Sleep Deprivation-Induced Changes in Baseline Brain Activity and Vigilant Attention Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Methods
2.3. Psychomotor Vigilance Task (PVT)
2.4. Electrophysiological Recordings and EEG Data Analysis
2.5. Event-Related Potentials
2.6. Pre-Stimulus, Baseline Activity
2.7. Power Spectrum Density (PSD) of the Baseline Activity
2.8. Statistical Analysis
3. Results
3.1. Descriptive Sleep Data
3.2. Vigilant Attention Performance
3.3. Pre-Stimulus, Baseline Activity
3.4. Pre-Stimulus, Baseline Activity and Behavioral Alertness
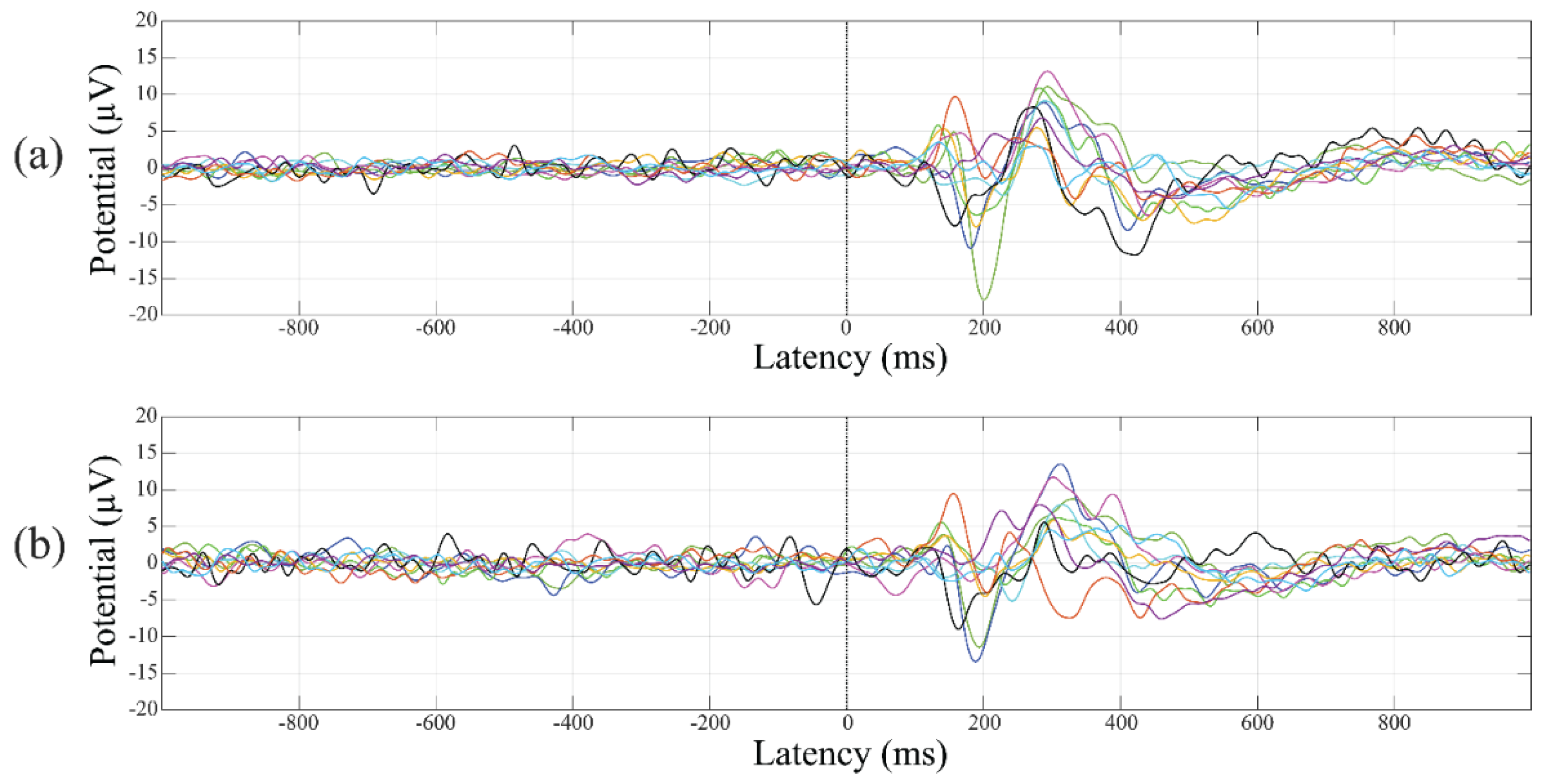
3.5. Event-Related Potentials
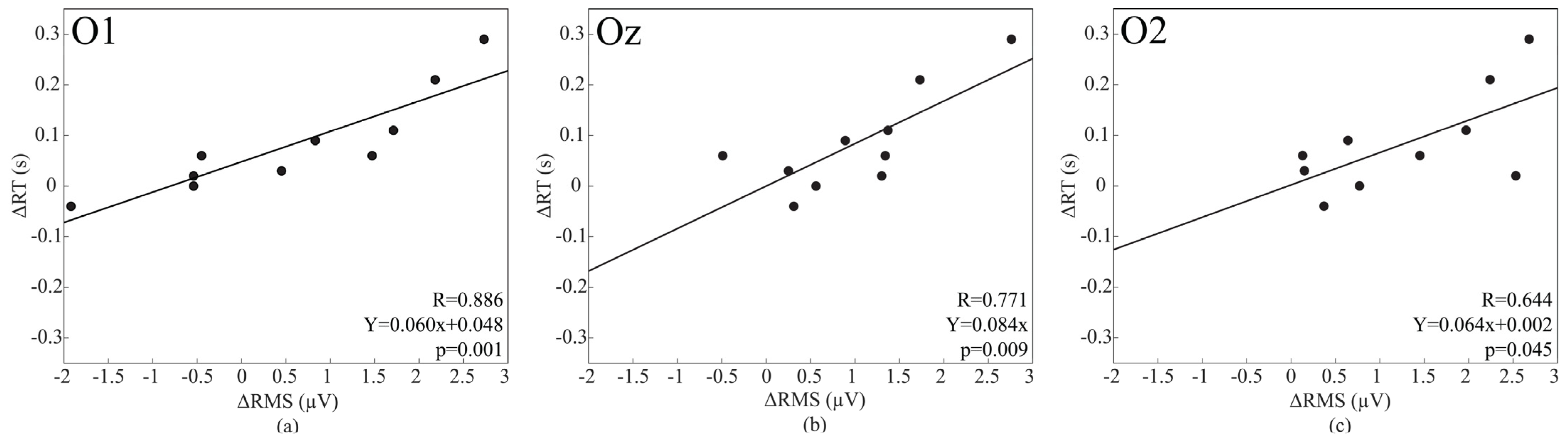
3.6. Correlation between Baseline Activity and ERPs Values
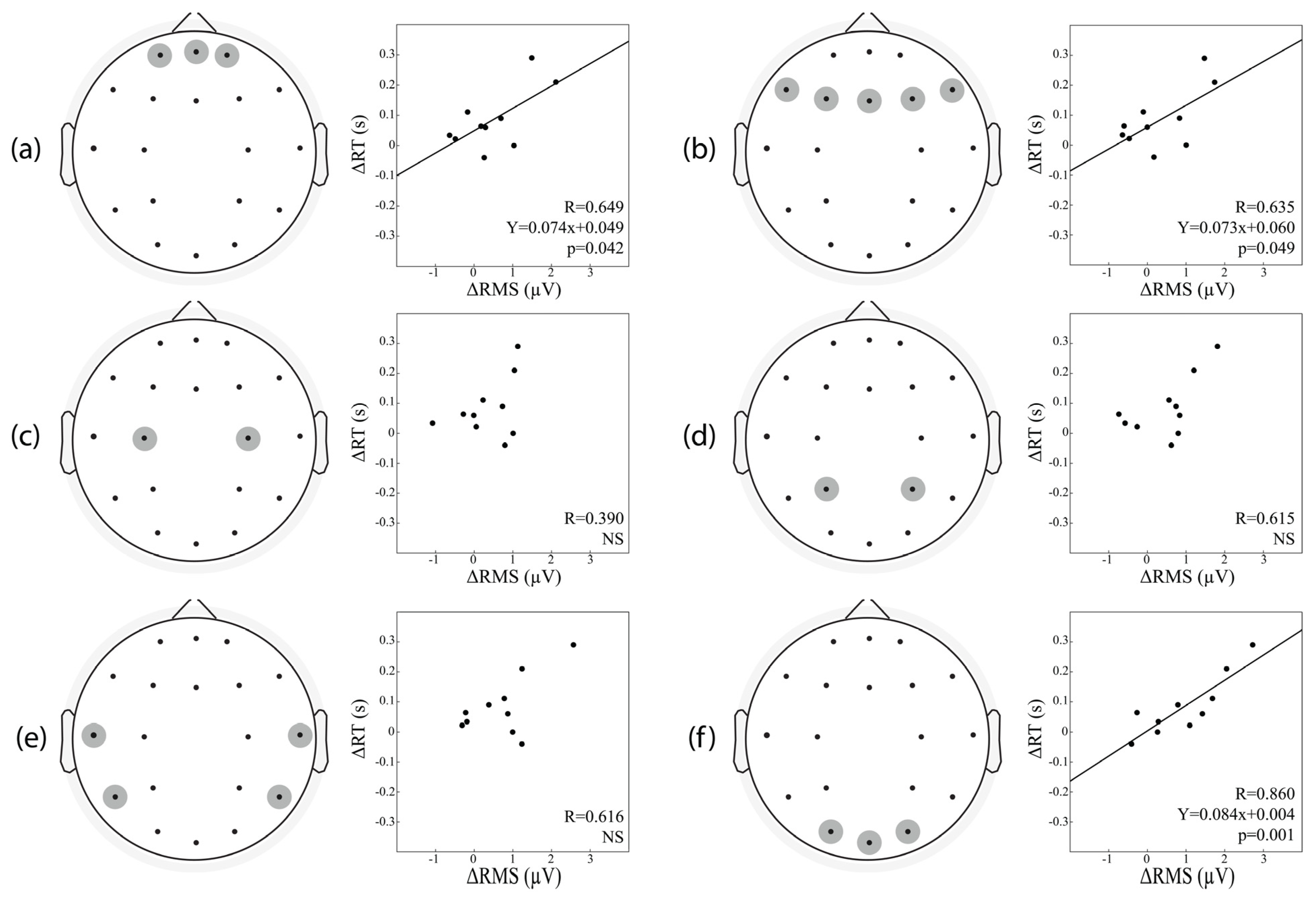
3.7. Correlation between Changes in the Baseline Activity and Those in Vigilant Attention Performance
3.8. PSD of the Baseline Activity
4. Discussion
4.1. Vigilant Attention Impairment
4.2. Event-Related Potentials Changes
4.3. Baseline Activity Changes
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clark, I.A.; Maguire, E.A. Do Questionnaires Reflect Their Purported Cognitive Functions? Cognition 2020, 195, 104114. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.S.; Trezza, B.M.; Busse, A.L.; Jacob Filho, W. Use of Computerized Tests to Assess the Cognitive Impact of Interventions in the Elderly. Dement. Neuropsychol. 2014, 8, 107–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kane, R.L.; Kay, G.G. Computerized Assessment in Neuropsychology: A Review of Tests and Test Batteries. Neuropsychol. Rev. 1992, 3, 1–117. [Google Scholar] [CrossRef]
- Rabbi, A.F.; Ivanca, K.; Putnam, A.V.; Musa, A.; Thaden, C.B.; Fazel-Rezai, R. Human Performance Evaluation Based on EEG Signal Analysis: A Prospective Review. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2009, 2009, 1879–1882. [Google Scholar] [CrossRef] [PubMed]
- Alhola, P.; Polo-Kantola, P. Sleep Deprivation: Impact on Cognitive Performance. Neuropsychiatr. Dis. Treat. 2007, 3, 553–567. [Google Scholar]
- Ward, L.M. Synchronous Neural Oscillations and Cognitive Processes. Trends Cogn. Sci. 2003, 7, 553–559. [Google Scholar] [CrossRef]
- Patel, S.H.; Azzam, P.N. Characterization of N200 and P300: Selected Studies of the Event-Related Potential. Int. J. Med. Sci. 2005, 2, 147–154. [Google Scholar] [CrossRef] [Green Version]
