Clinical Cognitive Motor Dissociation: A Case Report Showing How Pitfalls Can Hinder Early Clinical Detection of Awareness
Abstract
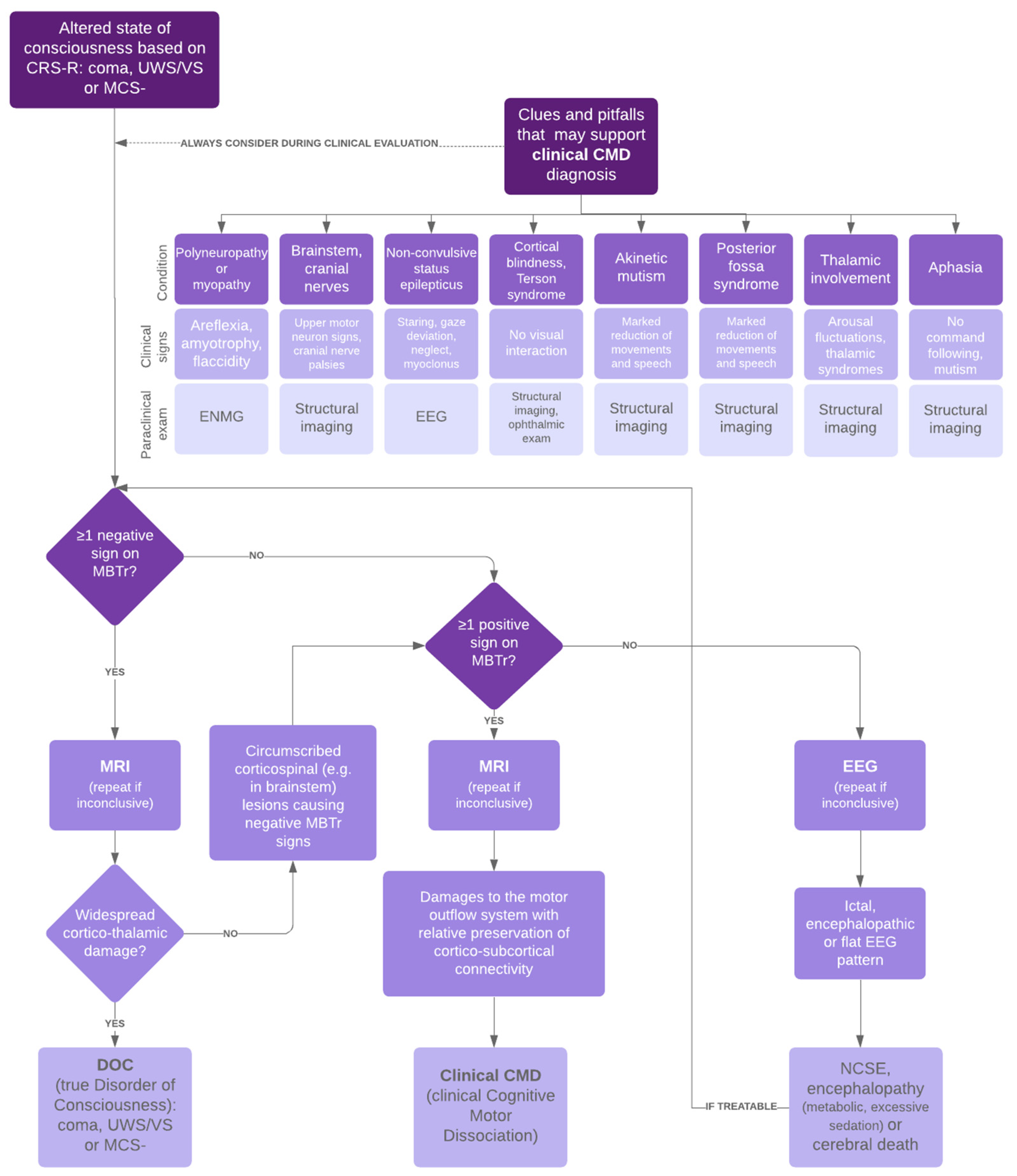
:1. Background
2. Case Presentation
2.1. History
2.2. Clinical Examination
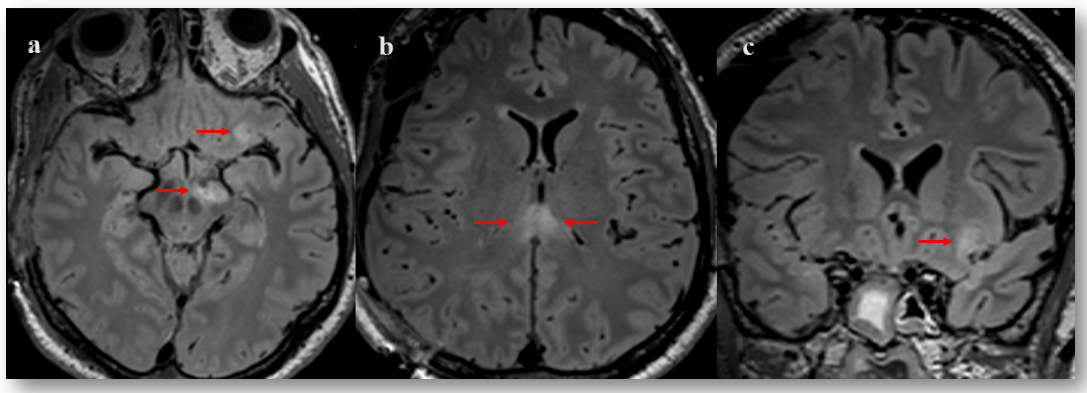
2.3. Pitfall Identification Process
2.4. Therapeutic Interventions, Outcomes at Discharge and Follow-Up
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Giacino, J.T.; Kalmar, K.; Whyte, J. The JFK Coma Recovery Scale-Revised: Measurement characteristics and diagnostic utility. Arch. Phys. Med. Rehabil. 2004, 85, 2020–2029. [Google Scholar] [CrossRef] [PubMed]
- Schiff, N.D. Cognitive Motor Dissociation Following Severe Brain Injuries. JAMA Neurol. 2015, 72, 1413. [Google Scholar] [CrossRef] [PubMed]
- Luauté, J.; Maucort-Boulch, D.; Tell, L.; Quelard, F.; Sarraf, T.; Iwaz, J.; Boisson, D.; Fischer, C. Long-term outcomes of chronic minimally conscious and vegetative states. Neurology 2010, 75, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Pincherle, A.; Jöhr, J.; Chatelle, C.; Pignat, J.-M.; Pasquier, R.D.; Ryvlin, P.; Oddo, M.; Diserens, K. Motor behavior unmasks residual cognition in disorders of consciousness. Ann. Neurol. 2019, 85, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Jöhr, J.; Halimi, F.; Pasquier, J.; Pincherle, A.; Schiff, N.; Diserens, K. Recovery in cognitive motor dissociation after severe brain injury: A cohort study. PLoS ONE 2020, 15, e0228474. [Google Scholar] [CrossRef] [PubMed]
- Pincherle, A.; Rossi, F.; Jöhr, J.; Dunet, V.; Ryvlin, P.; Oddo, M.; Schiff, N.; Diserens, K. Early discrimination of cognitive motor dissociation from disorders of consciousness: Pitfalls and clues. J. Neurol. 2021, 268, 178–188. [Google Scholar] [CrossRef]
- Plum, F.; Posner, J. The Diagnosis of Stupor and Coma; Davis: Philadelphia, PA, USA, 1980. [Google Scholar]
- Pozeg, P.; Jöhr, J.; Pincherle, A.; Marie, G.; Ryvlin, P.; Meuli, R.; Hagmann, P.; Diserens, K.; Dunet, V. Discriminating cognitive motor dissociation from disorders of consciousness using structural MRI. Neuroimage Clin. 2021, 30, 102651. [Google Scholar] [CrossRef] [PubMed]
