Res. Methods 2008, 40, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Croy, I.; Hummel, T. Olfaction as a marker for depression. J. Neurol. 2017, 264, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Buron, E.; Bulbena, A. Olfaction in affective and anxiety disorders: A review of the literature. Psychopathology 2013, 46, 63–74. [Google Scholar] [CrossRef]
- Khil, L.; Rahe, C.; Wellmann, J.; Baune, B.T.; Wersching, H.; Berger, K. Association between major depressive disorder and odor identification impairment. J. Affect. Disord. 2016, 203, 332–338. [Google Scholar] [CrossRef]
- Steffens, D.C.; McQuoid, D.R.; Payne, M.E.; Potter, G.G. Change in hippocampal volume on magnetic resonance imaging and cognitive decline among older depressed and nondepressed subjects in the neurocognitive outcomes of depression in the elderly study. Am. J. Geriatr. Psychiatry 2011, 19, 4–12. [Google Scholar] [CrossRef]
- Sapolsky, R.M.; Krey, L.C.; McEwen, B.S. The neuroendocrinology of stress and aging: The glucocorticoid cascade hypothesis. Endocr. Rev. 1986, 7, 284–301. [Google Scholar] [CrossRef]
- Bingham, K.S.; Flint, A.J.; Mulsant, B.H. Management of Late-Life Depression in the Context of Cognitive Impairment: A Review of the Recent Literature. Curr. Psychiatry Rep. 2019, 21, 74. [Google Scholar] [CrossRef]
- Empana, J.P.; Boutouyrie, P.; Lemogne, C.; Jouven, X.; van Sloten, T.T. Microvascular Contribution to Late-Onset Depression: Mechanisms, Current Evidence, Association with Other Brain Diseases, and Therapeutic Perspectives. Biol. Psychiatry 2021, 90, 214–225. [Google Scholar] [CrossRef]
- Alexopoulos, G.S.; Kiosses, D.N.; Klimstra, S.; Kalayam, B.; Bruce, M.L. Clinical Presentation of the “Depression–Executive Dysfunction Syndrome” of Late Life. Am. J. Geriatr. Psychiatry 2002, 10, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Xekardaki, A.; Santos, M.; Hof, P.; Kovari, E.; Bouras, C.; Giannakopoulos, P. Neuropathological substrates and structural changes in late-life depression: The impact of vascular burden. Acta Neuropathol. 2012, 124, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Paranthaman, R.; Burns, A.S.; Cruickshank, J.K.; Jackson, A.; Scott, M.L.; Baldwin, R.C. Age at onset and vascular pathology in late-life depression. Am. J. Geriatr. Psychiatry 2012, 20, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Dillon, C.; Allegri, R.F.; Serrano, C.M.; Iturry, M.; Salgado, P.; Glaser, F.B.; Taragano, F.E. Late- versus early-onset geriatric depression in a memory research center. Neuropsychiatr. Dis. Treat. 