- Lijffijt, M.; Lane, S.D.; Meier, S.L.; Boutros, N.N.; Burroughs, S.; Steinberg, J.L.; Moeller, F.G.; Swann, A.C. P50, N100, and P200 Sensory Gating: Relationships with Behavioral Inhibition, Attention, and Working Memory. Psychophysiology 2009, 46, 1059. [Google Scholar] [CrossRef] [Green Version]
- Hillyard, S.A.; Hink, R.F.; Schwent, V.L.; Picton, T.W. Electrical Signs of Selective Attention in the Human Brain. Science 1973, 182, 177–180. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Zhou, R.; Fu, L. Working Memory Updating Function Training Influenced Brain Activity. PLoS ONE 2013, 8, e71063. [Google Scholar] [CrossRef]
- Lee, E.-Y.; Cowan, N.; Vogel, E.K.; Rolan, T.; Valle-Inclán, F.; Hackley, S.A. Visual Working Memory Deficits in Patients with Parkinson’s Disease Are Due to Both Reduced Storage Capacity and Impaired Ability to Filter out Irrelevant Information. Brain 2010, 133, 2677–2689. [Google Scholar] [CrossRef] [PubMed]
- Näätänen, R.; Picton, T. The N1 Wave of the Human Electric and Magnetic Response to Sound: A Review and an Analysis of the Component Structure. Psychophysiology 1987, 24, 375–425. [Google Scholar] [CrossRef] [PubMed]
- Perrault, N.; Picton, T.W. Event-Related Potentials Recorded from the Scalp and Nasopharynx. I. N1 and P2. Electroencephalogr. Clin. Neurophysiol. 1984, 59, 177–194. [Google Scholar] [CrossRef] [PubMed]
- Yurgil, K.A.; Golob, E.J. Cortical Potentials in an Auditory Oddball Task Reflect Individual Differences in Working Memory Capacity. Psychophysiology 2013, 50, 1263–1274. [Google Scholar] [CrossRef] [Green Version]
- Polich, J. Updating P300: An Integrative Theory of P3a and P3b. Clinical. Neurophysiol. 2007, 118, 2128–2148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Dinteren, R.; Arns, M.; Jongsma, M.L.A.; Kessels, R.P.C. P300 Development across the Lifespan: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e87347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patat, A.; Rosenzweig, P.; Enslen, M.; Trocherie, S.; Miget, N.; Bozon, M.-C.; Allain, H.; Gandon, J.-M. Effects of a New Slow Release Formulation of Caffeine on EEG, Psychomotor and Cognitive Functions in Sleep-Deprived Subjects. Hum. Psychopharmacol. 2000, 15, 153–170. [Google Scholar] [CrossRef]
