Sex Differences of the Functional Brain Activity in Treatment-Resistant Depression: A Resting-State Functional Magnetic Resonance Study
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Scan Acquisition
2.3. Image Processing
2.3.1. fMRI Data Preprocessing
2.3.2. ALFF Analysis
2.4. Statistical Analyses
2.4.1. Clinical Data
2.4.2. fMRI Data
3. Results
3.1. Characteristics of Research Samples
3.2. Main Effects of Group, Sex, and Sex-by-Group Interaction in ALFF among the Four Groups
3.3. Post Hoc Analyses in ALFF among the Four Groups
3.4. Relationships between ALFF and Clinical Symptoms
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kverno, K.S.; Mangano, E. Treatment-Resistant Depression: Approaches to Treatment. J. Psychosoc. Nurs. Ment. Health Serv. 2021, 59, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Gaynes, B.N.; Lux, L.; Gartlehner, G.; Asher, G.; Forman-Hoffman, V.; Green, J.; Boland, E.; Weber, R.; Randolph, C.; Bann, C.; et al. Defining treatment-resistant depression. Depress. Anxiety 2020, 37, 134–145. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Millson, B.; Power, G.S. Burden of Treatment Resistant Depression (TRD) in patients with major depressive disorder in Ontario using Institute for Clinical Evaluative Sciences (ICES) databases: Economic burden and healthcare resource utilization. J. Affect. Disord. 2020, 277, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ma, Y.; Chen, L.; Wang, Z.; Guo, C.; Luo, Y.; Gao, D.; Li, X.; Xu, K.; Hong, Y.; et al. Altered Brain Function in Treatment-Resistant and Non-treatment-resistant Depression Patients: A Resting-State Functional Magnetic Resonance Imaging Study. Front. Psychiatry 2022, 13, 904139. [Google Scholar] [CrossRef] [PubMed]
- Pilon, D.; Sheehan, J.J.; Szukis, H.; Morrison, L.; Zhdanava, M.; Lefebvre, P.; Joshi, K. Is clinician impression of depression symptom severity associated with incremental economic burden in privately insured US patients with treatment resistant depression? J. Affect. Disord. 2019, 255, 50–59. [Google Scholar] [CrossRef]
- Moderie, C.; Nuñez, N.; Fielding, A.; Comai, S.; Gobbi, G. Sex Differences in Responses to Antidepressant Augmentations in Treatment-Resistant Depression. Int. J. Neuropsychopharmacol. 2022, 25, 479–488. [Google Scholar] [CrossRef]
- Kuehner, C. Why is depression more common among women than among men? Lancet Psychiatry 2017, 4, 146–158. [Google Scholar] [CrossRef]
- Aziz, R.; Steffens, D.C. What Are the Causes of Late-Life Depression? Psychiatr. Clin. North Am. 2013, 36, 497–516. [Google Scholar] [CrossRef] [Green Version]
- Bangasser, D.A.; Cuarenta, A. Sex differences in anxiety and depression: Circuits and mechanisms. Nat. Rev. Neurosci. 2021, 22, 674–684. [Google Scholar] [CrossRef]
