An fNIRS Study of Applicability of the Unity–Diversity Model of Executive Functions in Preschoolers
Abstract
1. Introduction
1.1. Behavioral Study of the Three Components of EF
1.2. The Neural Correlates of Executive Function
1.3. The Unity–Diversity Framework of Executive Function
1.4. The Present Study
2. Materials and Methods
2.1. Participants
2.2. Behavioral Task
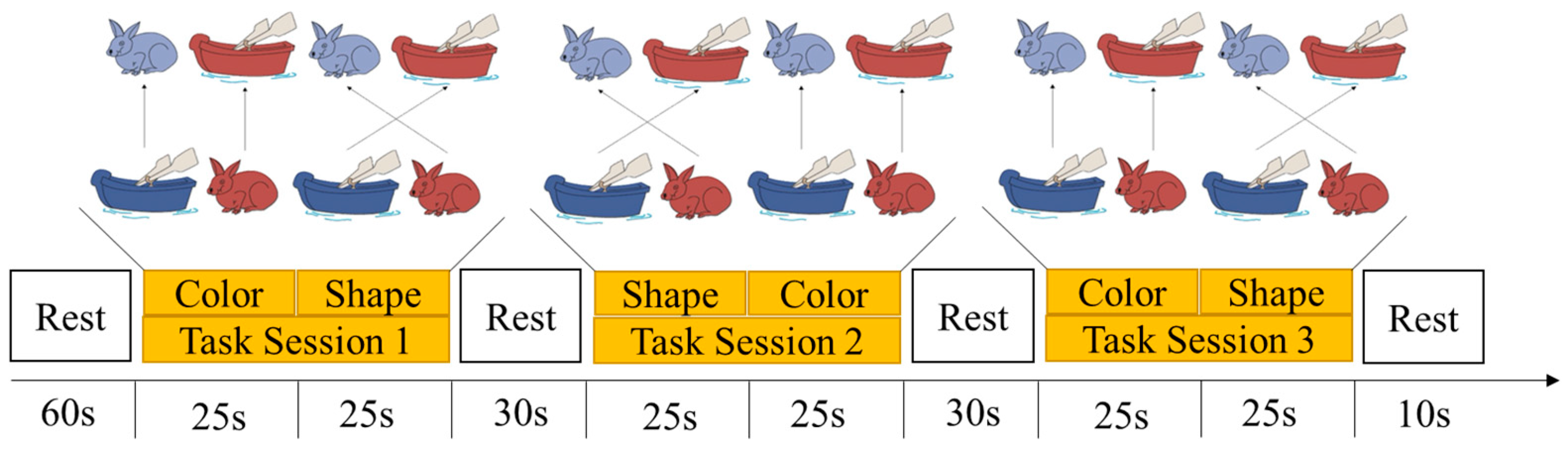
2.2.1. Dimensional Change Card Sort Task
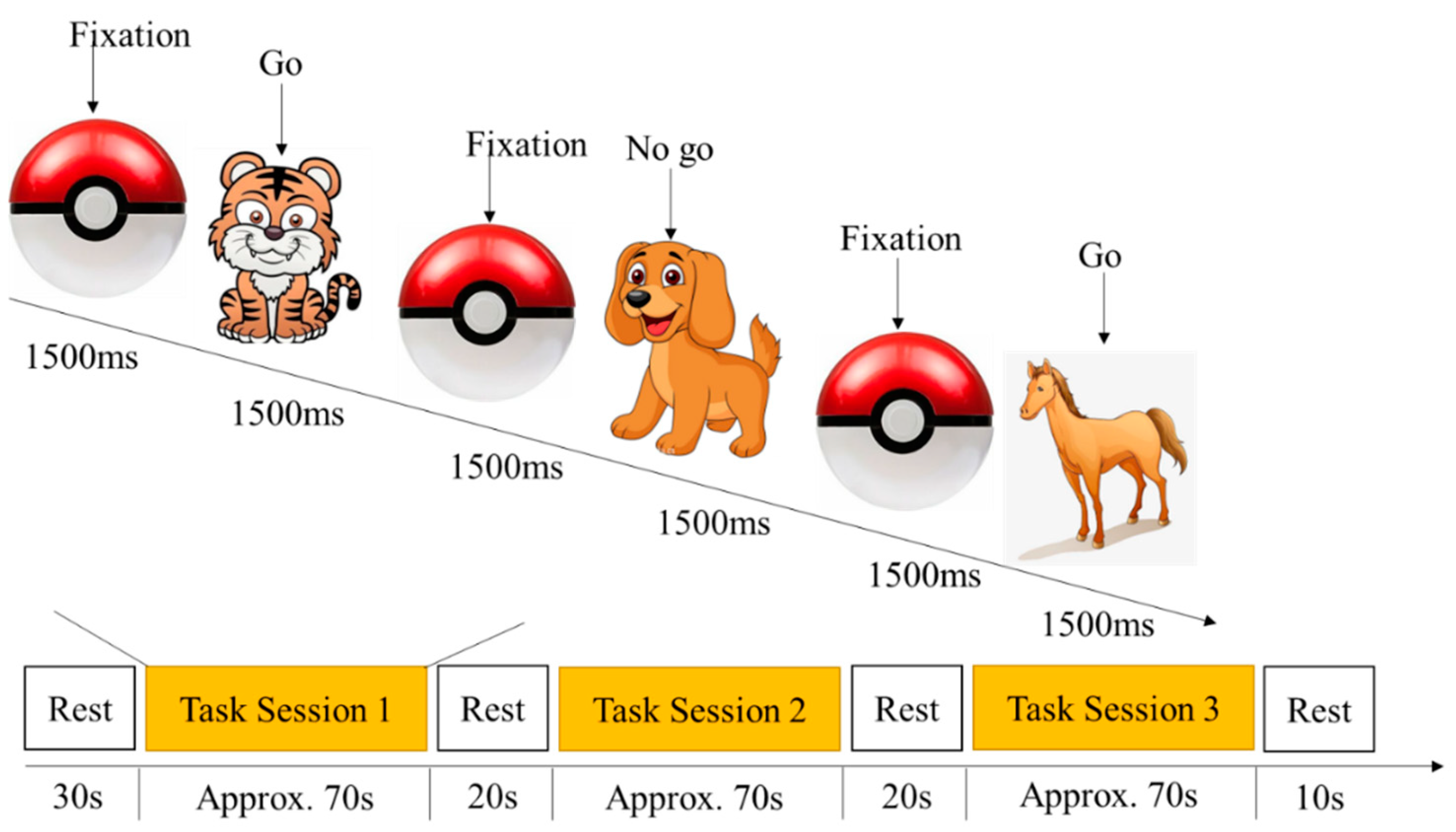
2.2.2. Go/No-Go Task
2.2.3. Missing Scan Task
2.3. Functional Near-Infrared Spectroscopy Recordings
2.4. Procedure
2.5. Analytic Plan
3. Results
3.1. Behavioral Results
3.2. The fNIRS Results
4. Discussion
4.1. Working Memory as the Common Executive Process of EF
4.2. Applicability of the Unity–Diversity Model
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zelazo, P.D.; Müller, U. Executive function in typical and atypical development. In Blackwell Handbook of Childhood Cognitive Development; Goswami, U., Ed.; Wiley-Blackwell: Malden, MA, USA, 2010; pp. 574–603. [Google Scholar]
- Griffin, J.A.; Freund, L.S.; McCARDLE, P.; DelCarmen-Wiggins, R.; Haydon, A. Introduction to executive function in preschool-age children. Exec. Funct. Presch. Child. Integr. Meas. Neurodev. Transl. Res. 2016, 3, 1–7. [Google Scholar]
- Wiebe, S.A.; Karbach, J. Development and plasticity of executive function across the life span. In Executive Function: Development Across the Life Span; Wiebe, S.A., Karbach, J., Eds.; Routledge: New York, NY, USA, 2018; pp. 1–7. [Google Scholar]
- Casey, B.J.; Tottenham, N.; Liston, C.; Durston, S. Imaging the developing brain: What have we learned about cognitive development? Trends Cogn. Sci. 2005, 9, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Moriguchi, Y. Neural mechanisms of executive function development during early childhood. In Executive Function: Development across the Life Span; Wiebe, S.A., Karbach, J., Eds.; Taylor & Francis: New York, NY, USA, 2017; pp. 75–90. [Google Scholar]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cogn. Psychol. 2000, 41, 49–100. [Google Scholar] [CrossRef] [PubMed]
- Miyake, A.; Friedman, N.P. The nature and organization of individual differences in executive functions: Four general conclusions. Curr. Dir. Psychol. Sci. 2012, 21, 8–14. [Google Scholar] [CrossRef]
- McKenna, R.; Rushe, T.; Woodcock, K.A. Informing the structure of executive function in children: A meta-analysis of functional neuroimaging data. Front. Hum. Neurosci. 2017, 11, 154. [Google Scholar] [CrossRef]
- Huizinga, M.; Dolan, C.V.; Van der Molen, M.W. Age-related change in executive function: Developmental trends and a latent variable analysis. Neuropsychologia 2006, 44, 2017–2036. [Google Scholar] [CrossRef]
