Heterogeneity of White Matter Hyperintensity and Cognitive Impairment in Patients with Acute Lacunar Stroke
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Data Collection and Baseline Evaluation
2.3. MRI Imaging
2.4. CSVD Imaging Markers
2.5. Neuropsychological Assessment and Follow-Up Visit
2.6. Statistical Analysis
3. Results
3.1. Baseline Clinical Features and MRI Characteristics
3.2. Independent Risk Factors for Cognitive Impairment in ALS Patients
3.3. Comparison of Clinical Features and CSVD Characteristics between Mismatch and Match Types
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Jiang, S.; Wu, S.; Zhang, S.; Wu, B. Advances in Understanding the Pathogenesis of Lacunar Stroke: From Pathology and Pathophysiology to Neuroimaging. Cerebrovasc. Dis. 2021, 50, 588–596. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Smith, E.E.; Biessels, G.J.; Cordonnier, C.; Fazekas, F.; Frayne, R.; Lindley, R.I.; O’Brien, J.T.; Barkhof, F.; Benavente, O.R.; et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013, 12, 822–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wardlaw, J.M.; Smith, C.; Dichgans, M. Mechanisms of sporadic cerebral small vessel disease: Insights from neuroimaging. Lancet Neurol. 2013, 12, 483–497. [Google Scholar] [CrossRef] [Green Version]
- Edwards, J.D.; Jacova, C.; Sepehry, A.A.; Pratt, B.; Benavente, O.R. A quantitative systematic review of domain-specific cognitive impairment in lacunar stroke. Neurology 2013, 80, 315–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Groot, J.C.; de Leeuw, F.E.; Oudkerk, M.; van Gijn, J.; Hofman, A.; Jolles, J.; Breteler, M.M. Cerebral white matter lesions and cognitive function: The Rotterdam Scan Study. Ann Neurol. 2000, 47, 145–151. [Google Scholar] [CrossRef]
- Inzitari, D.; Pracucci, G.; Poggesi, A.; Carlucci, G.; Barkhof, F.; Chabriat, H.; Erkinjuntti, T.; Fazekas, F.; Ferro, J.M.; Hennerici, M.; et al. Changes in white matter as determinant of global functional decline in older independent outpatients: Three year follow-up of LADIS (leukoaraiosis and disability) study cohort. BMJ 2009, 339, b2477. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.L.; Chen, W.; Cai, W.J.; Hu, H.; Xu, W.; Wang, Z.T.; Cao, X.P.; Tan, L.; Yu, J.T. Associations of White Matter Hyperintensities with Cognitive Decline: A Longitudinal Study. J. Alzheimer’s Dis. 2020, 73, 759–768. [Google Scholar] [CrossRef]
- Hu, H.Y.; Ou, Y.N.; Shen, X.N.; Qu, Y.; Ma, Y.H.; Wang, Z.T.; Dong, Q.; Tan, L.; Yu, J.T. White matter hyperintensities and risks of cognitive impairment and dementia: A systematic review and meta-analysis of 36 prospective studies. Neurosci. Biobehav. Rev. 2021, 120, 16–27. [Google Scholar] [CrossRef]
- Garde, E.; Mortensen, E.L.; Krabbe, K.; Rostrup, E.; Larsson, H.B. Relation between age-related decline in intelligence and cerebral white-matter hyperintensities in healthy octogenarians: A longitudinal study. Lancet 2000, 356, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Fruhwirth, V.; Enzinger, C.; Fandler-Hofler, S.; Kneihsl, M.; Eppinger, S.; Ropele, S.; Schmidt, R.; Gattringer, T.; Pinter, D. Baseline white matter hyperintensities affect the course of cognitive function after small vessel disease-related stroke: A prospective observational study. Eur. J. Neurol. 