Anodal-TDCS over Left-DLPFC Modulates Motor Cortex Excitability in Chronic Lower Back Pain
Abstract
1. Introduction
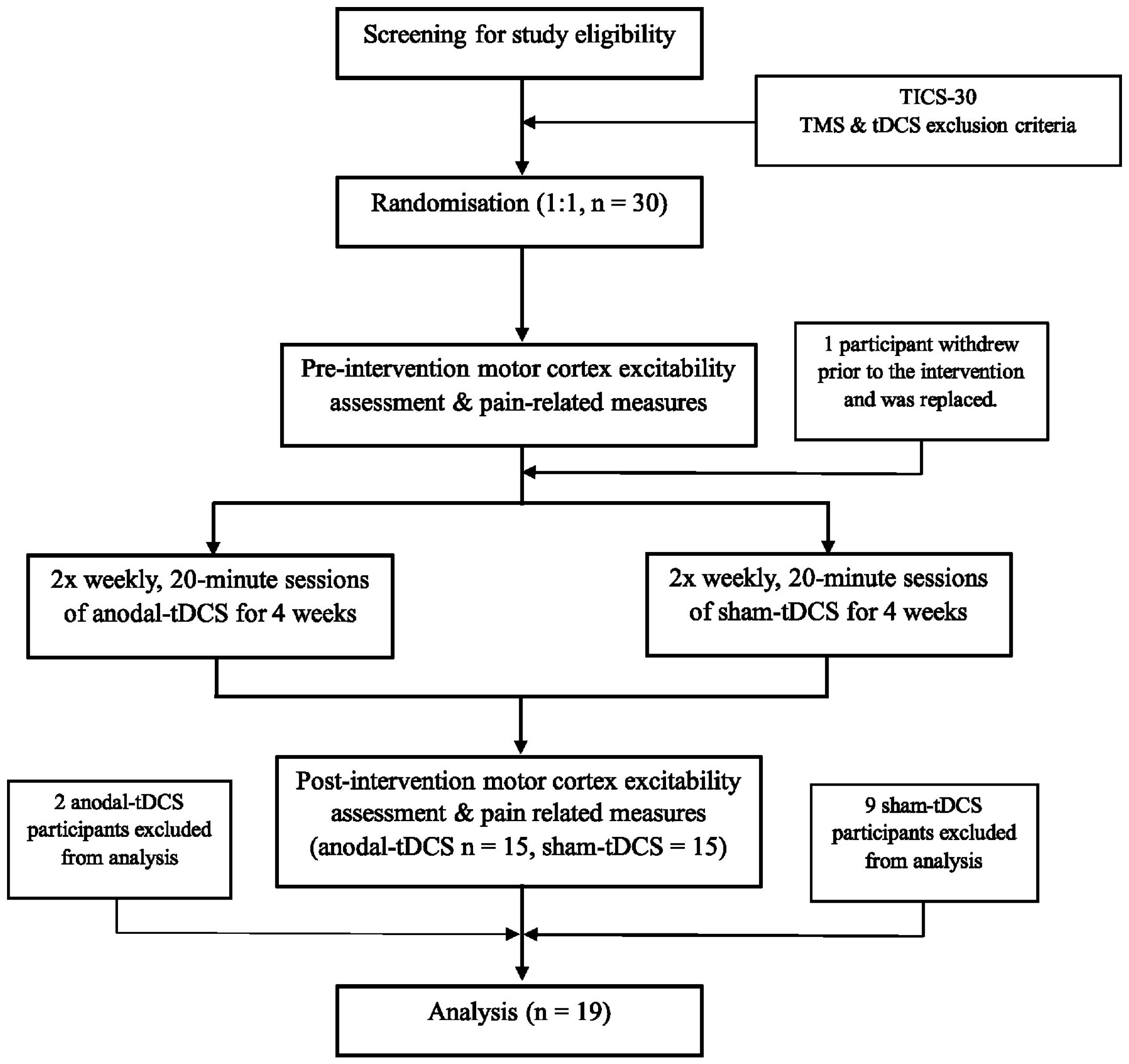
2. Materials and Methods
2.1. Participants
2.2. Measures
2.2.1. Motor Cortex Excitability Measures
2.2.2. Clinical Measures
2.3. Brain Stimulation
2.4. Statistical Analysis
3. Results
3.1. Test Pulse
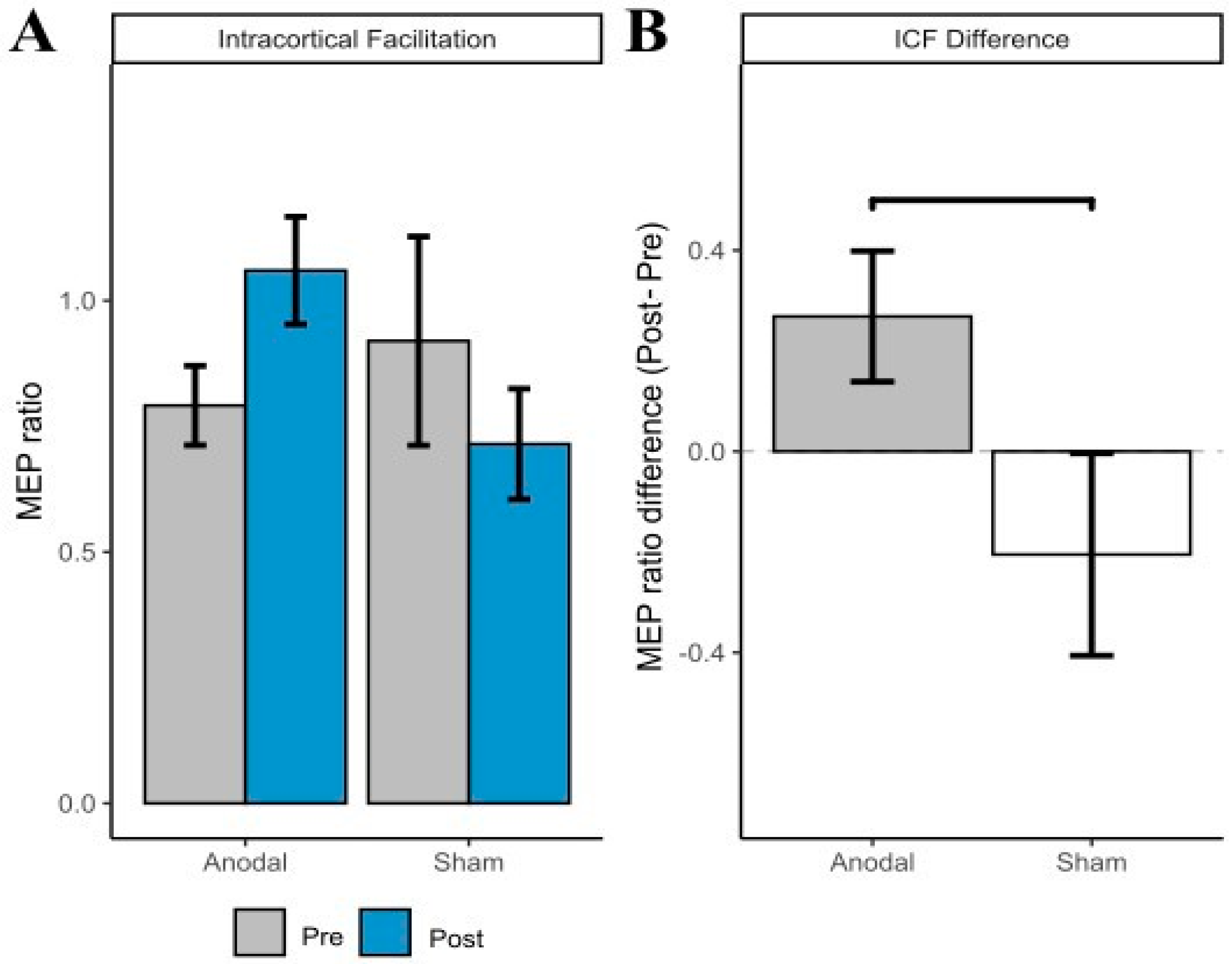
3.2. ICF
3.3. SICI
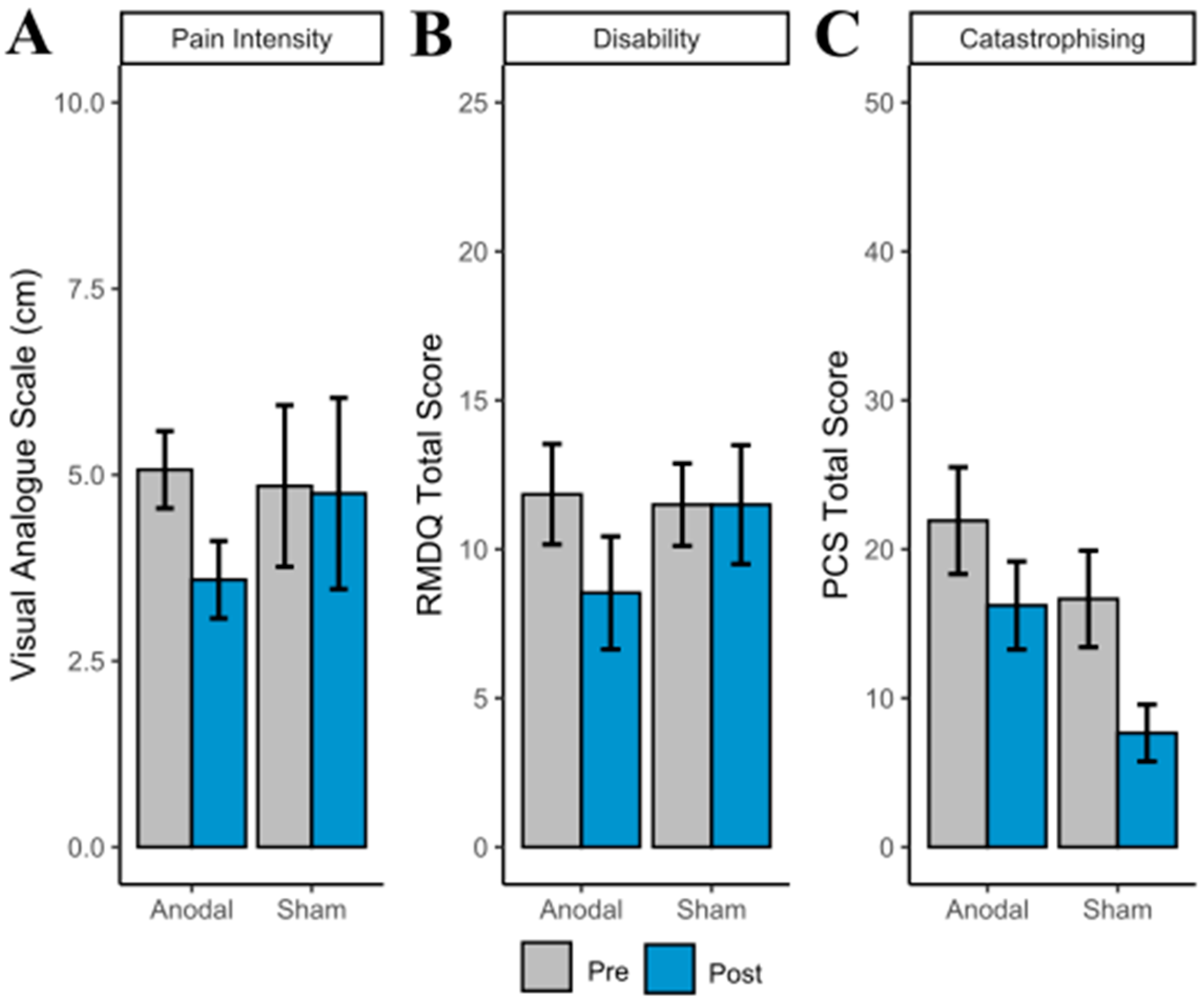
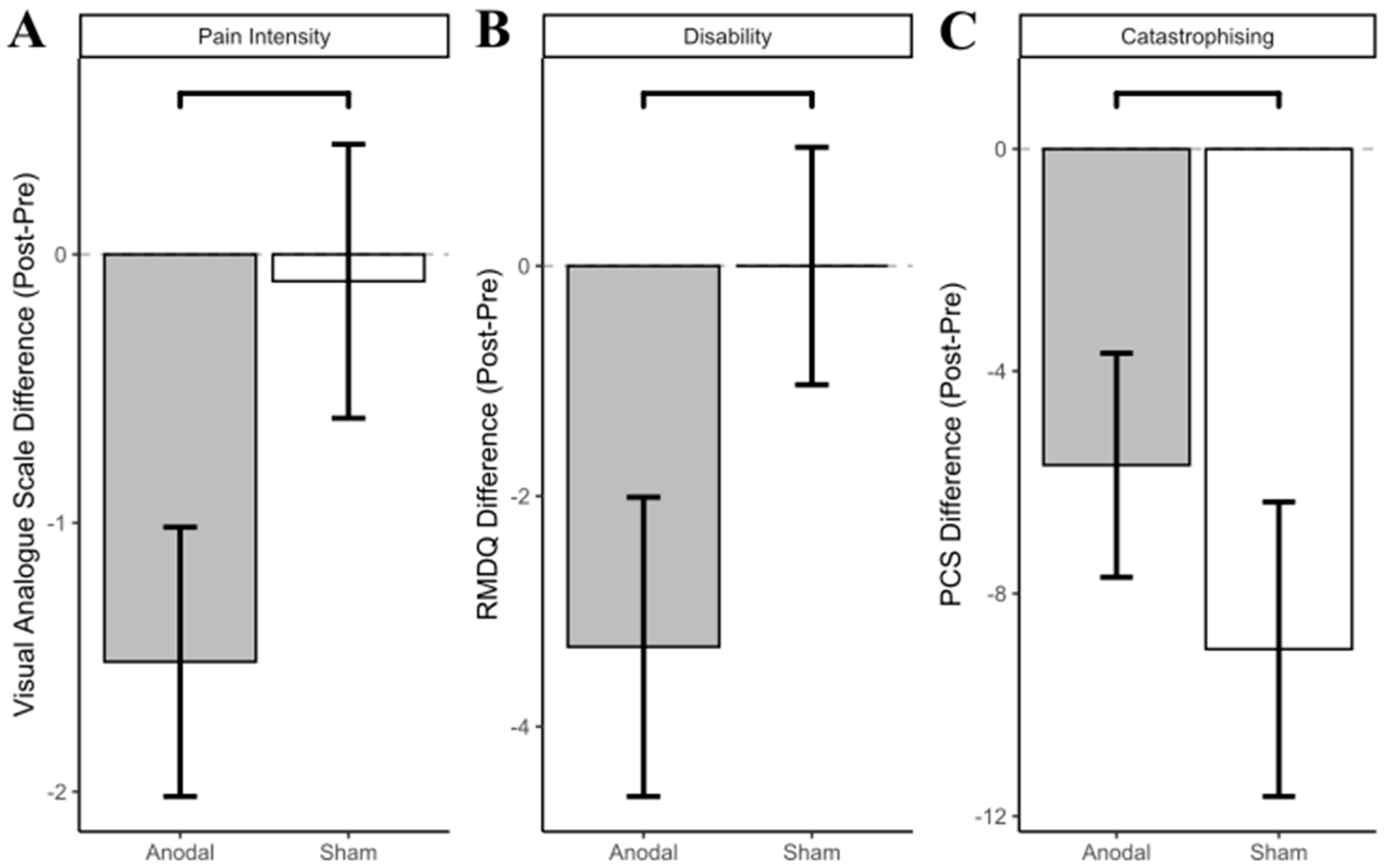
3.4. Clinical Outcomes
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoy, D.; March, L.; Brooks, P.; Blyth, F.; Woolf, A.; Bain, C.; Williams, G.; Smith, E.; Vos, T.; Barendregt, J.; et al. The global burden of low back pain: Estimates from the Global Burden of Disease 2010 study. Ann. Rheum. Dis. 2014, 73, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.; Deyo, R.; Friedly, J.; Skelly, A.; Weimer, M.; Fu, R.; Dana, T.; Kraegel, P.; Griffin, J.; Grusing, S. Systemic pharmacologic therapies for low back pain: A systematic review for an American College of Physicians clinical practice guideline. Ann. Intern. Med. 2017, 166, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.; Deyo, R.; Friedly, J.; Skelly, A.; Hashimoto, R.; Weimer, D.M.; Fu, R.; Dana, M.T.; Kraegel, M.P.; Griffin, M.J.; et al. Nonpharmacologic therapies for low back pain: A systematic review for an American College of Physicians clinical practice guideline. Ann. Intern. Med. 2017, 166, 493–505. [Google Scholar] [CrossRef]