- Wiebe, S.A.; Espy, K.A.; Charak, D. Using confirmatory factor analysis to understand executive control in preschool children: I. Latent structure. Dev. Psychol. 2008, 44, 575. [Google Scholar] [CrossRef]
- Willoughby, M.T.; Wirth, R.J.; Blair, C.B. Executive function in early childhood: Longitudinal measurement invariance and developmental change. Psychol. Assess. 2012, 24, 418. [Google Scholar] [CrossRef]
- Wiebe, S.A.; Sheffield, T.; Nelson, J.M.; Clark, C.A.C.; Chevalier, N.; Espy, K.A. The structure of executive function in 3-year-olds. J. Exp. Child Psychol. 2011, 108, 436–452. [Google Scholar] [CrossRef]
- Zelazo, P.D.; Blair, C.B.; Willoughby, M.T. Executive Function: Implications for Education; NCER 2017–2000; National Center for Education Research (NCER): Washington, DC, USA, 2016; p. 148.
- Miller, M.R.; Giesbrecht, G.F.; Müller, U.; McInerney, R.J.; Kerns, K.A. A latent variable approach to determining the structure of executive function in preschool children. J. Cogn. Dev. 2012, 13, 395–423. [Google Scholar] [CrossRef]
- Usai, M.C.; Viterbori, P.; Traverso, L.; De Franchis, V. Latent structure of executive function in five-and six-year-old children: A longitudinal study. Eur. J. Dev. Psychol. 2014, 11, 447–462. [Google Scholar] [CrossRef]
- Lee, K.; Bull, R.; Ho, R.M.H. Developmental changes in executive functioning. Child Dev. 2013, 84, 1933–1953. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, N.; Clark, C.A.C. Executive function in ealry and middle childhood. In Executive Function: Development across the Life Span; Wiebe, S.A., Karbach, J., Eds.; Routledge: New York, NY, USA, 2018; pp. 29–43. [Google Scholar]
- Karr, J.E.; Areshenkoff, C.N.; Rast, P.; Hofer, S.M.; Iverson, G.L.; Garcia-Barrera, M.A. The unity and diversity of executive functions: A systematic review and re-analysis of latent variable studies. Psychol. Bull. 2018, 144, 1147. [Google Scholar] [CrossRef] [PubMed]
- Garon, N.; Bryson, S.E.; Smith, I.M. Executive function in preschoolers: A review using an integrative framework. Psychol. Bull. 2008, 134, 31. [Google Scholar] [CrossRef]
- Reilly, S.E.; Downer, J.T.; Grimm, K.J. Developmental trajectories of executive functions from preschool to kindergarten. Dev. Sci. 2022, 25, e13236. [Google Scholar] [CrossRef]
- Zelazo, P.D. The Dimensional Change Card Sort (DCCS): A method of assessing executive function in children. Nat. Protoc. 2006, 1, 297–301. [Google Scholar] [CrossRef]
- Zelazo, P.D.; Frye, D.; Rapus, T. An age-related dissociation between knowing rules and using them. Cogn. Dev. 1996, 11, 37–63. [Google Scholar] [CrossRef]