2021, 28, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Silbert, L.C.; Howieson, D.B.; Dodge, H.; Kaye, J.A. Cognitive impairment risk: White matter hyperintensity progression matters. Neurology 2009, 73, 120–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debette, S.; Bombois, S.; Bruandet, A.; Delbeuck, X.; Lepoittevin, S.; Delmaire, C.; Leys, D.; Pasquier, F. Subcortical hyperintensities are associated with cognitive decline in patients with mild cognitive impairment. Stroke 2007, 38, 2924–2930. [Google Scholar] [CrossRef] [Green Version]
- Debette, S.; Markus, H.S. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: Systematic review and meta-analysis. BMJ 2010, 341, c3666. [Google Scholar] [CrossRef] [Green Version]
- Kloppenborg, R.P.; Nederkoorn, P.J.; Geerlings, M.I.; van den Berg, E. Presence and progression of white matter hyperintensities and cognition: A meta-analysis. Neurology 2014, 82, 2127–2138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivakumar, L.; Riaz, P.; Kate, M.; Jeerakathil, T.; Beaulieu, C.; Buck, B.; Camicioli, R.; Butcher, K. White matter hyperintensity volume predicts persistent cognitive impairment in transient ischemic attack and minor stroke. Int. J. Stroke 2017, 12, 264–272. [Google Scholar] [CrossRef]
- Schmidt, R.; Ropele, S.; Enzinger, C.; Petrovic, K.; Smith, S.; Schmidt, H.; Matthews, P.M.; Fazekas, F. White matter lesion progression, brain atrophy, and cognitive decline: The Austrian stroke prevention study. Ann. Neurol. 2005, 58, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, X.; Guan, L.; Wang, Y. Role of White Matter Hyperintensities and Related Risk Factors in Vascular Cognitive Impairment: A Review. Biomolecules 2021, 11, 1102. [Google Scholar] [CrossRef]
- De Groot, M.; Verhaaren, B.F.; de Boer, R.; Klein, S.; Hofman, A.; van der Lugt, A.; Ikram, M.A.; Niessen, W.J.; Vernooij, M.W. Changes in normal-appearing white matter precede development of white matter lesions. Stroke 2013, 44, 1037–1042. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, R.; Berghold, A.; Jokinen, H.; Gouw, A.A.; van der Flier, W.M.; Barkhof, F.; Scheltens, P.; Petrovic, K.; Madureira, S.; Verdelho, A.; et al. White matter lesion progression in LADIS: Frequency, clinical effects, and sample size calculations. Stroke 2012, 43, 2643–2647. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Jin, A.; Fu, Y.; Zhang, Z.; Li, S.; Wang, D.; Wang, Y. Heterogeneity of White Matter Hyperintensities in Cognitively Impaired Patients With Cerebral Small Vessel Disease. Front. Immunol. 2021, 12, 803504. [Google Scholar] [CrossRef]
- Debette, S.; Schilling, S.; Duperron, M.G.; Larsson, S.C.; Markus, H.S. Clinical Significance of Magnetic Resonance Imaging Markers of Vascular Brain Injury: A Systematic Review and Meta-analysis. JAMA Neurol. 2019, 76, 81–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skrobot, O.A.; Black, S.E.; Chen, C.; DeCarli, C.; Erkinjuntti, T.; Ford, G.A.; Kalaria, R.N.; O’Brien, J.; Pantoni, L.; Pasquier, F.; et al. Progress toward standardized diagnosis of vascular cognitive impairment: Guidelines from the Vascular Impairment of Cognition Classification Consensus Study. Alzheimer’s Dement. 2018, 14, 280–292. [Google Scholar] [CrossRef] [Green Version]