- Amiri, S.; Arbabi, M.; Kazemi, K.; Parvaresh-Rizi, M.; Mirbagheri, M.M. Characterization of brain functional connectivity in treatment-resistant depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 111, 110346. [Google Scholar] [CrossRef]
- Kennedy, S.H.; Lam, R.W.; McIntyre, R.S.; Tourjman, S.V.; Bhat, V.; Blier, P.; Hasnain, M.; Jollant, F.; Levitt, A.J.; MacQueen, G.M.; et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: Section 3. Pharmacological Treatments. Can. J. Psychiatry 2016, 61, 540–560, Erratum in Can. J. Psychiatry 2017, 62, 356. [Google Scholar] [CrossRef] [PubMed]
- Seifert, J.; Führmann, F.; Reinhard, M.A.; Engel, R.R.; Bernegger, X.; Bleich, S.; Stübner, S.; Rüther, E.; Toto, S.; Grohmann, R.; et al. Sex differences in pharmacological treatment of major depressive disorder: Results from the AMSP pharmacovigilance program from 2001 to 2017. J. Neural Transm. 2021, 128, 827–843. [Google Scholar] [CrossRef] [PubMed]
- Johnston, B.A.; Steele, J.D.; Tolomeo, S.; Christmas, D.; Matthews, K. Structural MRI-Based Predictions in Patients with Treatment-Refractory Depression (TRD). PLoS ONE 2015, 10, e0132958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandu, A.-L.; Artiges, E.; Galinowski, A.; Gallarda, T.; Bellivier, F.; Lemaitre, H.; Granger, B.; Ringuenet, D.; Tzavara, E.T.; Martinot, J.-L.; et al. Amygdala and regional volumes in treatment-resistant versus nontreatment-resistant depression patients. Depress. Anxiety 2017, 34, 1065–1071. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Du, Z.; Ma, Y.; Chen, L.; Wang, Z.; Guo, C.; Luo, Y.; Gao, D.; Hong, Y.; Zhang, L.; et al. Altered functional connectivity in first-episode and recurrent depression: A resting-state functional magnetic resonance imaging study. Front. Neurol. 2022, 13. [Google Scholar] [CrossRef]
- Mei, L.; Wang, Y.; Liu, C.; Mou, J.; Yuan, Y.; Qiu, L.; Gong, Q. Study of Sex Differences in Unmedicated Patients with Major Depressive Disorder by Using Resting State Brain Functional Magnetic Resonance Imaging. Front. Neurosci. 2022, 16, 922207. [Google Scholar] [CrossRef]
- Yao, Z.; Yan, R.; Wei, M.; Tang, H.; Qin, J.; Lu, Q. Gender differences in brain activity and the relationship between brain activity and differences in prevalence rates between male and female major depressive disorder patients: A resting-state fMRI study. Clin. Neurophysiol. 2014, 125, 2232–2239. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, L.; Liang, K.; Cao, L.; Liu, J.; Li, H.; Gao, Y.; Hu, Y.; Kuang, W.; Sweeney, J.A.; et al. Sex-specific alterations of cortical morphometry in treatment-naïve patients with major depressive disorder. Neuropsychopharmacology 2022, 47, 2002–2009. [Google Scholar] [CrossRef]