- Li, H.; Wu, D.; Yang, J.; Xie, S.; Luo, J.; Chang, C. A Functional Near-Infrared Spectroscopy Examination of the Neural Correlates of Cognitive Shifting in Dimensional Change Card Sort Task. Front. Hum. Neurosci. 2021, 14, 561223. [Google Scholar] [CrossRef]
- Li, Q.; Liu, P.; Yan, N.; Feng, T. Executive Function Training Improves Emotional Competence for Preschool Children: The Roles of Inhibition Control and Working Memory. Front. Psychol. 2020, 11, 347. [Google Scholar] [CrossRef]
- Li, H.; Subrahmanyam, K.; Bai, X.; Xie, X.; Liu, T. Viewing fantastical events versus touching fantastical events: Short-term effects on children’s inhibitory control. Child Dev. 2018, 89, 48–57. [Google Scholar] [CrossRef]
- Mehnert, J.; Akhrif, A.; Telkemeyer, S.; Rossi, S.; Schmitz, C.H.; Steinbrink, J.; Wartenburger, I.; Obrig, H.; Neufang, S. Developmental changes in brain activation and functional connectivity during response inhibition in the early childhood brain. Brain Dev. 2013, 35, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Buss, A.T.; Fox, N.; Boas, D.A.; Spencer, J.P. Probing the early development of visual working memory capacity with functional near-infrared spectroscopy. Neuroimage 2014, 85, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Roman, A.S.; Pisoni, D.B.; Kronenberger, W.G. Assessment of working memory capacity in preschool children using the missing scan task. Infant Child Dev. 2014, 23, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Bull, R.; Espy, K.A.; Wiebe, S.A. Short-Term Memory, Working Memory, and Executive Functioning in Preschoolers: Longitudinal Predictors of Mathematical Achievement at Age 7 Years. Dev. Neuropsychol. 2008, 33, 205–228. [Google Scholar] [CrossRef] [PubMed]
- Zelazo, P.D.; Müller, U. The balance beam in the balance: Reflections on rules, relational complexity, and developmental processes. J. Exp. Child Psychol. 2002, 81, 458–465. [Google Scholar] [CrossRef]
- Aron, A.R.; Robbins, T.W.; Poldrack, R.A. Right inferior frontal cortex: Addressing the rebuttals. Front. Hum. Neurosci. 2014, 8, 905. [Google Scholar] [CrossRef]
- Wager, T.D.; Smith, E.E. Neuroimaging studies of working memory. Cogn. Affect. Behav. Neurosci. 2003, 3, 255–274. [Google Scholar] [CrossRef]
- Konishi, S.; Nakajima, K.; Uchida, I.; Kameyama, M.; Nakahara, K.; Sekihara, K.; Miyashita, Y. Transient activation of inferior prefrontal cortex during cognitive set shifting. Nat. Neurosci. 1998, 1, 80–84. [Google Scholar] [CrossRef]
- Moriguchi, Y.; Hiraki, K. Longitudinal development of prefrontal function during early childhood. Dev. Cogn. Neurosci. 2011, 1, 153–162. [Google Scholar] [CrossRef]