- Tang, A.; Liu, S.; Wang, Z.; Shao, H.; Cai, X.; Li, T. A New Nomogram Model for Individualized Prediction of Cognitive Impairment in Patients with Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2022, 31, 106515. [Google Scholar] [CrossRef] [PubMed]
- Pendlebury, S.T.; Rothwell, P.M. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: A systematic review and meta-analysis. Lancet Neurol. 2009, 8, 1006–1018. [Google Scholar] [CrossRef]
- Surawan, J.; Areemit, S.; Tiamkao, S.; Sirithanawuthichai, T.; Saensak, S. Risk factors associated with post-stroke dementia: A systematic review and meta-analysis. Neurol. Int. 2017, 9, 7216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, Y.; Ohara, T.; Nagakane, Y.; Tanaka, E.; Morii, F.; Koizumi, T.; Akiguchi, I. Chronic kidney disease, 24-h blood pressure and small vessel diseases are independently associated with cognitive impairment in lacunar infarct patients. Hypertens. Res. 2011, 34, 1276–1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akinyemi, R.O.; Allan, L.M.; Oakley, A.; Kalaria, R.N. Hippocampal Neurodegenerative Pathology in Post-stroke Dementia Compared to Other Dementias and Aging Controls. Front. Neurosci. 2017, 11, 717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sperling, R.A.; Aisen, P.S.; Beckett, L.A.; Bennett, D.A.; Craft, S.; Fagan, A.M.; Iwatsubo, T.; Jack, C.R., Jr.; Kaye, J.; Montine, T.J.; et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 280–292. [Google Scholar] [CrossRef] [Green Version]
- Low, A.; Mak, E.; Rowe, J.B.; Markus, H.S.; O’Brien, J.T. Inflammation and cerebral small vessel disease: A systematic review. Ageing Res. Rev. 2019, 53, 100916. [Google Scholar] [CrossRef] [PubMed]
- Van Oijen, M.; Witteman, J.C.; Hofman, A.; Koudstaal, P.J.; Breteler, M.M. Fibrinogen is associated with an increased risk of Alzheimer disease and vascular dementia. Stroke 2005, 36, 2637–2641. [Google Scholar] [CrossRef]
- Marioni, R.E.; Stewart, M.C.; Murray, G.D.; Deary, I.J.; Fowkes, F.G.; Lowe, G.D.; Rumley, A.; Price, J.F. Peripheral levels of fibrinogen, C-reactive protein, and plasma viscosity predict future cognitive decline in individuals without dementia. Psychosom. Med. 2009, 71, 901–906. [Google Scholar] [CrossRef] [Green Version]
- Rafnsson, S.; Deary, I.J.; Whiteman, M.C.; Rumley, A.; Lowe, G.D.; Fowkes, F.G. Haemorheological predictors of cognitive decline: The Edinburgh Artery Study. Age Ageing. 2010, 39, 217–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamori, M.; Hosomi, N.; Tachiyama, K.; Kamimura, T.; Matsushima, H.; Hayashi, Y.; Imamura, E.; Wakabayashi, S.; Maruyama, H. Lobar microbleeds are associated with cognitive impairment in patients with lacunar infarction. Sci. Rep. 2020, 10, 16410. [Google Scholar] [CrossRef] [PubMed]
- Yakushiji, Y.; Noguchi, T.; Hara, M.; Nishihara, M.; Eriguchi, M.; Nanri, Y.; Nishiyama, M.; Hirotsu, T.; Nakajima, J.; Kuroda, Y.; et al. Distributional impact of brain microbleeds on global cognitive function in adults without neurological disorder. Stroke 2012, 43, 1800–1805. [Google Scholar] [CrossRef] [Green Version]
- Firbank, M.J.; Burton, E.J.; Barber, R.; Stephens, S.; Kenny, R.A.; Ballard, C.; Kalaria, R.N.; O’Brien, J.T. Medial temporal atrophy rather than white matter hyperintensities predict cognitive decline in stroke survivors. Neurobiol. Aging 2007, 28, 1664–1669. [Google Scholar] [CrossRef]