2009, 5, 517–526. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Larsson, M.; Nilsson, L.G.; Olofsson, J.K.; Nordin, S. Demographic and cognitive predictors of cued odor identification: Evidence from a population-based study. Chem. Senses 2004, 29, 547–554. [Google Scholar] [CrossRef]
- Dulay, M.F.; Gesteland, R.C.; Shear, P.K.; Ritchey, P.N.; Frank, R.A. Assessment of the influence of cognition and cognitive processing speed on three tests of olfaction. J. Clin. Exp. Neuropsychol 2008, 30, 327–337. [Google Scholar] [CrossRef]
- Wilson, R.S.; Arnold, S.E.; Tang, Y.; Bennett, D.A. Odor identification and decline in different cognitive domains in old age. Neuroepidemiology 2006, 26, 61–67. [Google Scholar] [CrossRef]
- Mohedano-Moriano, A.; Martinez-Marcos, A.; Munoz, M.; Arroyo-Jimenez, M.M.; Marcos, P.; Artacho-Perula, E.; Blaizot, X.; Insausti, R. Reciprocal connections between olfactory structures and the cortex of the rostral superior temporal sulcus in the Macaca fascicularis monkey. Eur. J. Neurosci. 2005, 22, 2503–2518. [Google Scholar] [CrossRef]
- Porter, J.; Anand, T.; Johnson, B.; Khan, R.M.; Sobel, N. Brain mechanisms for extracting spatial information from smell. Neuron 2005, 47, 581–592. [Google Scholar] [CrossRef]
- Milardi, D.; Cacciola, A.; Calamuneri, A.; Ghilardi, M.F.; Caminiti, F.; Cascio, F.; Andronaco, V.; Anastasi, G.; Mormina, E.; Arrigo, A.; et al. The Olfactory System Revealed: Non-Invasive Mapping by using Constrained Spherical Deconvolution Tractography in Healthy Humans. Front. Neuroanat. 2017, 11, 32. [Google Scholar] [CrossRef] [PubMed]


| EOD (n = 92) | LOD (n = 87) | NC (n = 189) | F/t/χ2 | p | Post hoc | |
|---|---|---|---|---|---|---|
| Male (%) | 16 (17.4%) | 24 (27.6%) | 70 (37.0%) | 11.69 | 0.003 | A < C |
| Age | 64.85 ± 5.02 | 70.92 ± 6.91 | 67.29 ± 7.49 | 20.65 | 0.001 | B > C > A |
| Years of education (years) | 9.04 ± 3.66 | 8.38 ± 4.11 | 10.38 ± 3.36 | 10.30 | 0.001 | C > A, B |
| Number of people taking antidepressants | 81 | 68 | NA | 3.13 | 0.07 | —— |
| Age at first onset | 53.50 (49.00–57.00) | 66.00 (62.00–73.00) | NA | −10.00 | 0.001 | A < B |
| Disease duration (years) | 12.10 ± 12.71 | 4.38 ± 5.47 | NA | 22.90 | 0.001 | A > B |
| Number of episodes | 3.06 ± 3.75 | 1.54 ± 1.26 | NA | 15.98 | 0.002 | A > B |