- Xie, S.; Gong, C.; Lu, J.; Li, H.; Wu, D.; Chi, X.; Chang, C. Enhancing Chinese preschoolers’ executive function via mindfulness training: An fNIRS study. Front. Behav. Neurosci. 2022, 16, 961797. [Google Scholar] [CrossRef]
- Ezekiel, F.; Bosma, R.; Morton, J.B. Dimensional change card sort performance associated with age-related differences in functional connectivity of lateral prefrontal cortex. Dev. Cogn. Neurosci. 2013, 5, 40–50. [Google Scholar] [CrossRef]
- Zhou, X.; Planalp, E.M.; Heinrich, L.; Pletcher, C.; DiPiero, M.; Alexander, A.L.; Litovsky, R.Y.; Dean, D.C., III. Inhibitory Control in Children 4–10 Years of Age: Evidence From Functional Near-Infrared Spectroscopy Task-Based Observations. Front. Hum. Neurosci. 2021, 15, 798358. [Google Scholar] [CrossRef] [PubMed]
- Nee, D.E.; Brown, J.W. Dissociable frontal–striatal and frontal–parietal networks involved in updating hierarchical contexts in working memory. Cereb. Cortex 2013, 23, 2146–2158. [Google Scholar] [CrossRef] [PubMed]
- Rottschy, C.; Langner, R.; Dogan, I.; Reetz, K.; Laird, A.R.; Schulz, J.B.; Fox, P.T.; Eickhoff, S.B. Modelling neural correlates of working memory: A coordinate-based meta-analysis. Neuroimage 2012, 60, 830–846. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.D.; Perlstein, W.M.; Braver, T.S.; Nystrom, L.E.; Noll, D.C.; Jonides, J.; Smith, E.E. Temporal dynamics of brain activation during a working memory task. Nature 1997, 386, 604–608. [Google Scholar] [CrossRef]
- Perlman, S.B.; Huppert, T.J.; Luna, B. Functional near-infrared spectroscopy evidence for development of prefrontal engagement in working memory in early through middle childhood. Cereb. Cortex 2016, 26, 2790–2799. [Google Scholar] [CrossRef]
- Lenartowicz, A.; Kalar, D.J.; Congdon, E.; Poldrack, R.A. Towards an ontology of cognitive control. Top. Cogn. Sci. 2010, 2, 678–692. [Google Scholar] [CrossRef]
- Herd, S.A.; Hazy, T.E.; Chatham, C.H.; Brant, A.M.; Friedman, N.P. A neural network model of individual differences in task switching abilities. Neuropsychologia 2014, 62, 375–389. [Google Scholar] [CrossRef]
- Niendam, T.A.; Laird, A.R.; Ray, K.L.; Dean, Y.M.; Glahn, D.C.; Carter, C.S. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn. Affect. Behav. Neurosci. 2012, 12, 241–268. [Google Scholar] [CrossRef]
- Baker, J.M.; Bruno, J.L.; Gundran, A.; Hosseini, S.M.H.; Reiss, A.L. fNIRS measurement of cortical activation and functional connectivity during a visuospatial working memory task. PLoS ONE 2018, 13, e0201486. [Google Scholar]
- Morton, J.B.; Bosma, R.; Ansari, D. Age-related changes in brain activation associated with dimensional shifts of attention: An fMRI study. Neuroimage 2009, 46, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.W.; Foxe, J.J.; Molholm, S. Neuro-oscillatory mechanisms of intersensory selective attention and task switching in school-aged children, adolescents and young adults. Dev. Sci. 2016, 19, 469–487. [Google Scholar] [CrossRef] [PubMed]