- Van der Flier, W.M.; van Buchem, M.A.; van Buchem, H.A. Volumetric MRI predicts rate of cognitive decline related to AD and cerebrovascular disease. Neurology 2003, 60, 1558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huijts, M.; Duits, A.; van Oostenbrugge, R.J.; Kroon, A.A.; de Leeuw, P.W.; Staals, J. Accumulation of MRI Markers of Cerebral Small Vessel Disease is Associated with Decreased Cognitive Function. A Study in First-Ever Lacunar Stroke and Hypertensive Patients. Front. Aging Neurosci. 2013, 5, 72. [Google Scholar] [CrossRef] [Green Version]
- Zhi, N.; Zhang, L.; Wang, Y.; Bai, S.; Geng, J.; Yu, L.; Cao, W.; Zhuang, L.; Zhou, Y.; Guan, Y. Modified cerebral small vessel disease score is associated with vascular cognitive impairment after lacunar stroke. Aging 2021, 13, 9510–9521. [Google Scholar] [CrossRef]
- Smith, E.E.; Blacker, D.; Killiany, R.J.; Muzikansky, A.; Dickerson, B.C.; Tanzi, R.E.; Albert, M.S.; Greenberg, S.M.; Guttmann, C.R. Magnetic resonance imaging white matter hyperintensities and brain volume in the prediction of mild cognitive impairment and dementia. Arch. Neurol. 2008, 65, 94–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dufouil, C.; Godin, O.; Chalmers, J.; Coskun, O.; MacMahon, S.; Tzourio-Mazoyer, N.; Bousser, M.G.; Anderson, C.; Mazoyer, B.; Tzourio, C.; et al. Severe cerebral white matter hyperintensities predict severe cognitive decline in patients with cerebrovascular disease history. Stroke 2009, 40, 2219–2221. [Google Scholar] [CrossRef]
- Jokinen, H.; Kalska, H.; Ylikoski, R.; Madureira, S.; Verdelho, A.; van der Flier, W.M.; Scheltens, P.; Barkhof, F.; Visser, M.C.; Fazekas, F.; et al. Longitudinal cognitive decline in subcortical ischemic vascular disease--the LADIS Study. Cerebrovasc. Dis. 2009, 27, 384–391. [Google Scholar] [CrossRef]
- Gouw, A.A.; Seewann, A.; van der Flier, W.M.; Barkhof, F.; Rozemuller, A.M.; Scheltens, P.; Geurts, J.J. Heterogeneity of small vessel disease: A systematic review of MRI and histopathology correlations. J. Neurol. Neurosurg. Psychiatry 2011, 82, 126–135. [Google Scholar] [CrossRef]
- Jung, K.H.; Stephens, K.A.; Yochim, K.M.; Riphagen, J.M.; Kim, C.M.; Buckner, R.L.; Salat, D.H. Heterogeneity of Cerebral White Matter Lesions and Clinical Correlates in Older Adults. Stroke 2021, 52, 620–630. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Chappell, F.M.; Valdes Hernandez, M.D.C.; Makin, S.D.J.; Staals, J.; Shuler, K.; Thrippleton, M.J.; Armitage, P.A.; Munoz-Maniega, S.; Heye, A.K.; et al. White matter hyperintensity reduction and outcomes after minor stroke. Neurology 2017, 89, 1003–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyden, P. Using the National Institutes of Health Stroke Scale: A Cautionary Tale. Stroke 2017, 48, 513–519. [Google Scholar] [CrossRef]
- Einstad, M.S.; Saltvedt, I.; Lydersen, S.; Ursin, M.H.; Munthe-Kaas, R.; Ihle-Hansen, H.; Knapskog, A.B.; Askim, T.; Beyer, M.K.; Naess, H.; et al. Associations between post-stroke motor and cognitive function: A cross-sectional study. BMC Geriatr. 2021, 21, 103. [Google Scholar] [CrossRef] [PubMed]