- Corsi-Cabrera, M.; Sánchez, A.I.; del-Río-Portilla, Y.; Villanueva, Y.; Pérez-Garci, E. Effect of 38 h of Total Sleep Deprivation on the Waking EEG in Women: Sex Differences. Int. J. Psychophysiol. 2003, 50, 213–224. [Google Scholar] [CrossRef]
- Ferreira, C.; Deslandes, A.; Moraes, H.; Cagy, M.; Pompeu, F.; Basile, L.F.; Piedade, R.; Ribeiro, P. Electroencephalographic Changes after One Night of Sleep Deprivation. Arq. Neuropsiquiatr. 2006, 64, 388–393. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, Q.; Li, J.; Chen, Y.; Shao, S.; Xiao, Y. Decreased Resting-State Alpha-Band Activation and Functional Connectivity after Sleep Deprivation. Sci. Rep. 2021, 11, 484. [Google Scholar] [CrossRef]
- Hung, C.-S.; Sarasso, S.; Ferrarelli, F.; Riedner, B.; Ghilardi, M.F.; Cirelli, C.; Tononi, G. Local Experience-Dependent Changes in the Wake EEG after Prolonged Wakefulness. Sleep 2013, 36, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Kim, L.; Kim, Y.-K.; Suh, K.-Y.; Han, J.; Park, M.-K.; Park, K.-W.; Lee, D.-H. Auditory Event-Related Potentials and Psychological Changes during Sleep Deprivation. Neuropsychobiology 2004, 50, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, A.; De Koninck, J.; Campbell, K.B. Total Sleep Deprivation and Novelty Processing: Implications for Frontal Lobe Functioning. Clin. Neurophysiol. 2005, 116, 211–222. [Google Scholar] [CrossRef]
- Zukerman, G.; Goldstein, A.; Babkoff, H. The Effect of 24-40 Hours of Sleep Deprivation on the P300 Response to Auditory Target Stimuli. Aviat. Space Environ. Med. 2007, 78, B216–B223. [Google Scholar]
- Ray, K.; Chatterjee, A.; Panjwani, U.; Kumar, S.; Sahu, S.; Ghosh, S.; Thakur, L.; Anand, J.P. Modafinil Improves Event Related Potentials P300 and Contingent Negative Variation after 24 h Sleep Deprivation. Life Sci. 2012, 91, 94–99. [Google Scholar] [CrossRef]
- Zhang, L.; Shao, Y.; Liu, Z.; Li, C.; Chen, Y.; Zhou, Q. Decreased Information Replacement of Working Memory After Sleep Deprivation: Evidence From an Event-Related Potential Study. Front. Neurosci. 2019, 13, 408. [Google Scholar] [CrossRef]
- Peng, Z.; Dai, C.; Ba, Y.; Zhang, L.; Shao, Y.; Tian, J. Effect of Sleep Deprivation on the Working Memory-Related N2-P3 Components of the Event-Related Potential Waveform. Front. Neurosci. 2020, 14, 469. [Google Scholar] [CrossRef]