- McKay, C.; Wijeakumar, S.; Rafetseder, E.; Shing, Y.L. Disentangling age and schooling effects on inhibitory control development: An fNIRS investigation. Dev. Sci. 2021, 25, e13205. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Fox, S.; Blasi, A.; Elwell, C.E. Illuminating the developing brain: The past, present and future of functional near infrared spectroscopy. Neurosci. Biobehav. Rev. 2010, 34, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Wu, D.D.; Yang, J.F.; Luo, J.T.; Chang, C.Q.; Li, H. An fNIRS examination of executive function in bilingual young children. Int. J. Biling. 2021, 25, 516–530. [Google Scholar] [CrossRef]
- Moriguchi, Y. Relationship between cool and hot executive function in young children: A near-infrared spectroscopy study. Dev. Sci. 2022, 25, e13165. [Google Scholar] [CrossRef]
- Lahat, A.; Todd, R.; Mahy, C.E.V.; Lau, K.; Zelazo, P.D. Neurophysiological correlates of executive function: A comparison of European-Canadian and Chinese-Canadian 5-year-olds. Front. Hum. Neurosci. 2010, 3, 72. [Google Scholar] [CrossRef]
- Wiebe, S.A.; Sheffield, T.D.; Espy, K.A. Separating the fish from the sharks: A longitudinal study of preschool response inhibition. Child Dev. 2012, 83, 1245–1261. [Google Scholar] [CrossRef]
- Gu, Y.; Miao, S.; Han, J.; Zeng, K.; Ouyang, G.; Yang, J.; Li, X. Complexity analysis of fNIRS signals in ADHD children during working memory task. Sci. Rep. 2017, 7, 829. [Google Scholar] [CrossRef]
- Schecklmann, M.; Romanos, M.; Bretscher, F.; Plichta, M.M.; Warnke, A.; Fallgatter, A.J. Prefrontal oxygenation during working memory in ADHD. J. Psychiatr. Res. 2010, 44, 621–628. [Google Scholar] [CrossRef]
- Duncan, A.; Meek, J.H.; Clemence, M.; Elwell, C.E.; Fallon, P.; Tyszczuk, L.; Cope, M.; Delpy, D.T. Measurement of cranial optical path length as a function of age using phase resolved near infrared spectroscopy. Pediatr. Res. 1996, 39, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Strangman, G.; Culver, J.P.; Thompson, J.H.; Boas, D.A. A quantitative comparison of simultaneous BOLD fMRI and NIRS recordings during functional brain activation. Neuroimage 2002, 17, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, Y. Functional near-infrared optical imaging: Utility and limitations in human brain mapping. Psychophysiology 2003, 40, 511–520. [Google Scholar] [CrossRef]