- Hackett, M.L.; Pickles, K. Part I: Frequency of depression after stroke: An updated systematic review and meta-analysis of observational studies. Int. J. Stroke 2014, 9, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Villa, R.F.; Ferrari, F.; Moretti, A. Post-stroke depression: Mechanisms and pharmacological treatment. Pharmacol. Ther. 2018, 184, 131–144. [Google Scholar] [CrossRef]
- Nys, G.M.; van Zandvoort, M.J.; van der Worp, H.B.; de Haan, E.H.; de Kort, P.L.; Kappelle, L.J. Early depressive symptoms after stroke: Neuropsychological correlates and lesion characteristics. J. Neurol. Sci. 2005, 228, 27–33. [Google Scholar] [CrossRef]
- Miller, M.D.; Lenze, E.J.; Dew, M.A.; Whyte, E.; Weber, E.; Begley, A.E.; Reynolds, C.F., 3rd. Effect of cerebrovascular risk factors on depression treatment outcome in later life. Am. J. Geriatr. Psychiatry 2002, 10, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Moorhouse, P.; Song, X.; Rockwood, K.; Black, S.; Kertesz, A.; Gauthier, S.; Feldman, H. Executive dysfunction in vascular cognitive impairment in the consortium to investigate vascular impairment of cognition study. J. Neurol. Sci. 2010, 288, 142–146. [Google Scholar] [CrossRef]
- De Groot, J.C.; De Leeuw, F.E.; Oudkerk, M.; Van Gijn, J.; Hofman, A.; Jolles, J.; Breteler, M.M. Periventricular cerebral white matter lesions predict rate of cognitive decline. Ann. Neurol. 2002, 52, 335–341. [Google Scholar] [CrossRef]
- De Groot, J.C.; de Leeuw, F.E.; Oudkerk, M.; Hofman, A.; Jolles, J.; Breteler, M.M. Cerebral white matter lesions and depressive symptoms in elderly adults. Arch. Gen. Psychiatry 2000, 57, 1071–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mungas, D.; Harvey, D.; Reed, B.R.; Jagust, W.J.; DeCarli, C.; Beckett, L.; Mack, W.J.; Kramer, J.H.; Weiner, M.W.; Schuff, N.; et al. Longitudinal volumetric MRI change and rate of cognitive decline. Neurology 2005, 65, 565–571. [Google Scholar] [CrossRef] [Green Version]
- Nordahl, C.W.; Ranganath, C.; Yonelinas, A.P.; Decarli, C.; Fletcher, E.; Jagust, W.J. White matter changes compromise prefrontal cortex function in healthy elderly individuals. J. Cogn. Neurosci. 2006, 18, 418–429. [Google Scholar] [CrossRef]
- Alonso, A.; Mosley, T.H., Jr.; Gottesman, R.F.; Catellier, D.; Sharrett, A.R.; Coresh, J. Risk of dementia hospitalisation associated with cardiovascular risk factors in midlife and older age: The Atherosclerosis Risk in Communities (ARIC) study. J. Neurol. Neurosurg. Psychiatry 2009, 80, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Englund, E. Neuropathology of white matter lesions in vascular cognitive impairment. Cerebrovasc. Dis. 2002, 13 (Suppl. S2), 11–15. [Google Scholar] [CrossRef]
- Mascalchi, M.; Ginestroni, A.; Toschi, N.; Poggesi, A.; Cecchi, P.; Salvadori, E.; Tessa, C.; Cosottini, M.; De Stefano, N.; Pracucci, G.; et al. The burden of microstructural damage modulates cortical activation in elderly subjects with MCI and leuko-araiosis. A DTI and fMRI study. Hum. Brain Mapp. 2014, 35, 819–830. [Google Scholar] [CrossRef]
- Duering, M.; Finsterwalder, S.; Baykara, E.; Tuladhar, A.M.; Gesierich, B.; Konieczny, M.J.; Malik, R.; Franzmeier, N.; Ewers, M.; Jouvent, E.; et al. Free water determines diffusion alterations and clinical status in cerebral small vessel disease. Alzheimer’s Dement. 2018, 14, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Maillard, P.; Fletcher, E.; Harvey, D.; Carmichael, O.; Reed, B.; Mungas, D.; DeCarli, C. White matter hyperintensity penumbra. Stroke 2011, 42, 1917–1922. [Google Scholar] [CrossRef] [PubMed]