| Lifetime duration (years) | 9.29 ± 8.90 | 2.75 ± 2.82 | NA | 42.91 | 0.001 | A > B |
| Untreated time (years) | 3.22 ± 7.77 | 1.56 ± 3.28 | NA | 5.70 | 0.11 | —— |
| GDS | 5.55 ± 4.14 | 4.35 ± 3.73 | 2.28 ± 2.30 | 28.37 | 0.001 | A > B > C |
| EOD (n = 92) | LOD (n = 87) | NC (n = 189) | F | p | Post hoc | |
|---|---|---|---|---|---|---|
| OI | 10.87 ± 2.78 | 9.29 ± 2.62 | 10.75 ± 2.46 | 4.10 | 0.018 | A, C < B |
| OI dysfunction (%) | 23(25.0%) | 44(50.6%) | 52(27.5%) | 17.50 | 0.001 | A, C < B |
| Global cognition | ||||||
| MMSE | 23.72 ± 4.69 | 22.10 ± 5.56 | 25.78 ± 2.81 | 9.94 | <0.001 | A, C > B |
| Memory | ||||||
| AVLT N1–3 | 17.69 ± 6.13 | 15.82 ± 4.80 | 18.51 ± 4.73 | 0.794 | 0.45 | —— |
| AVLT N4 | 5.83 ± 2.46 | 5.08 ± 2.59 | 6.52 ± 2.33 | 1.44 | 0.24 | —— |
| AVLT N5 | 4.91 ± 2.93 | 4.17 ± 2.75 | 5.51 ± 2.66 | 0.34 | 0.72 | —— |
| AVLT N6 | 20.68 ± 2.62 | 20.38 ± 2.43 | 21.07 ± 2.66 | 0.40 | 0.67 | —— |
| Executive function | ||||||
| TMTA | 61.53 ± 21.03 | 68.25 ± 28.75 | 51.80 ± 21.54 | 3.52 | 0.03 | B > C |
| TMTB | 76.77 ± 34.13 | 91.69 ± 36.76 | 71.16 ± 31.13 | 2.84 | 0.06 | —— |
| Stroop A | 33.36 ± 9.20 | 35.37 ± 9.43 | 31.27 ± 8.76 | 2.17 | 0.12 | —— |
| Stroop B | 47.44 ± 15.77 | 45.97 ± 14.27 | 43.76 ± 13.77 | 1.08 | 0.34 | —— |
| Stroop C | 92.73 ± 30.99 | 94.04 ± 33.71 | 88.96 ± 36.59 | 0.71 | 0.49 | —— |
| Language | ||||||
| BNT | 20.68 ± 3.82 | 19.25 ± 4.89 | 21.44 ± 3.37 | 2.85 | 0.06 | —— |
| VFT | 7.92 ± 3.18 | 8.02 ± 3.73 | 9.55 ± 3.67 | 3.64 | 0.03 | C > A |
| Attention | ||||||
| SDMT | 28.55 ± 10.33 | 23.61 ± 12.72 | 33.15 ± 10.68 | 7.49 | 0.001 | A, B < C |
| DST | 5.03 ± 1.04 | 4.80 ± 1.27 | 5.18 ± 1.07 | 0.35 | 0.71 | —— |
| Visuospatial skill | ||||||
| ROCF | 24.98 ± 5.81 | 22.81 ± 7.90 | 25.83 ± 5.31 | 0.77 | 0.46 | —— |
| CDT4 | 3.52 ± 0.73 | 3.25 ± 0.82 | 3.53 ± 0.77 | 2.56 | 0.08 | —— |
| − | EOD | LOD | NC | ||||||
|---|---|---|---|---|---|---|---|---|---|
| r | p | q | r | p | q | r | p | q | |
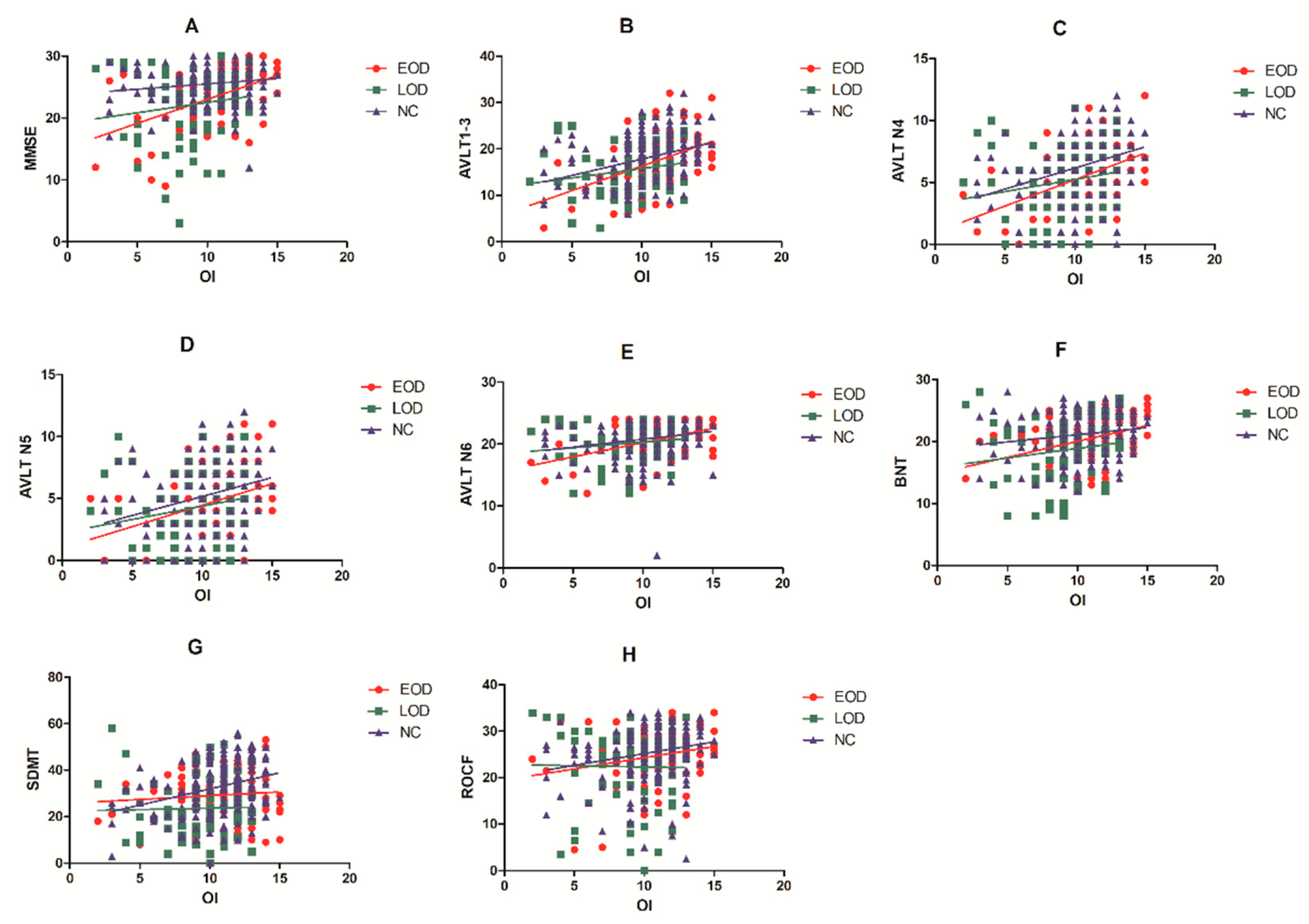
| MMSE | 0.385 | 0.001 | 0.008 | 0.095 | 0.453 | 0.906 | 0.030 | 0.692 | 0.738 |
| AVLT N1–3 | 0.442 | p < 0.001 | q < 0.001 | 0.123 | 0.368 | 0.981 | 0.286 | p < 0.001 | q < 0.001 |
| AVLT N4 | 0.396 | 0.002 | 0.008 | 0.070 | 0.606 | 0.693 | 0.299 | p < 0.001 | q < 0.001 |
| AVLT N5 | 0.238 | 0.065 | 0.130 | 0.091 | 0.507 | 0.737 | 0.203 | 0.008 | 0.032 |