- Kong, L.; Chen, K.; Womer, F.; Jiang, W.; Luo, X.; Driesen, N.; Liu, J.; Blumberg, H.; Tang, Y.; Xu, K.; et al. Sex differences of gray matter morphology in cortico-limbic-striatal neural system in major depressive disorder. J. Psychiatr. Res. 2013, 47, 733–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hastings, R.; Parsey, R.V.; Oquendo, A.M.; Arango, V.; Mann, J.J. Volumetric Analysis of the Prefrontal Cortex, Amygdala, and Hippocampus in Major Depression. Neuropsychopharmacology 2004, 29, 952–959. [Google Scholar] [CrossRef] [Green Version]
- Glover, G.H. Overview of Functional Magnetic Resonance Imaging. Neurosurg. Clin. N. Am. 2011, 22, 133–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, J.; Wang, J.; Qiu, S.; Chen, P.; Luo, Z.; Wang, J.; Huang, L.; Wang, Y. Common and distinct patterns of intrinsic brain activity alterations in major depression and bipolar disorder: Voxel-based meta-analysis. Transl. Psychiatry 2020, 10, 353. [Google Scholar] [CrossRef]
- Mei, T.; Ma, Z.-H.; Guo, Y.-Q.; Lu, B.; Cao, Q.-J.; Chen, X.; Yang, L.; Wang, H.; Tang, X.-Z.; Ji, Z.-Z.; et al. Frequency-specific age-related changes in the amplitude of spontaneous fluctuations in autism. Transl. Pediatr. 2022, 11, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yang, J.; Yin, Z.; Duan, J.; Zhang, R.; Sun, J.; Xu, Y.; Liu, L.; Chen, X.; Li, H.; et al. Amplitude of low-frequency fluctuation (ALFF) may be associated with cognitive impairment in schizophrenia: A correlation study. BMC Psychiatry 2019, 19, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, C. DPARSF: A MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front. Syst. Neurosci. 2010, 4, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajah, M.N.; Languay, R.; Grady, C.L. Age-Related Changes in Right Middle Frontal Gyrus Volume Correlate with Altered Episodic Retrieval Activity. J. Neurosci. 2011, 31, 17941–17954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quidé, Y.; O’Reilly, N.; Watkeys, O.J.; Carr, V.J.; Green, M.J. Effects of childhood trauma on left inferior frontal gyrus function during response inhibition across psychotic disorders. Psychol. Med. 2018, 48, 1454–1463. [Google Scholar] [CrossRef] [PubMed]
- Amidfar, M.; Ko, Y.-H.; Kim, Y.-K. Neuromodulation and Cognitive Control of Emotion. Adv. Exp. Med. Biol. 2019, 1192, 545–564. [Google Scholar] [CrossRef] [PubMed]
- Kupfer, D.J.; Frank, E.; Phillips, M.L. Major depressive disorder: New clinical, neurobiological, and treatment perspectives. Lancet 2012, 379, 1045–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, M.L.; Ladouceur, C.D.; Drevets, W.C. A neural model of voluntary and automatic emotion regulation: Implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol. Psychiatry 2008, 13, 833–857. [Google Scholar] [CrossRef]