- Maillard, P.; Carmichael, O.; Harvey, D.; Fletcher, E.; Reed, B.; Mungas, D.; DeCarli, C. FLAIR and diffusion MRI signals are independent predictors of white matter hyperintensities. AJNR Am. J. Neuroradiol. 2013, 34, 54–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Promjunyakul, N.O.; Lahna, D.L.; Kaye, J.A.; Dodge, H.H.; Erten-Lyons, D.; Rooney, W.D.; Silbert, L.C. Comparison of cerebral blood flow and structural penumbras in relation to white matter hyperintensities: A multi-modal magnetic resonance imaging study. J. Cereb. Blood Flow Metab. 2016, 36, 1528–1536. [Google Scholar] [CrossRef] [PubMed]
- Ter Telgte, A.; van Leijsen, E.M.C.; Wiegertjes, K.; Klijn, C.J.M.; Tuladhar, A.M.; de Leeuw, F.E. Cerebral small vessel disease: From a focal to a global perspective. Nat. Rev. Neurol. 2018, 14, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Duering, M.; Csanadi, E.; Gesierich, B.; Jouvent, E.; Herve, D.; Seiler, S.; Belaroussi, B.; Ropele, S.; Schmidt, R.; Chabriat, H.; et al. Incident lacunes preferentially localize to the edge of white matter hyperintensities: Insights into the pathophysiology of cerebral small vessel disease. Brain 2013, 136, 2717–2726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiaerken, Y.; Lian, C.; Huang, P.; Yu, X.; Zhang, R.; Wang, S.; Hong, H.; Luo, X.; Yap, P.T.; Shen, D.; et al. Dilated perivascular space is related to reduced free-water in surrounding white matter among healthy adults and elderlies but not in patients with severe cerebral small vessel disease. J. Cereb. Blood Flow Metab. 2021, 41, 2561–2570. [Google Scholar] [CrossRef] [PubMed]

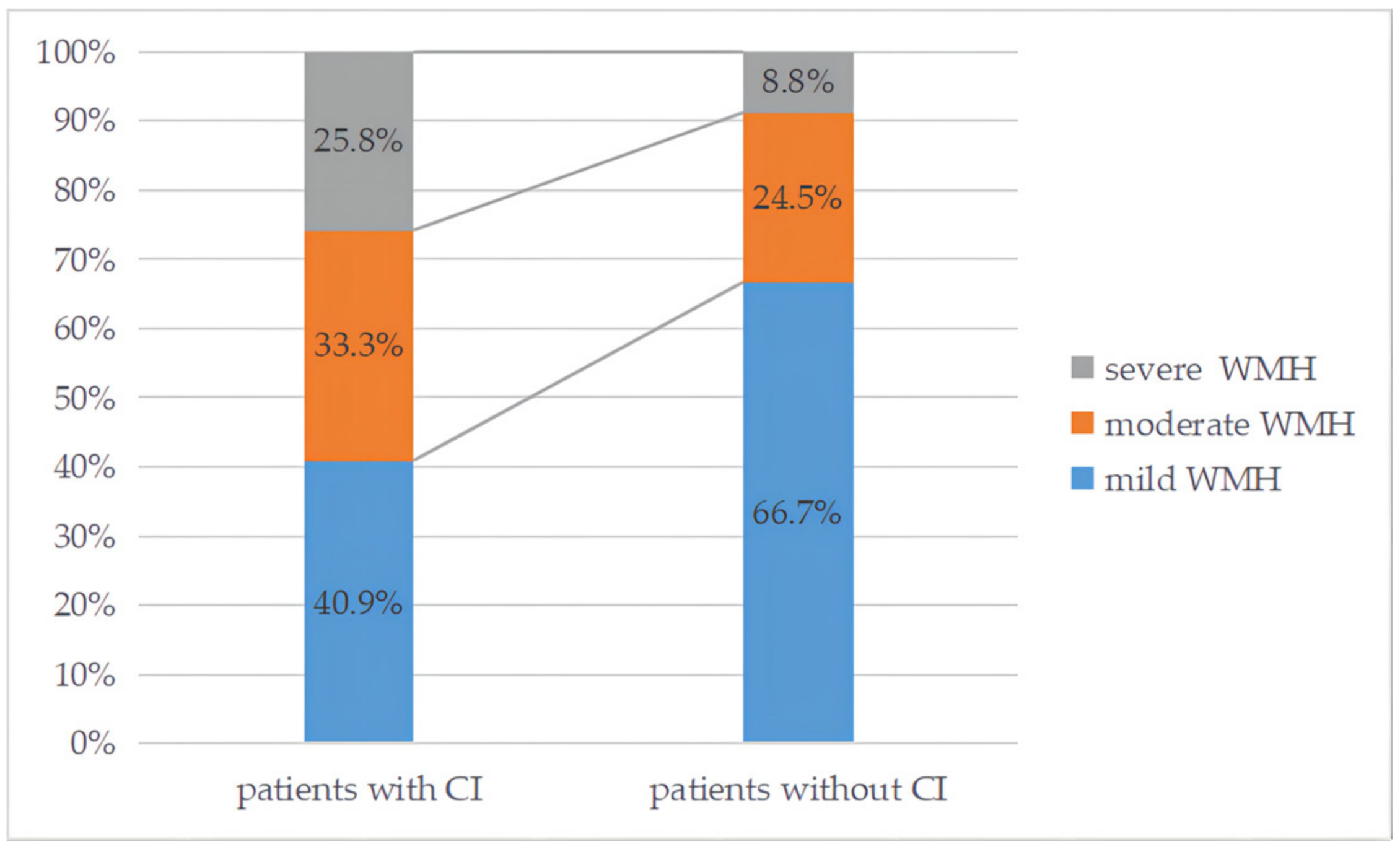

| Total Patients (n = 213) | With Cognitive Impairment (n = 66) | Without Cognitive Impairment (n = 147) | p Value | BH-Adjusted p Values (q Value) | |
|---|---|---|---|---|---|
| Demographic and clinical characteristics | |||||
| Age (years) | 64 (56–72) | 68 (62–76) | 62 (53–72) | p = 0.001 * | q = 0.004 # |
| Male, n (%) | 136 (63.8%) | 35 (53.0%) | 101 (68.7%) | p = 0.028 * | q = 0.061 # |
| Medical history | |||||
| Cerebral infarction, n (%) | 46 (21.6%) | 21 (31.8%) | 25 (17.0%) | p = 0.015 * | q = 0.043 # |
| Hypertension, n (%) | 159 (74.6%) | 53 (80.3%) | 106 (72.1%) | p = 0.204 | q = 0.295 |
| Diabetes mellitus, n (%) | 59 (27.7%) | 22 (33.3%) | 37 (25.2%) | p = 0.218 | q = 0.298 |
| MOCA score | 22 (19–25) | 20 (16–24) | 24 (23–26) | p < 0.001 * | q = 0.001 # |
| Depression, n (%) | 18 (8.5%) | 8 (12.1%) | 10 (6.8%) | p = 0.197 | q = 0.295 |
| NIHSS score | 3 (1–3) | 4 (1–5) | 2(1–3) | p = 0.018 * | q = 0.043 # |
| Laboratory examination | |||||
| Thrombocyte (109/L) | 201 ± 58 | 201 ± 60 | 202 ± 59 | p = 0.793 | q = 0.825 |
| LDL-C (mmol/L) | 2.64 (2.07–3.14) | 2.63 (2.08–3.05) | 2.65 (2.07–3.16) | p = 0.360 | q = 0.446 |
| Hemoglobin (g/L) | 134 (124–144) | 131 (121–142) | 135 (126–146) | p = 0.035 * | q = 0.070 # |
| Creatinine (μmol/L) | 71 (57–80) | 70 (60–81) | 71 (57–80) | p = 0.685 | q = 0.759 |