- Massé-Alarie, H.; Schneider, C. Revisiting the Corticomotor Plasticity in Low Back Pain: Challenges and Perspectives. Healthcare 2016, 4, 67. [Google Scholar] [CrossRef] [PubMed]
- Moseley, G.L.; Flor, H. Targeting cortical representations in the treatment of chronic pain: A review. Neurorehabilit. Neural Repair 2012, 26, 646–652. [Google Scholar] [CrossRef]
- Parker, R.S.; Lewis, G.N.; Rice, D.A.; McNair, P.J. Is motor cortical excitability altered in people with chronic pain? A systematic review and meta-analysis. Brain Stimul. 2016, 9, 488–500. [Google Scholar] [CrossRef]
- Apkarian, A.V.; Baliki, M.N.; Geha, P.Y. Towards a theory of chronic pain. Prog. Neurobiol. 2009, 87, 81–97. [Google Scholar] [CrossRef]
- Baliki, M.N.; Chialvo, D.R.; Geha, P.Y.; Levy, R.M.; Harden, R.N.; Parrish, T.B.; Apkarian, A.V. Chronic pain and the emotional brain: Specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J. Neurosci. 2006, 26, 12165–12173. [Google Scholar] [CrossRef]
- Konno, S.I.; Sekiguchi, M. Association between brain and low back pain. J. Orthop. Sci. 2018, 23, 3–7. [Google Scholar] [CrossRef]
- Tracey, I.; Bushnell, M.C. How neuroimaging studies have challenged us to rethink: Is chronic pain a disease? J. Pain 2009, 10, 1113–1120. [Google Scholar] [CrossRef]
- Qu, S.; Wei, J.; Wang, Q.; Li, Y.; Jin, X.; Chaib, L. Maladaptive motor cortical excitability and connectivity in polyneuropathy with neuropathic pain. Eur. J. Neurol. 2022, 29, 1465–1476. [Google Scholar] [CrossRef]