- Strijkstra, A.M.; Beersma, D.G.M.; Drayer, B.; Halbesma, N.; Daan, S. Subjective Sleepiness Correlates Negatively with Global Alpha (8-12 Hz) and Positively with Central Frontal Theta (4–8 Hz) Frequencies in the Human Resting Awake Electroencephalogram. Neurosci. Lett. 2003, 340, 17–20. [Google Scholar] [CrossRef]
- Vyazovskiy, V.V.; Tobler, I. Theta Activity in the Waking EEG Is a Marker of Sleep Propensity in the Rat. Brain Res. 2005, 1050, 64–71. [Google Scholar] [CrossRef]
- Lee, H.-J.; Kim, L.; Suh, K.-Y. Cognitive Deterioration and Changes of P300 during Total Sleep Deprivation. Psychiatry Clin. Neurosci. 2003, 57, 490–496. [Google Scholar] [CrossRef]
- Klimesch, W. EEG Alpha and Theta Oscillations Reflect Cognitive and Memory Performance: A Review and Analysis. Brain Res. Rev. 1999, 29, 169–195. [Google Scholar] [CrossRef] [PubMed]
- Gorgoni, M.; Ferlazzo, F.; Ferrara, M.; Moroni, F.; D’Atri, A.; Fanelli, S.; Gizzi Torriglia, I.; Lauri, G.; Marzano, C.; Rossini, P.M.; et al. Topographic Electroencephalogram Changes Associated with Psychomotor Vigilance Task Performance after Sleep Deprivation. Sleep Med. 2014, 15, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Posada-Quintero, H.F.; Reljin, N.; Bolkhovsky, J.B.; Orjuela-Cañón, A.D.; Chon, K.H. Brain Activity Correlates With Cognitive Performance Deterioration During Sleep Deprivation. Front. Neurosci. 2019, 13, 1001. [Google Scholar] [CrossRef] [PubMed]
- Dinges, D.F.; Powell, J.W. Microcomputer Analyses of Performance on a Portable, Simple Visual RT Task during Sustained Operations. Behav. Res. Methods Instrum. Comput. 1985, 17, 652–655. [Google Scholar] [CrossRef]
- Lim, J.; Dinges, D.F. Sleep Deprivation and Vigilant Attention. Ann. N. Y. Acad. Sci. 2008, 1129, 305–322. [Google Scholar] [CrossRef]
- Jewett, M.E.; Dijk, D.J.; Kronauer, R.E.; Dinges, D.F. Dose-Response Relationship between Sleep Duration and Human Psychomotor Vigilance and Subjective Alertness. Sleep 1999, 22, 171–179. [Google Scholar] [CrossRef] [Green Version]
- Corsi-Cabrera, M.; Arce, C.; Ramos, J.; Lorenzo, I.; Guevara, M.A. Time Course of Reaction Time and EEG While Performing a Vigilance Task during Total Sleep Deprivation. Sleep 1996, 19, 563–569. [Google Scholar] [CrossRef] [Green Version]
- Hoedlmoser, K.; Griessenberger, H.; Fellinger, R.; Freunberger, R.; Klimesch, W.; Gruber, W.; Schabus, M. Event-Related Activity and Phase Locking during a Psychomotor Vigilance Task over the Course of Sleep Deprivation. J. Sleep Res. 2011, 20, 377–385. [Google Scholar] [CrossRef]