| hs-CRP (mg/L) | 3.75 (0.76–4.71) | 4.21 (0.85–4.97) | 3.59 (0.66–4.59) | p = 0.136 | q = 0.236 |
| Fibrinogen (g/L) | 2.72 (2.14–3.13) | 1.75 (2.37–3.37) | 1.64 (2.00–3.04) | p = 0.001 * | q = 0.004 # |
| Imaging features | |||||
| Infarction lesions | |||||
| Thalamus | 25 (11.7%) | 8 (12.1%) | 17 (11.6%) | p = 0.907 | q = 0.907 |
| Basal ganglia/internal capsule | 82 (38.5) | 27 (40.9%) | 55 (37.4%) | p = 0.628 | q = 0.742 |
| Centrum ovale/corona radiata | 70 (32.9%) | 18 (27.3%) | 52 (35.4%) | p = 0.244 | q = 0.317 |
| Medulla/midbrain/pons/cerebellum | 52 (24.4%) | 15 (22.7%) | 37 (25.2%) | p = 0.701 | q = 0.759 |
| WMH Fazekas score (0–6) | 2 (0–4) | 3 (1–4) | 2 (0–3) | p = 0.067 | q = 0.124 |
| Lacune, n (%) | 114 (53.5%) | 44 (66.7%) | 70 (47.6%) | p = 0.011 * | q = 0.036 # |
| Microbleeds, n (%) | 52 (24.4%) | 28 (42.4%) | 24 (16.3%) | p < 0.001 * | q = 0.001 # |
| EPVS (N > 10), n (%) | 109 (51.2%) | 42 (63.6%) | 67 (45.6%) | p = 0.017 * | q = 0.043 # |
| Moderate to severe WMH, n (%) | 76 (35.7%) | 37 (56.1%) | 39 (26.5%) | p < 0.001 * | q = 0.001 # |
| Periventricular WMH | 1 (0–2) | 2 (1–3) | 1 (0–2) | p = 0.189 | q = 0.295 |
| Deep WMH | 1 (0–1) | 1 (0–2) | 0 (0–1) | p < 0.001 * | q = 0.001 # |
| Total CSVD score (0–4) | 2 (1–3) | 2 (1–3) | 1 (0–2) | p = 0.001 * | q = 0.004 # |
| OR | 95% CI | B | p Value | |
|---|---|---|---|---|
| Age(years) | 1.044 | 1.009–1.080 | 0.043 | p = 0.014 * |
| Male, n (%) | 0.379 | 0.176–0.817 | −0.970 | p = 0.013 * |
| NIHSS score | 1.124 | 0.995–1.268 | 0.116 | p = 0.060 |
| Cerebral infarction history, n (%) | 2.359 | 1.027–5.419 | 0.858 | p = 0.043 * |
| Fibrinogen (g/L) | 1.810 | 1.242–2.639 | 0.594 | p = 0.002 * |
| Moderate to severe WMH | 3.485 | 1.656–7.333 | 1.248 | p = 0.001 * |
| Deep WMH | 6.037 | 2.600–14.020 | 1.798 | p < 0.001 * |
| Total CSVD score (0–4) | p = 0.005 * | |||
| 0 | Ref. | Ref. | Ref. | Ref. |
| 1 | 0.558 | 0.189–1.654 | −0.583 | p = 0.293 |
| 2 | 1.116 | 0.388–3.211 | 0.110 | p = 0.839 |
| 3 | 1.878 | 0.598–5.898 | 0.630 | p = 0.281 |
| 4 | 7.309 | 1.872–28.530 | 1.989 | p = 0.004 * |
| Mismatch Type (n = 40) | Match Type (n = 115) | p Value | |
|---|---|---|---|
| Demographic and clinical characteristics | |||
| Age (years) | 67 ± 10 | 61 ± 12 | p = 0.012 * |
| Male, n (%) | 25(62.5%) | 73 (63.5%) | p = 0.912 |
| Depression, n (%) | 7(17.5%) | 7 (6.1%) | p = 0.030 * |
| Medical history | |||
| Cerebral infarction, n (%) | 12(30.0%) | 22 (19.1%) | p = 0.152 |
| Hypertension, n (%) | 31(77.5%) | 83 (72.2%) | p = 0.511 |
| Diabetes mellitus, n (%) | 13(32.5%) | 31 (27.0%) | p = 0.503 |
| Laboratory examination | |||
| Thrombocyte (109/L) | 194 ± 45 | 204 ± 63 | p = 0.285 |
| LDL-C (mmol/L) | 2.59 (1.91–3.11) | 2.65 (2.08–3.10) | p = 0.343 |
| Hemoglobin (g/L) | 135 (125–145) | 134 (125–145) | p = 0.864 |
| Creatinine (μmol/L) | 72 (59–81) | 71 (56–79) | p = 0.193 |
| hs-CRP (mg/L) | 3.70 (0.79–4.92) | 3.77 (0.77–4.47) | p = 0.852 |
| Fibrinogen (g/L) | 2.55 (2.09–2.91) | 2.70 (2.15–3.06) | p = 0.422 |
| Imaging features | |||
| Periventricular WMH | 1 (0–3) | 1 (0–1) | p = 0.109 |
| Deep WMH | 1 (0–2) | 1 (0–1) | p = 0.038 * |
| Lacune, n (%) | 22 (55.0%) | 59 (51.3%) | p = 0.724 |
| Microbleeds, n (%) | 10 (25.0%) | 25 (21.7%) | p = 0.690 |
| EPVS (N > 10), n (%) | 22 (55.0%) | 49 (42.6%) | p = 0.190 |