- Schabrun, S.M.; Hodges, P.W. Muscle pain differentially modulates short interval intracortical inhibition and intracortical facilitation in primary motor cortex. J. Pain 2012, 13, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lei, Y.; Tian, Y.; Xu, S.; Shen, X.; Wu, H.; Bao, S.; Wang, F. The etiological contribution of GABAergic plasticity to the pathogenesis of neuropathic pain. Mol. Pain 2019, 15, 1744806919847366. [Google Scholar] [CrossRef] [PubMed]
- Apkarian, A.V.; Sosa, Y.; Sonty, S.; Levy, R.M.; Harden, R.N.; Parrish, T.B.; Gitelman, D.R. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J. Neurosci. 2004, 24, 10410–10415. [Google Scholar] [CrossRef] [PubMed]
- Ivo, R.; Nicklas, A.; Dargel, J.; Sobottke, R.; Delank, K.S.; Eysel, P.; Weber, B. Brain structural and psychometric alterations in chronic low back pain. Eur. Spine J. 2013, 22, 1958–1964. [Google Scholar] [CrossRef] [PubMed]
- Luedtke, K.; Rushton, A.; Wright, C.; Geiss, B.; Juergens, T.P.; May, A. Transcranial direct current stimulation for the reduction of clinical and experimentally induced pain: A systematic review and meta-analysis. Clin. J. Pain 2012, 28, 452–461. [Google Scholar] [CrossRef]
- O’Connell, N.E.; Marston, L.; Spencer, S.; DeSouza, L.H.; Wand, B.M. Non-invasive brain stimulation techniques for chronic pain. Cochrane Database Syst. Rev. 2018, 4, CD008208. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Cohen, L.G.; Wassermann, E.M.; Priori, A.; Lang, N.; Antal, A.; Paulus, W.; Hummel, F.; Boggio, P.S.; Fregni, F.; et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008, 1, 206–223. [Google Scholar] [CrossRef]