- Khitrov, M.Y.; Laxminarayan, S.; Thorsley, D.; Ramakrishnan, S.; Rajaraman, S.; Wesensten, N.J.; Reifman, J. PC-PVT: A Platform for Psychomotor Vigilance Task Testing, Analysis, and Prediction. Behav. Res. Methods 2014, 46, 140–147. [Google Scholar] [CrossRef] [Green Version]
- Delorme, A.; Makeig, S. EEGLAB: An Open Source Toolbox for Analysis of Single-Trial EEG Dynamics Including Independent Component Analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Yi, C.; Jiang, Y.; Liao, Y.; Si, Y.; Dai, J.; Yao, D.; Zhang, Y.; Xu, P. Different Contexts in the Oddball Paradigm Induce Distinct Brain Networks in Generating the P300. Front. Hum. Neurosci. 2019, 12, 520. [Google Scholar] [CrossRef] [Green Version]
- Tramonti Fantozzi, M.P.; Artoni, F.; Di Galante, M.; Briscese, L.; De Cicco, V.; Bruschini, L.; d’Ascanio, P.; Manzoni, D.; Faraguna, U.; Carboncini, M.C. Effect of the Trigeminal Nerve Stimulation on Auditory Event-Related Potentials. Cereb. Cortex Commun. 2021, 2, tgab012. [Google Scholar] [CrossRef]
- Saito, H.; Yamazaki, H.; Matsuoka, H.; Matsumoto, K.; Numachi, Y.; Yoshida, S.; Ueno, T.; Sato, M. Visual Event-Related Potential in Mild Dementia of the Alzheimer’s Type. Psychiatry Clin. Neurosci. 2001, 55, 365–371. [Google Scholar] [CrossRef]
- Potts, G.F. An ERP Index of Task Relevance Evaluation of Visual Stimuli. Brain Cogn. 2004, 56, 5–13. [Google Scholar] [CrossRef]
- Breznitz, Z.; Meyler, A. Speed of Lower-Level Auditory and Visual Processing as a Basic Factor in Dyslexia: Electrophysiological Evidence. Brain Lang. 2003, 85, 166–184. [Google Scholar] [CrossRef]
- Anjana, Y.; Khaliq, F.; Vaney, N. Event-Related Potentials Study in Attention Deficit Hyperactivity Disorder. Funct. Neurol. 2010, 25, 87–92. [Google Scholar]
- Martinelli, V.; Locatelli, T.; Comi, G.; Lia, C.; Alberoni, M.; Bressi, S.; Rovaris, M.; Franceschi, M.; Canal, N. Pattern Visual Evoked Potential Mapping in Alzheimer’s Disease: Correlations with Visuospatial Impairment. Dementia 1996, 7, 63–68. [Google Scholar] [CrossRef]
- Wascher, E.; Verleger, R.; Vieregge, P.; Jaskowski, P.; Koch, S.; Kömpf, D. Responses to Cued Signals in Parkinson’s Disease. Distinguishing between Disorders of Cognition and of Activation. Brain 1997, 120 Pt 8, 1355–1375. [Google Scholar] [CrossRef]