| Total CSVD score (0–4) | 2 (1–3) | 1 (0–2) | p = 0.082 |
| Type 1 | Type 2 | |||||
|---|---|---|---|---|---|---|
| Mismatch (n = 27) | Match (n = 98) | p Value | Mismatch (n = 13) | Match (n = 17) | p Value | |
| Age (years) | 64 (55–73) | 59 (51–68) | p = 0.081 | 73 ± 7 | 73 ± 9 | p = 0.971 |
| Male, n (%) | 13 (48.2%) | 63 (64.3%) | p = 0.128 | 12 (92.3%) | 10 (58.8%) | p = 0.040 * |
| NIHSS score | 4 (1–4) | 2 (1–3) | p = 0.018 * | 3 (0–3) | 2 (0–4) | p = 0.915 |
| Depression, n (%) | 6 (22.2%) | 6 (6.1%) | p = 0.012 * | 1 (7.7%) | 1 (5.9%) | p = 0.844 |
| Medical history | ||||||
| Cerebral infarction, n (%) | 8 (29.6%) | 17 (17.4%) | p = 0.158 | 4 (30.8%) | 5 (29.4%) | p = 0.936 |
| Hypertension, n (%) | 20 (74.1%) | 69 (70.4%) | p = 0.710 | 11 (84.6%) | 14 (82.4%) | p = 0.869 |
| Diabetes mellitus, n (%) | 11 (40.7%) | 25 (25.5%) | p = 0.122 | 2 (15.4%) | 6 (35.3%) | p = 0.222 |
| Laboratory examination | ||||||
| Thrombocyte (109/L) | 195 ± 48 | 204 ± 63 | p = 0.275 | 191 ± 38 | 203 ± 66 | p = 0.572 |
| LDL-C (mmol/L) | 2.62 ± 1.04 | 2.64 ± 0.77 | p = 0.091 | 2.54 (2.28–3.12) | 2.71 (2.37–3.10) | p = 0.967 |
| Hemoglobin (g/L) | 134 (124–143) | 135 (126–146) | p = 0.477 | 136 ± 13 | 129 ± 15 | p = 0.184 |
| Creatinine (μmol/L) | 69 (57–78) | 71 (56–78) | p = 0.732 | 78 ± 13 | 72 ± 16 | p = 0.287 |
| hs-CRP (mg/L) | 3.42 (0.79–3.57) | 3.67 (0.67–4.43) | p = 0.859 | 4.26 (0.75–7.23) | 4.36 (0.92–4.97) | p = 0.706 |
| Fibrinogen (g/L) | 2.85 (2.27–3.14) | 2.67 (2.08–3.06) | p = 0.339 | 1.93 (1.36–2.45) | 2.89 (2.48–3.11) | p = 0.005 * |
| Imaging features | ||||||
| Periventricular WMH | 1 (0–1) | 0 (0–1) | p = 0.613 | 3 (3–3) | 3 (2–3) | p = 0.035 * |
| Deep WMH | 0 (0–0) | 0 (0–0) | p = 0.560 | 2 (2–3) | 3 (2–3) | p = 0.026 * |
| Lacune, n (%) | 15 (55.6%) | 45 (45.9%) | p = 0.399 | 7 (53.8%) | 14 (82.4%) | p = 0.091 |
| Microbleeds, n (%) | 8 (29.6%) | 13 (13.3%) | p = 0.047 * | 2 (15.4%) | 12 (70.6%) | p = 0.003 * |
| EPVS (N > 10), n (%) | 13 (48.1%) | 36 (36.7%) | p = 0.300 | 9 (69.2%) | 13 (76.5%) | p = 0.657 |
| Total CSVD score | 1 (0–2) | 1 (0–2) | p = 0.184 | 2 (2–3) | 3 (3–4) | p = 0.017 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, M.; Zhou, Y.; Chen, H.; Zhu, S.; Diao, S.; Zhao, J.; Kong, Y.; Li, T. Heterogeneity of White Matter Hyperintensity and Cognitive Impairment in Patients with Acute Lacunar Stroke. Brain Sci. 2022, 12, 1674. https://doi.org/10.3390/brainsci12121674
Ye M, Zhou Y, Chen H, Zhu S, Diao S, Zhao J, Kong Y, Li T. Heterogeneity of White Matter Hyperintensity and Cognitive Impairment in Patients with Acute Lacunar Stroke. Brain Sciences. 2022; 12(12):1674. https://doi.org/10.3390/brainsci12121674
Chicago/Turabian StyleYe, Mengfan, Yun Zhou, Huiru Chen, Sijia Zhu, Shanshan Diao, Jieji Zhao, Yan Kong, and Tan Li. 2022. "Heterogeneity of White Matter Hyperintensity and Cognitive Impairment in Patients with Acute Lacunar Stroke" Brain Sciences 12, no. 12: 1674. https://doi.org/10.3390/brainsci12121674
APA StyleYe, M., Zhou, Y., Chen, H., Zhu, S., Diao, S., Zhao, J., Kong, Y., & Li, T. (2022). Heterogeneity of White Matter Hyperintensity and Cognitive Impairment in Patients with Acute Lacunar Stroke. Brain Sciences, 12(12), 1674. https://doi.org/10.3390/brainsci12121674