- Dos Santos, M.F.; Love, T.M.; Martikainen, I.K.; Nascimento, T.D.; Fregni, F.; Cummiford, C.; Deboer, M.D.; Zubieta, J.-K.; DaSilva, A.F.M. Immediate effects of tDCS on the μ-opioid system of a chronic pain patient. Front. Psychiatry 2012, 3, 93. [Google Scholar] [CrossRef]
- Garcia-Larrea, L.; Peyron, R. Motor cortex stimulation for neuropathic pain: From phenomenology to mechanisms. Neuroimage 2007, 37 (Suppl. S1), S71–S79. [Google Scholar] [CrossRef]
- Antal, A.; Terney, D.; Kühnl, S.; Paulus, W. Anodal transcranial direct current stimulation of the motor cortex ameliorates chronic pain and reduces short intracortical inhibition. J. Pain Symptom Manag. 2010, 39, 890–903. [Google Scholar] [CrossRef] [PubMed]
- Fregni, F.; Boggio, P.; Lima, M.; Ferreira, M.J.; Wagner, T.; Rigonatti, S.P.; Castro, A.W.; Souza, D.R.; Riberto, M.; Freedman, S.D.; et al. A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain 2006, 122, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Fregni, F.; Gimenes, R.; Valle, A.C.; Ferreira, M.J.L.; Rocha, R.R.; Natalle, L.; Bravo, R.; Rigonatti, S.P.; Freedman, S.D.; Nitsche, M.A.; et al. A randomized, sham-controlled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgia. Arthritis Rheum. 2006, 54, 3988–3998. [Google Scholar] [CrossRef] [PubMed]