| AVLT N6 | 0.391 | 0.002 | 0.008 | 0.019 | 0.892 | 0.892 | 0.175 | 0.022 | 0.059 |
| TMTA | 0.185 | 0.163 | 0.261 | 0.053 | 0.636 | 0.678 | 0.048 | 0.214 | 0.285 |
| TMTB | −0.008 | 0.950 | 0.950 | −0.142 | 0.306 | 1.000 | −0.062 | 0.423 | 0.483 |
| Stroop A | −0.269 | 0.034 | 0.078 | −0.251 | 0.065 | 1.000 | −0.181 | 0.018 | 0.058 |
| Stroop B | −0.198 | 0.123 | 0.219 | −0.091 | 0.515 | 0.687 | −0.100 | 0.197 | 0.287 |
| Stroop C | −0.022 | 0.868 | 0.992 | −0.078 | 0.578 | 0.711 | −0.021 | 0.788 | 0.788 |
| BNT | 0.381 | 0.002 | 0.008 | 0.145 | 0.286 | 1.000 | 0.115 | 0.135 | 0.240 |
| VFT | −0.015 | 0.906 | 0.966 | −0.093 | 0.496 | 0.794 | 0.130 | 0.091 | 0.182 |
| SDMT | 0.029 | 0.822 | 1.000 | −0.131 | 0.353 | 1.000 | 0.211 | 0.006 | 0.032 |
| DST | 0.162 | 0.209 | 0.304 | 0.205 | 0.131 | 1.000 | 0.087 | 0.274 | 0.337 |
| ROCF | 0.347 | 0.006 | 0.016 | −0.097 | 0.477 | 0.848 | 0.149 | 0.052 | 0.119 |
| CDT4 | 0.109 | 0.399 | 0.532 | 0.119 | 0.381 | 0.871 | 0.106 | 0.167 | 0.267 |
| R2 | Variables | B | β | t | p | 95% CI | |
|---|---|---|---|---|---|---|---|
| EOD | 0.220 | AVLT N1–3 | 0.209 | 0.469 | 4.805 | 0.001 | (0.123, 0.296) |
| NC | 0.168 | AVLT N1–3 | 0.144 | 0.276 | 3.707 | 0.001 | (0.068, 0.221) |
| SDMT | 0.049 | 0.211 | 2.838 | 0.005 | (0.015, 0.083) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Chen, B.; Zhong, X.; Zhang, M.; Wang, Q.; Zhou, H.; Wu, Z.; Hou, L.; Peng, Q.; Zhang, S.; et al. Differences in Odor Identification in Early-Onset and Late-Onset Depression. Brain Sci. 2022, 12, 276. https://doi.org/10.3390/brainsci12020276
Liu M, Chen B, Zhong X, Zhang M, Wang Q, Zhou H, Wu Z, Hou L, Peng Q, Zhang S, et al. Differences in Odor Identification in Early-Onset and Late-Onset Depression. Brain Sciences. 2022; 12(2):276. https://doi.org/10.3390/brainsci12020276
Chicago/Turabian StyleLiu, Meiling, Ben Chen, Xiaomei Zhong, Min Zhang, Qiang Wang, Huarong Zhou, Zhangying Wu, Le Hou, Qi Peng, Si Zhang, and et al. 2022. "Differences in Odor Identification in Early-Onset and Late-Onset Depression" Brain Sciences 12, no. 2: 276. https://doi.org/10.3390/brainsci12020276
APA StyleLiu, M., Chen, B., Zhong, X., Zhang, M., Wang, Q., Zhou, H., Wu, Z., Hou, L., Peng, Q., Zhang, S., Yang, M., Lin, G., & Ning, Y. (2022). Differences in Odor Identification in Early-Onset and Late-Onset Depression. Brain Sciences, 12(2), 276. https://doi.org/10.3390/brainsci12020276