- Leocani, L.; Gonzalez-Rosa, J.J.; Comi, G. Neurophysiological Correlates of Cognitive Disturbances in Multiple Sclerosis. Neurol. Sci. 2010, 31, S249–S253. [Google Scholar] [CrossRef]
- Scalise, A.; Desiato, M.T.; Gigli, G.L.; Romigi, A.; Tombini, M.; Marciani, M.G.; Izzi, F.; Placidi, F. Increasing Cortical Excitability: A Possible Explanation for the Proconvulsant Role of Sleep Deprivation. Sleep 2006, 29, 1595–1598. [Google Scholar] [CrossRef] [Green Version]
- De Gennaro, L.; Marzano, C.; Veniero, D.; Moroni, F.; Fratello, F.; Curcio, G.; Ferrara, M.; Ferlazzo, F.; Novelli, L.; Concetta Pellicciari, M.; et al. Neurophysiological Correlates of Sleepiness: A Combined TMS and EEG Study. Neuroimage 2007, 36, 1277–1287. [Google Scholar] [CrossRef]
- Civardi, C.; Boccagni, C.; Vicentini, R.; Bolamperti, L.; Tarletti, R.; Varrasi, C.; Monaco, F.; Cantello, R. Cortical Excitability and Sleep Deprivation: A Transcranial Magnetic Stimulation Study. J. Neurol. Neurosurg. Psychiatry 2001, 71, 809–812. [Google Scholar] [CrossRef]
- Kreuzer, P.; Langguth, B.; Popp, R.; Raster, R.; Busch, V.; Frank, E.; Hajak, G.; Landgrebe, M. Reduced Intra-Cortical Inhibition after Sleep Deprivation: A Transcranial Magnetic Stimulation Study. Neurosci. Lett. 2011, 493, 63–66. [Google Scholar] [CrossRef]
- Huber, R.; Mäki, H.; Rosanova, M.; Casarotto, S.; Canali, P.; Casali, A.G.; Tononi, G.; Massimini, M. Human Cortical Excitability Increases with Time Awake. Cereb. Cortex 2013, 23, 332–338. [Google Scholar] [CrossRef] [Green Version]
- Hupfeld, K.E.; Swanson, C.W.; Fling, B.W.; Seidler, R.D. TMS-Induced Silent Periods: A Review of Methods and Call for Consistency. J. Neurosci. Methods 2020, 346, 108950. [Google Scholar] [CrossRef]
- Vyazovskiy, V.V.; Olcese, U.; Cirelli, C.; Tononi, G. Prolonged Wakefulness Alters Neuronal Responsiveness to Local Electrical Stimulation of the Neocortex in Awake Rats. J. Sleep Res. 2013, 22, 239–250. [Google Scholar] [CrossRef] [Green Version]
- Bliss, T.V.; Lomo, T. Long-Lasting Potentiation of Synaptic Transmission in the Dentate Area of the Anaesthetized Rabbit Following Stimulation of the Perforant Path. J. Physiol. 1973, 232, 331–356. [Google Scholar] [CrossRef]
- Vyazovskiy, V.V.; Cirelli, C.; Pfister-Genskow, M.; Faraguna, U.; Tononi, G. Molecular and Electrophysiological Evidence for Net Synaptic Potentiation in Wake and Depression in Sleep. Nat. Neurosci. 2008, 11, 200–208. [Google Scholar] [CrossRef]