- Schabrun, S.M.; Burns, E.; Thapa, T.; Hodges, P. The response of the primary motor cortex to neuromodulation is altered in chronic low back pain: A preliminary study. Pain Med. 2018, 19, 1227–1236. [Google Scholar] [CrossRef]
- Luedtke, K.; Rushton, A.; Wright, C.; Jürgens, T.; Polzer, A.; Mueller, G.; May, A. Effectiveness of transcranial direct current stimulation preceding cognitive behavioural management for chronic low back pain: Sham controlled double blinded randomised controlled trial. BMJ 2015, 350, h1640. [Google Scholar] [CrossRef]
- Hazime, F.A.; Baptista, A.F.; de Freitas, D.G.; Monteiro, R.L.; Maretto, R.L.; Hasue, R.H.; João, S. Treating low back pain with combined cerebral and peripheral electrical stimulation: A randomized, double-blind, factorial clinical trial. Eur. J. Pain 2017, 21, 1132–1143. [Google Scholar] [CrossRef]
- Baptista, A.F.; Fernandes, A.M.B.; Sá, K.N.; Okano, A.H.; Brunoni, A.R.; Lara-Solares, A.; Iskandar, A.J.; Guerrero, C.; Amescua-García, C.; Kraychete, D.C.; et al. Latin American and Caribbean consensus on noninvasive central nervous system neuromodulation for chronic pain management (LAC2-NIN-CP). Pain Rep. 2019, 4, e692. [Google Scholar] [CrossRef]
- Brighina, F.; Piazza, A.; Vitello, G.; Aloisio, A.; Palermo, A.; Daniele, O.; Fierro, B. rTMS of the prefrontal cortex in the treatment of chronic migraine: A pilot study. J. Neurol. Sci. 2004, 227, 67–71. [Google Scholar] [CrossRef]