- Claassen, J.; Doyle, K.; Matory, A.; Couch, C.; Burger, K.M.; Velazquez, A.; Okonkwo, J.U.; King, J.R.; Park, S.; Agarwal, S.; et al. Detection of Brain Activation in Unresponsive Patients with Acute Brain Injury. N. Engl. J. Med. 2019, 380, 2497–2505. [Google Scholar] [CrossRef]
- Schiff, N.D. Central thalamic contributions to arousal regulation and neurological disorders of consciousness. Ann. N. Y. Acad. Sci. 2008, 1129, 105–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Espejo, D.; Rossit, S.; Owen, A.M. A Thalamocortical Mechanism for the Absence of Overt Motor Behavior in Covertly Aware Patients. JAMA Neurol. 2015, 72, 1442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mega, M.S.; Cohenour, R.C. Akinetic mutism: Disconnection of frontal-subcortical circuits. Neuropsychiatry Neuropsychol. Behav. Neurol. 1997, 10, 254–259. [Google Scholar]
- Vergani, F.; Lacerda, L.; Martino, J.; Attems, J.; Morris, C.; Mitchell, P.; Thiebaut de Schotten, M.; Dell’Acqua, F. White matter connections of the supplementary motor area in humans. J. Neurol. Neurosurg. Psychiatry 2014, 85, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Schiff, N.D. Recovery of consciousness after brain injury: A mesocircuit hypothesis. Trends Neurosci. 2010, 33, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Z.S.; Reggente, N.; Lutkenhoff, E.; Owen, A.M.; Monti, M.M. Disentangling disorders of consciousness: Insights from diffusion tensor imaging and machine learning. Hum. Brain Mapp. 2017, 38, 431–443. [Google Scholar] [CrossRef]
- Latronico, N.; Bolton, C.F. Critical illness polyneuropathy and myopathy: A major cause of muscle weakness and paralysis. Lancet Neurol. 2011, 10, 931–941. [Google Scholar] [CrossRef]
| Clinical Assessment Post-Sedation Withdrawal to Discharge from Acute Rehabilitation Unit | |||
| Time after Onset (days) | CRS-R Diagnosis | CRS-R Subscale Scores | MBTr Classification |
| 9 | Coma | A0-V0-M0-O0-C0-Ar0 | |