- Disner, S.; Beevers, C.; Haigh, E.A.P.; Beck, A.T. Neural mechanisms of the cognitive model of depression. Nat. Rev. Neurosci. 2011, 12, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Burgess, P.W.; Gilbert, S.; Dumontheil, I. Function and localization within rostral prefrontal cortex (area 10). Philos. Trans. R. Soc. B Biol. Sci. 2007, 362, 887–899. [Google Scholar] [CrossRef] [Green Version]
- Benadhira, R.; Thomas, F.; Bouaziz, N.; Braha, S.; Andrianisaina, P.S.-K.; Isaac, C.; Moulier, V.; Januel, D. A randomized, sham-controlled study of maintenance rTMS for treatment-resistant depression (TRD). Psychiatry Res. 2017, 258, 226–233. [Google Scholar] [CrossRef]
- Wada, M.; Nakajima, S.; Honda, S.; Takano, M.; Taniguchi, K.; Tsugawa, S.; Mimura, Y.; Hattori, N.; Koike, S.; Zomorrodi, R.; et al. Reduced signal propagation elicited by frontal transcranial magnetic stimulation is associated with oligodendrocyte abnormalities in treatment-resistant depression. J. Psychiatry Neurosci. 2022, 47, E325–E335. [Google Scholar] [CrossRef] [PubMed]
- Kaas, J.H.; Collins, C.E. The organization of sensory cortex. Curr. Opin. Neurobiol. 2001, 11, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Wager, T.D.; Smith, E.E. Neuroimaging studies of working memory. Cogn. Affect. Behav. Neurosci. 2003, 3, 255–274. [Google Scholar] [CrossRef] [Green Version]
- Nitsche, M.A.; Schauenburg, A.; Lang, N.; Liebetanz, D.; Exner, C.; Paulus, W.; Tergau, F. Facilitation of Implicit Motor Learning by Weak Transcranial Direct Current Stimulation of the Primary Motor Cortex in the Human. J. Cogn. Neurosci. 2003, 15, 619–626. [Google Scholar] [CrossRef]
- Liu, P.; Li, G.; Zhang, A.; Yang, C.; Liu, Z.; Sun, N.; Kerang, Z. Brain structural and functional alterations in MDD patient with gastrointestinal symptoms: A resting-state MRI study. J. Affect. Disord. 2020, 273, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; He, Y.; Cui, X.; Liu, F.; Li, H.; Huang, R.; Tang, Y.; Chen, J.; Zhao, J.; Xie, G.; et al. Disrupted Regional Homogeneity in Melancholic and Non-melancholic Major Depressive Disorder at Rest. Front. Psychiatry 2021, 12, 618805. [Google Scholar] [CrossRef]
- Liu, P.; Tu, H.; Zhang, A.; Yang, C.; Liu, Z.; Lei, L.; Wu, P.; Sun, N.; Zhang, K. Brain functional alterations in MDD patients with somatic symptoms: A resting-state fMRI study. J. Affect. Disord. 2021, 295, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Klok, M.P.C.; van Eijndhoven, P.; Argyelan, M.; Schene, A.H.; Tendolkar, I. Structural brain characteristics in treatment-resistant depression: Review of magnetic resonance imaging studies. BJ Psych. Open 2019, 5, e76. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.; Liu, F.; Xue, Z.; Gao, K.; Liu, Z.; Xiao, C.; Chen, H.; Zhao, J. Abnormal resting-state cerebellar–cerebral functional connectivity in treatment-resistant depression and treatment sensitive depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 44, 51–57. [Google Scholar] [CrossRef]