- O’Reardon, J.P.; Fontecha, J.F.; Cristancho, M.A.; Newman, S. Unexpected reduction in migraine and psychogenic headaches following rTMS treatment for major depression: A report of two cases. CNS Spectr. 2007, 12, 921–925. [Google Scholar] [CrossRef]
- Sampson, S.M.; Rome, J.D.; Rummans, T.A. Slow-frequency rTMS reduces fibromyalgia pain. Pain Med. 2006, 7, 115–118. [Google Scholar] [CrossRef]
- Brighina, F.; De Tommaso, M.; Giglia, F.; Scalia, S.; Cosentino, G.; Puma, A.; Panetta, M.; Giglia, G.; Fierro, B. Modulation of pain perception by transcranial magnetic stimulation of left prefrontal cortex. J. Headache Pain 2011, 12, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, J.; Minoshima, S.; Casey, K.L. Keeping pain out of mind: The role of the dorsolateral prefrontal cortex in pain modulation. Brain 2003, 126, 1079–1091. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, J.; Cross, D.J.; Minoshima, S.; Morrow, T.J.; Paulson, P.E.; Casey, K.L. A unique representation of heat allodynia in the human brain. Neuron 2002, 35, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Cao, N.; Pi, Y.; Liu, K.; Meng, H.; Tan, X. Inhibitory and facilitatory connections from dorsolateral prefrontal to primary motor cortex in healthy humans at rest—An rTMS study. Neurosci. Lett. 2018, 687, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.-P.; Aleman, A.; Baeken, C.; Benninger, D.H.; Brunelin, J.; Di Lazzaro, V.; Filipović, S.R.; Grefkes, C.; Hasan, A.; Hummel, F.C.; et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014–2018). Clin. Neurophysiol. 2020, 131, 474–528. [Google Scholar] [CrossRef]