| 11 | Coma | A0-V0-M0-O0-C0-Ar0 | |
| 16 | UWS | A0-V0-M0-O1-C0-Ar1 | |
| 23 | UWS | A1-V0-M0-O1-C0-Ar1 | |
| 24 | UWS | A1-V0-M2-O1-C0-Ar1 | Clinical CMD with 2 positive signs |
| 44 | MCS- | A2-V3-M2-O2-C0-Ar2 | |
| 51 | MCS+ | A3-V4-M5-O2-C1-Ar2 | |
| 58 | MCS+ | A3-V4-M5-O3-C1-Ar2 | |
| 65 | EMCS | A4-V5-M6-O3-C2-Ar2 | |
| Neurological examination and outcome at discharge | |||
| Neurological exam | |||
| Mental status | Cranial nerve Sensory system | Motor/Functional level | |
| Dysexecutive syndrome with loss of spontaneity | Oculomotricity preserved in all planes | Upper-right limb functional in distal part | |
| Speech and comprehension disorders | Absence of paresis or facial hypoesthesia | Upper-left limb improved in strength | |
| Attentional disturbances | Sensation coarsely preserved in 4 limbs | Right-lower limb functional | |
| Disorientation in space | Mobilization of left-lower limb limited | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jöhr, J.; Aureli, V.; Meyer, I.; Cossu, G.; Diserens, K. Clinical Cognitive Motor Dissociation: A Case Report Showing How Pitfalls Can Hinder Early Clinical Detection of Awareness. Brain Sci. 2022, 12, 157. https://doi.org/10.3390/brainsci12020157
Jöhr J, Aureli V, Meyer I, Cossu G, Diserens K. Clinical Cognitive Motor Dissociation: A Case Report Showing How Pitfalls Can Hinder Early Clinical Detection of Awareness. Brain Sciences. 2022; 12(2):157. https://doi.org/10.3390/brainsci12020157
Chicago/Turabian StyleJöhr, Jane, Viviana Aureli, Ivo Meyer, Giulia Cossu, and Karin Diserens. 2022. "Clinical Cognitive Motor Dissociation: A Case Report Showing How Pitfalls Can Hinder Early Clinical Detection of Awareness" Brain Sciences 12, no. 2: 157. https://doi.org/10.3390/brainsci12020157
APA StyleJöhr, J., Aureli, V., Meyer, I., Cossu, G., & Diserens, K. (2022). Clinical Cognitive Motor Dissociation: A Case Report Showing How Pitfalls Can Hinder Early Clinical Detection of Awareness. Brain Sciences, 12(2), 157. https://doi.org/10.3390/brainsci12020157