- Raichle, M.E.; MacLeod, A.M.; Snyder, A.Z.; Powers, W.J.; Gusnard, D.A.; Shulman, G.L. A default mode of brain function. Proc. Natl. Acad. Sci. USA 2001, 98, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Rossbach, K.; Zhang, A.; Liu, P.; Zhang, K. Resting-state functional changes in the precuneus within first-episode drug-naive patients with MDD. Neuropsychiatr. Dis. Treat. 2018, 14, 1991–1998. [Google Scholar] [CrossRef] [Green Version]
- De Kwaasteniet, B.P.; Rive, M.M.; Ruhé, H.G.; Schene, A.H.; Veltman, D.J.; Fellinger, L.; Van Wingen, G.A.; Denys, D. Decreased Resting-State Connectivity between Neurocognitive Networks in Treatment Resistant Depression. Front. Psychiatry 2015, 6, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamura, T.; Okamoto, Y.; Okada, G.; Takaishi, Y.; Takamura, M.; Mantani, A.; Kurata, A.; Otagaki, Y.; Yamashita, H.; Yamawaki, S. Association of thalamic hyperactivity with treatment-resistant depression and poor response in early treatment for major depression: A resting-state fMRI study using fractional amplitude of low-frequency fluctuations. Transl. Psychiatry 2016, 6, e754. [Google Scholar] [CrossRef] [Green Version]
- Sahib, A.K.; Loureiro, J.; Vasavada, M.M.; Kubicki, A.; Joshi, S.H.; Wang, K.; Woods, R.P.; Congdon, E.; Wang, D.; Boucher, M.L.; et al. Single and repeated ketamine treatment induces perfusion changes in sensory and limbic networks in major depressive disorder. Eur. Neuropsychopharmacol. 2020, 33, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Shin, J.-H.; Myung, W.; Fava, M.; Mischoulon, D.; Papakostas, G.I.; Choi, K.W.; Na, E.J.; Seo, S.W.; Seong, J.-K.; et al. Deformities of the Globus Pallidus are Associated with Severity of Suicidal Ideation and Impulsivity in Patients with Major Depressive Disorder. Sci. Rep. 2019, 9, 7462. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Yin, Y.; Svob, C.; Long, J.; He, X.; Zhang, Y.; Xu, Z.; Li, L.; Liu, J.; Dong, J.; et al. Amygdala Atrophy and Its Functional Disconnection with the Cortico-Striatal-Pallidal-Thalamic Circuit in Major Depressive Disorder in Females. PLoS ONE 2017, 12, e0168239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kupchik, Y.M.; Brown, R.; Heinsbroek, J.; Lobo, M.K.; Schwartz, D.J.; Kalivas, P.W. Coding the direct/indirect pathways by D1 and D2 receptors is not valid for accumbens projections. Nat. Neurosci. 2015, 18, 1230–1232. [Google Scholar] [CrossRef] [PubMed]
- Root, D.H.; Melendez, R.I.; Zaborszky, L.; Napier, T.C. The ventral pallidum: Subregion-specific functional anatomy and roles in motivated behaviors. Prog. Neurobiol. 2015, 130, 29–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Guo, C.; Ma, Y.; Du, Z.; Wang, Z.; Luo, Y.; Chen, L.; Gao, D.; Li, X.; Xu, K.; et al. A comparative study of amplitude of low-frequence fluctuation of resting-state fMRI between the younger and older treatment-resistant depression in adults. Front. Neurosci. 2022, 16, 949698. [Google Scholar] [CrossRef] [PubMed]