- Fierro, B.; De Tommaso, M.; Giglia, F.; Giglia, G.; Palermo, A.; Brighina, F. Repetitive transcranial magnetic stimulation (rTMS) of the dorsolateral prefrontal cortex (DLPFC) during capsaicin-induced pain: Modulatory effects on motor cortex excitability. Exp. Brain Res. 2010, 203, 31–38. [Google Scholar] [CrossRef]
- Vaseghi, B.; Zoghi, M.; Jaberzadeh, S. How does anodal transcranial direct current stimulation of the pain neuromatrix affect brain excitability and pain perception? A randomised, double-blind, sham-control study. PLoS ONE 2015, 10, e0118340. [Google Scholar] [CrossRef]
- Lefaucheur, J.P.; Drouot, X.; Ménard-Lefaucheur, I.; Keravel, Y.; Nguyen, J.P. Motor cortex rTMS restores defective intracortical inhibition in chronic neuropathic pain. Neurology 2006, 67, 1568–1574. [Google Scholar] [CrossRef]
- Schwenkreis, P.; Witscher, K.; Janssen, F.; Addo, A.; Dertwinkel, R.; Zenz, M.; Malin, J.-P.; Tegenthoff, M. Influence of the N-methyl-D-aspartate antagonist memantine on human motor cortex excitability. Neurosci. Lett. 1999, 270, 137–140. [Google Scholar] [CrossRef]
- Rossi, S.; Hallett, M.; Rossini, P.M.; Pascual-Leone, A. Safety of TMS Consensus Group Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 2009, 120, 2008–2039. [Google Scholar] [CrossRef]
- Trans Cranial Technologies. 10/20 System Positioning Manual. 2012. Available online: https://www.trans-cranial.com/docs/10_20_pos_man_v1_0_pdf.pdf (accessed on 1 August 2014).