- Drummond, S.P.; Brown, G.G.; Gillin, J.C.; Stricker, J.L.; Wong, E.C.; Buxton, R.B. Altered Brain Response to Verbal Learning Following Sleep Deprivation. Nature 2000, 403, 655–657. [Google Scholar] [CrossRef]
- Chee, M.W.L.; Choo, W.C. Functional Imaging of Working Memory after 24 Hr of Total Sleep Deprivation. J. Neurosci. 2004, 24, 4560–4567. [Google Scholar] [CrossRef] [Green Version]
- Boly, M.; Balteau, E.; Schnakers, C.; Degueldre, C.; Moonen, G.; Luxen, A.; Phillips, C.; Peigneux, P.; Maquet, P.; Laureys, S. Baseline Brain Activity Fluctuations Predict Somatosensory Perception in Humans. Proc. Natl. Acad. Sci. USA 2007, 104, 12187–12192. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.-W.; Yu, Y.W.-Y.; Wu, H.-C.; Chen, T.-J. Do Resting Brain Dynamics Predict Oddball Evoked-Potential? BMC Neurosci. 2011, 12, 121. [Google Scholar] [CrossRef] [Green Version]
- Cajochen, C.; Brunner, D.P.; Kräuchi, K.; Graw, P.; Wirz-Justice, A. Power Density in Theta/Alpha Frequencies of the Waking EEG Progressively Increases during Sustained Wakefulness. Sleep 1995, 18, 890–894. [Google Scholar] [CrossRef]
- Aeschbach, D.; Matthews, J.R.; Postolache, T.T.; Jackson, M.A.; Giesen, H.A.; Wehr, T.A. Dynamics of the Human EEG during Prolonged Wakefulness: Evidence for Frequency-Specific Circadian and Homeostatic Influences. Neurosci. Lett. 1997, 239, 121–124. [Google Scholar] [CrossRef] [PubMed]




| A | B | |||||
|---|---|---|---|---|---|---|
| Electrodes | A1. P200 Latency Pre-SD (ms) | A2. P200 Latency Post-SD (ms) | A1vs. A2 p= | B1. P200 Amplitude Pre-SD (µV) | B2. P200 Amplitude Post-SD (µV) | B1vs. B2 p= |
| Fp1 | 252.15 ± 33.23 | 291.99 ± 45.01 | 0.003 | 3.98 ± 3.32 | 3.80 ± 2.48 | 0.786 |
| Fpz | 249.03 ± 29.48 | 261.52 ± 41.77 | 0.252 | 3.75 ± 2.97 | 3.16 ± 2.33 | 0.351 |
| Fp2 | 253.91 ± 30.17 | 280.86 ± 40.16 | 0.003 | 3.98 ± 3.40 | 3.35 ± 2.44 | 0.437 |
| F7 | 260.55 ± 38.70 | 309.37 ± 46.99 | 0.011 | 5.81 ± 3.60 | 4.92 ± 2.14 | 0.330 |
| F3 | 244.53 ± 24.60 | 254.49 ± 25.00 | 0.011 | 3.29 ± 1.73 | 2.57 ± 1.55 | 0.160 |
| Fz | 234.38 ± 13.56 | 257.23 ± 35.71 | 0.025 | 2.12 ± 2.13 | 2.02 ± 1.59 | 0.841 |
| F4 | 247.27 ± 25.50 | 273.24 ± 41.70 | 0.018 | 4.05 ± 3.01 | 3.39 ± 2.22 | 0.415 |
| F8 | 258.59 ± 41.67 | 281.64 ± 47.67 | 0.024 | 5.71 ± 3.95 | 4.68 ± 2.95 | 0.164 |
| C3 | 302.34 ± 24.55 | 312.50 ± 34.38 | 0.464 | 1.84 ± 1.07 | 1.70 ± 1.05 | 0.477 |