- Mohr, P.; Rodriguez, M.; Slavíčková, A.; Hanka, J. The Application of Vagus Nerve Stimulation and Deep Brain Stimulation in Depression. Neuropsychobiology 2011, 64, 170–181. [Google Scholar] [CrossRef] [PubMed]


| Variable | Male TRD (n = 16) | Female TRD (n = 18) | Male HCs (n = 18) | Female HCs (n = 19) | t(F)/χ2 | p-Value |
|---|---|---|---|---|---|---|
| Age (years) | 40.37 ± 8.98 | 42.16 ± 10.34 | 41.72 ± 11.06 | 43.68 ± 10.98 | 0.300 | 0.825 a |
| Education (years) | 14.37 ± 2.91 | 14.05 ± 3.11 | 14.61 ± 3.16 | 13.47 ± 4.38 | 0.369 | 0.775 a |
| Duration of illness (months) | 46.00 ± 18.40 | 43.33 ± 14.90 | - | - | 0.467 | 0.644 b |
| HAMD-17 score | 22.18 ± 2.73 | 23.72 ± 3.26 | - | - | −1.474 | 0.150 b,* |
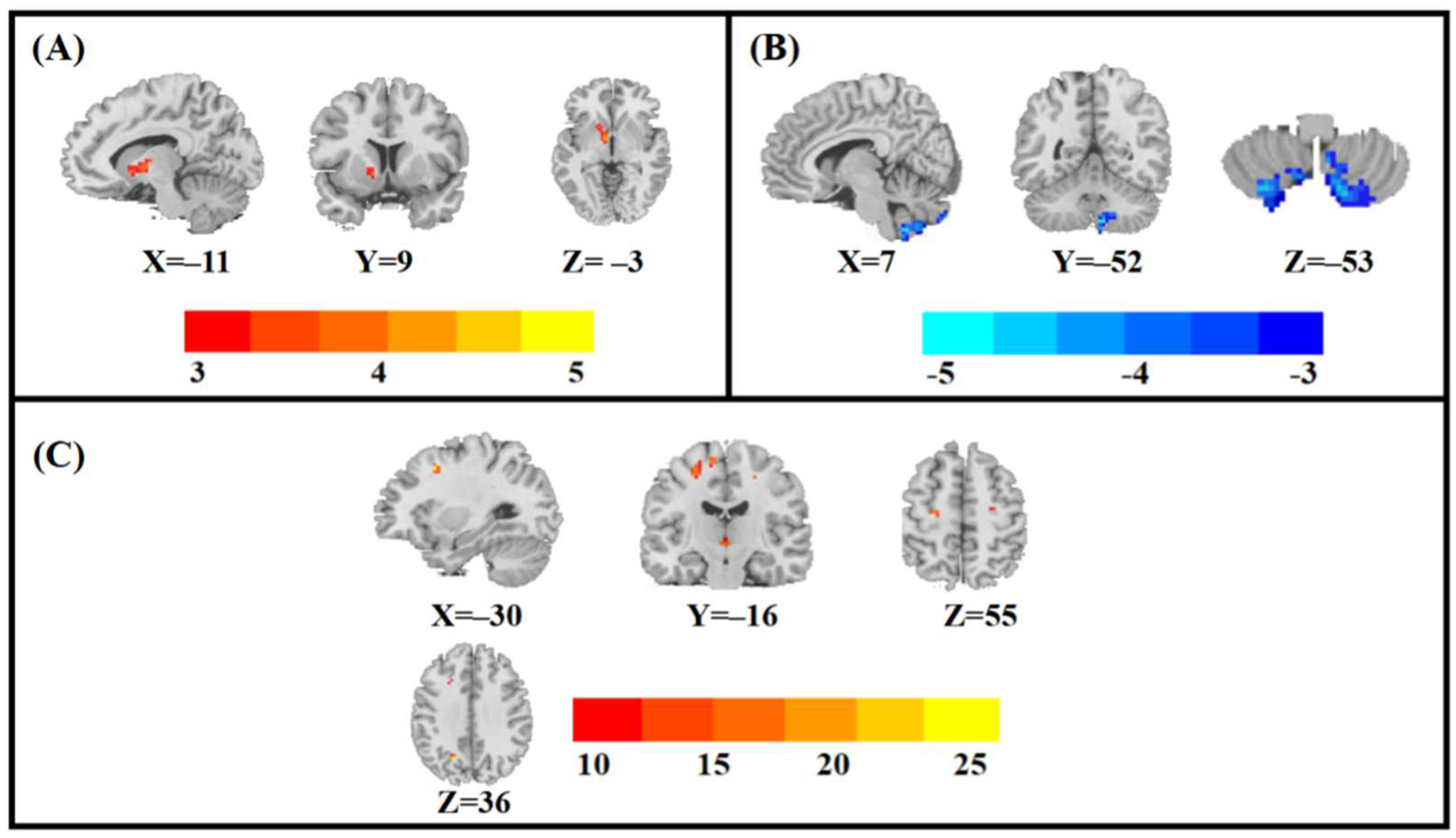
| Clusters | Brain Regions | Peak Coordinates (MNI) | Cluster Size | T/F-Values | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Main effects of the group | ||||||
| 1 | Left pallidum | −11 | 9 | −3 | 51 | 3.570 a |
| Main effects of sex | ||||||
| 1 | Right posterior cerebellar lobe | 7 | −52 | −53 | 22 | −4.091 a |
| Sex-by-group interaction effects | ||||||
| 1 | left middle frontal gyrus | −30 | 12 | 45 | 21 | 26.370 b |
| 2 | left precentral gyrus | −27 | −16 | 55 | 22 | 17.058 b |
| 3 | left precuneus | −18 | −63 | 36 | 24 | 27.699 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Luo, Y.; Ma, Y.; Guo, C.; Du, Z.; Gao, S.; Chen, L.; Wang, Z.; Li, X.; Xu, K.; et al. Sex Differences of the Functional Brain Activity in Treatment-Resistant Depression: A Resting-State Functional Magnetic Resonance Study. Brain Sci. 2022, 12, 1604. https://doi.org/10.3390/brainsci12121604
Sun J, Luo Y, Ma Y, Guo C, Du Z, Gao S, Chen L, Wang Z, Li X, Xu K, et al. Sex Differences of the Functional Brain Activity in Treatment-Resistant Depression: A Resting-State Functional Magnetic Resonance Study. Brain Sciences. 2022; 12(12):1604. https://doi.org/10.3390/brainsci12121604
Chicago/Turabian StyleSun, Jifei, Yi Luo, Yue Ma, Chunlei Guo, Zhongming Du, Shanshan Gao, Limei Chen, Zhi Wang, Xiaojiao Li, Ke Xu, and et al. 2022. "Sex Differences of the Functional Brain Activity in Treatment-Resistant Depression: A Resting-State Functional Magnetic Resonance Study" Brain Sciences 12, no. 12: 1604. https://doi.org/10.3390/brainsci12121604
APA StyleSun, J., Luo, Y., Ma, Y., Guo, C., Du, Z., Gao, S., Chen, L., Wang, Z., Li, X., Xu, K., Hong, Y., Yu, X., Xiao, X., & Fang, J. (2022). Sex Differences of the Functional Brain Activity in Treatment-Resistant Depression: A Resting-State Functional Magnetic Resonance Study. Brain Sciences, 12(12), 1604. https://doi.org/10.3390/brainsci12121604