- Plichta, M.M.; Herrmann, M.J.; Baehne, C.G.; Ehlis, A.-C.; Richter, M.M.; Pauli, P.; Fallgatter, A.J. Event-related functional near-infrared spectroscopy (fNIRS): Are the measurements reliable? Neuroimage 2006, 31, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Brigadoi, S.; Ceccherini, L.; Cutini, S.; Scarpa, F.; Scatturin, P.; Selb, J.; Gagnon, L.; Boas, D.A.; Cooper, R.J. Motion artifacts in functional near-infrared spectroscopy: A comparison of motion correction techniques applied to real cognitive data. Neuroimage 2014, 85, 181–191. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, Z.; Zhao, C.; Duan, L.; Gong, Y.; Li, Z.; Zhu, C. NIRS-KIT: A MATLAB toolbox for both resting-state and task fNIRS data analysis. Neurophotonics 2021, 8, 10802. [Google Scholar] [CrossRef] [PubMed]
- Fishburn, F.A.; Ludlum, R.S.; Vaidya, C.J.; Medvedev, A.V. Temporal derivative distribution repair (TDDR): A motion correction method for fNIRS. Neuroimage 2019, 184, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Pinti, P.; Scholkmann, F.; Hamilton, A.; Burgess, P.; Tachtsidis, I. Current status and issues regarding pre-processing of fNIRS neuroimaging data: An investigation of diverse signal filtering methods within a general linear model framework. Front. Hum. Neurosci. 2019, 12, 505. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Wang, Y.; Liao, Y.; Li, J.; Zhang, X.; Gao, Z.; Shen, M.; He, J. Development of information integration in the visual working memory of preschoolers. Child Dev. 2022, 93, 1793–1803. [Google Scholar] [CrossRef]
- Diamond, A. Executive functions. Annu. Rev. Psychol. 2013, 64, 135. [Google Scholar] [CrossRef]
- Garon, N.; Smith, I.M.; Bryson, S.E. A novel executive function battery for preschoolers: Sensitivity to age differences. Child Neuropsychol. 2014, 20, 713–736. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M.C.; Amso, D.; Anderson, L.C.; Diamond, A. Development of cognitive control and executive functions from 4 to 13 years: Evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia 2006, 44, 2037–2078. [Google Scholar] [CrossRef]
- Zanto, T.P.; Rubens, M.T.; Thangavel, A.; Gazzaley, A. Causal role of the prefrontal cortex in top-down modulation of visual processing and working memory. Nat. Neurosci. 2011, 14, 656–661. [Google Scholar] [CrossRef]
- Munakata, Y.; Herd, S.A.; Chatham, C.H.; Depue, B.E.; Banich, M.T.; O’Reilly, R.C. A unified framework for inhibitory control. Trends Cogn. Sci. 2011, 15, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Engle, R.W.; Kane, M.J. Executive attention, working memory capacity, and a two-factor theory of cognitive control. In The Psychology of Learning and Motivation: Advances in Research and Theory; Elsevier Science: Amsterdam, The Netherlands, 2004; Volume 44, pp. 145–199. [Google Scholar]