| C4 | 267.77 ± 28.58 | 283.20 ± 31.96 | 0.123 | 3.93 ± 1.74 | 3.21 ± 1.59 | 0.130 |
| T3 | 270.32 ± 38.93 | 311.53 ± 42.16 | 0.004 | 5.90 ± 3.50 | 5.20 ± 1.90 | 0.367 |
| T4 | 281.64 ± 20.12 | 309.38 ± 36.46 | 0.056 | 5.83 ± 2.76 | 5.26 ± 2.83 | 0.464 |
| T5 | 283.01 ± 23.75 | 314.45 ± 32.75 | 0.004 | 7.71 ± 4.59 | 7.18 ± 2.76 | 0.531 |
| T6 | 273.63 ± 24.44 | 299.42 ± 43.08 | 0.016 | 6.78 ± 4.32 | 6.44 ± 3.70 | 0.568 |
| P3 | 294.73 ± 18.29 | 310.55 ± 40.73 | 0.267 | 6.06 ± 2.36 | 5.62 ± 1.61 | 0.399 |
| P4 | 274.22 ± 23.93 | 291.60 ± 36.28 | 0.060 | 6.31 ± 2.43 | 5.54 ± 1.89 | 0.118 |
| O1 | 279.49 ± 20.49 | 309.96 ± 27.14 | 0.001 | 9.21 ± 2.79 | 8.57 ± 2.53 | 0.221 |
| Oz | 272.66 ± 23.40 | 293.56 ± 32.30 | 0.003 | 9.89 ± 2.21 | 9.47 ± 3.48 | 0.659 |
| O2 | 280.47 ± 13.73 | 297.66 ± 24.90 | 0.004 | 8.10 ± 3.24 | 7.65 ± 3.05 | 0.596 |
| ROIs | A. ΔRMS | B. ΔRMS, ΔP200 Amplitude |
|---|---|---|
| Frontopolar Area | R = 0.649 p = 0.042 | R = 0.845 p = 0.013 |
| Frontal Area | R = 0.635 p = 0.049 | R = 0.839 p = 0.014 |
| Central Area | R = 0.390 p = 0.266 | R = 882 p = 0.005 |
| Parietal Area | R = 0.615 p = 0.058 | R = 0.819 p = 0.020 |
| Temporal Area | R = 0.616 p = 0.058 | R = 0.752 p = 0.054 |
| Occipital Area | R = 0.860 p = 0.001 | R = 0.930 p = 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tramonti Fantozzi, M.P.; Banfi, T.; Di Galante, M.; Ciuti, G.; Faraguna, U. Sleep Deprivation-Induced Changes in Baseline Brain Activity and Vigilant Attention Performance. Brain Sci. 2022, 12, 1690. https://doi.org/10.3390/brainsci12121690
Tramonti Fantozzi MP, Banfi T, Di Galante M, Ciuti G, Faraguna U. Sleep Deprivation-Induced Changes in Baseline Brain Activity and Vigilant Attention Performance. Brain Sciences. 2022; 12(12):1690. https://doi.org/10.3390/brainsci12121690
Chicago/Turabian StyleTramonti Fantozzi, Maria Paola, Tommaso Banfi, Marco Di Galante, Gastone Ciuti, and Ugo Faraguna. 2022. "Sleep Deprivation-Induced Changes in Baseline Brain Activity and Vigilant Attention Performance" Brain Sciences 12, no. 12: 1690. https://doi.org/10.3390/brainsci12121690
APA StyleTramonti Fantozzi, M. P., Banfi, T., Di Galante, M., Ciuti, G., & Faraguna, U. (2022). Sleep Deprivation-Induced Changes in Baseline Brain Activity and Vigilant Attention Performance. Brain Sciences, 12(12), 1690. https://doi.org/10.3390/brainsci12121690