- Wais, P.E.; Gazzaley, A. The impact of auditory distraction on retrieval of visual memories. Psychon. Bull. Rev. 2011, 18, 1090–1097. [Google Scholar] [CrossRef] [PubMed]
- Cowan, N. Working Memory Maturation: Can We Get at the Essence of Cognitive Growth? Perspect. Psychol. Sci. 2016, 11, 239–264. [Google Scholar] [CrossRef]
- Demetriou, A.; Kazali, E.; Kazi, S.; Spanoudis, G. Cognition and cognizance in preschool predict school achievement in primary school. Cogn. Dev. 2020, 54, 100872. [Google Scholar] [CrossRef]
- Tourva, A.; Spanoudis, G.; Demetriou, A. Cognitive correlates of developing intelligence: The contribution of working memory, processing speed and attention. Intelligence 2016, 54, 136–146. [Google Scholar] [CrossRef]
- Dosenbach, N.U.F.; Fair, D.A.; Cohen, A.L.; Schlaggar, B.L.; Petersen, S.E. A dual-networks architecture of top-down control. Trends Cogn. Sci. 2008, 12, 99–105. [Google Scholar] [CrossRef]


| Task | M (%) (SD) | 1 | 2 | 3 |
|---|---|---|---|---|
| 1. DCCS | 0.97 (0.00) | - | ||
| 2. Go/No-Go | 0.93 (0.01) | 0.13 | - | |
| 3. Missing Scan | 0.62 (0.05) | 0.26 * | 0.53 *** | - |
| ROI | DCCS | Go/No-Go | Missing Scan |
|---|---|---|---|
| left VLPFC | −0.02 (0.08) | −0.01 (0.04) | 0.01 (0.06) |
| left VLPFC | 0.01 (0.13) | 0.03 (0.07) | −0.00 (0.01) |
| left DLPFC | 0.00 (0.11) | −0.00 (015) | 0.01 (0.03) |
| left DLPFC | −0.00 (0.12) | −0.06 (0.04) | 0.03 (0.07) |
| left PSFC | −0.08 (0.18) | −0.03 (0.07) | 0.06 (0.04) |
| left PSFC | −0.09 (0.13) | −0.07 (0.05) | −0.01 (0.05) |
| left TC | −0.03 (0.17) | −0.01 (0.01) | 0.00 (0.04) |
| left TC | −0.05 (0.12) | −0.14 (0.05) | 0.03 (0.03) |
| MFPC | −0.02 (0.13) | −0.01 (0.04) | 0.02 (0.02) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, S.; Gong, C.; Lu, J.; Zhang, H.; Wu, D.; Chi, X.; Li, H.; Chang, C. An fNIRS Study of Applicability of the Unity–Diversity Model of Executive Functions in Preschoolers. Brain Sci. 2022, 12, 1722. https://doi.org/10.3390/brainsci12121722
Xie S, Gong C, Lu J, Zhang H, Wu D, Chi X, Li H, Chang C. An fNIRS Study of Applicability of the Unity–Diversity Model of Executive Functions in Preschoolers. Brain Sciences. 2022; 12(12):1722. https://doi.org/10.3390/brainsci12121722
Chicago/Turabian StyleXie, Sha, Chaohui Gong, Jiahao Lu, Hao Zhang, Dandan Wu, Xinli Chi, Hui Li, and Chunqi Chang. 2022. "An fNIRS Study of Applicability of the Unity–Diversity Model of Executive Functions in Preschoolers" Brain Sciences 12, no. 12: 1722. https://doi.org/10.3390/brainsci12121722
APA StyleXie, S., Gong, C., Lu, J., Zhang, H., Wu, D., Chi, X., Li, H., & Chang, C. (2022). An fNIRS Study of Applicability of the Unity–Diversity Model of Executive Functions in Preschoolers. Brain Sciences, 12(12), 1722. https://doi.org/10.3390/brainsci12121722