- Strutton, P.H.; Theodorou, S.; Catley, M.; McGregor, A.H.; Davey, N.J. Corticospinal excitability in patients with chronic low back pain. J. Spinal Disord. Tech. 2005, 18, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Bouguetoch, A.; Grosprêtre, S.; Martin, A. Optimal stimulation parameters for spinal and corticospinal excitabilities during contraction, motor imagery and rest: A pilot study. PLoS ONE 2020, 15, e0235074. [Google Scholar] [CrossRef] [PubMed]
- Rossini, P.M.; Burke, D.; Chen, R.; Cohen, L.G.; Daskalakis, Z.; Di Iorio, R.; Di Lazzaro, V.; Ferreri, F.; Fitzgerald, P.B.; George, M.S.; et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin. Neurophysiol. 2015, 126, 1071–1107. [Google Scholar] [CrossRef] [PubMed]
- Kujirai, T.; Caramia, M.D.; Rothwell, J.C.; Day, B.L.; Thompson, P.D.; Ferbert, A.; Wroe, S.; Asselman, P.; Marsden, C.D. Corticocortical inhibition in human motor cortex. J. Physiol. 1993, 471, 501–519. [Google Scholar] [CrossRef] [PubMed]
- Garry, M.I.; Thomson, R.H. The effect of test TMS intensity on short-interval intracortical inhibition in different excitability states. Exp. Brain Res. 2009, 193, 267–274. [Google Scholar] [CrossRef]
- Melzack, R. The short-form McGill Pain Questionnaire. Pain 1987, 30, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Hawker, G.A.; Mian, S.; Kendzerska, T.; French, M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res. 2011, 63 (Suppl. S11), S240–S252. [Google Scholar] [CrossRef]
- Gallagher, E.J.; Bijur, P.E.; Latimer, C.; Silver, W. Reliability and validity of a visual analog scale for acute abdominal pain in the ED. Am. J. Emerg. Med. 2002, 20, 287–290. [Google Scholar] [CrossRef]
- Roland, M.; Morris, R. A study of the natural history of back pain. Part I: Development of a reliable and sensitive measure of disability in low-back pain. Spine 1983, 8, 141–144. [Google Scholar] [CrossRef]
- Rocchi, M.B.; Sisti, D.; Benedetti, P.; Valentini, M.; Bellagamba, S.; Federici, A. Critical comparison of nine different self-administered questionnaires for the evaluation of disability caused by low back pain. Eur. Med. 2005, 41, 275–281. [Google Scholar]
- Sullivan, M.J.L.; Bishop, S.R.; Pivik, J. The Pain Catastrophizing Scale: Development and validation. Psychol. Assess. 1995, 7, 524–532. [Google Scholar] [CrossRef]
- Osman, A.; Barrios, F.X.; Gutierrez, P.M.; Kopper, B.A.; Merrifield, T.; Grittmann, L. The Pain Catastrophizing Scale: Further psychometric evaluation with adult samples. J. Behav. Med. 2000, 23, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Ambrus, G.G.; Al-Moyed, H.; Chaieb, L.; Sarp, L.; Antal, A.; Paulus, W. The fade-in--short stimulation--fade out approach to sham tDCS--reliable at 1 mA for naïve and experienced subjects, but not investigators. Brain Stimul. 2012, 5, 499–504. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-155. 2022. Available online: https://CRAN.R-project.org/package=nlme (accessed on 13 October 2022).
- Goodrich, B.; Gabry, J.; Ali, I.; Brilleman, S. Rstanarm: Bayesian Applied Regression Modeling Via Stan. R Package Version 2.21.3. 2022. Available online: https://mc-stan.org/rstanarm/ (accessed on 13 October 2022).
- Makowski, D.; Ben-Shachar, M.; Patil, I.; Lüdecke, D. Automated Results Reporting as a Practical Tool to Improve Reproducibility and Methodological Best Practices Adoption. CRAN. 2021. Available online: https://github.com/easystats/report (accessed on 13 October 2022).
- Makowski, D.; Ben-Shachar, M.; Lüdecke, D. Bayestestr: Describing effects and their uncertainty, existence and significance within the bayesian framework. J. Open Source Softw. 2019, 4, 1541. [Google Scholar] [CrossRef]
- Koes, B.W.; van Tulder, M.W.; Ostelo, R.; Kim Burton, A.; Waddell, G. Clinical guidelines for the management of low back pain in primary care: An international comparison. Spine 2001, 26, 2504–2514. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, F.M.; Abraira, V.; Royuela, A.; Corcoll, J.; Alegre, L.; Cano, A.; Muriel, A.; Zamora, J.; Gil Del Real, M.T.; Gestoso, M.; et al. Minimal clinically important change for pain intensity and disability in patients with nonspecific low back pain. Spine 2007, 32, 2915–2920. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Aono, S.; Inoue, S.; Imajo, Y.; Nishida, N.; Funaba, M.; Harada, H.; Mori, A.; Matsumoto, M.; Higuchi, F.; et al. Clinically significant changes in pain along the Pain Intensity Numerical Rating Scale in patients with chronic low back pain. PLoS ONE 2020, 15, e0229228. [Google Scholar] [CrossRef]
- Cash, R.F.H.; Noda, Y.; Zomorrodi, R.; Radhu, N.; Farzan, F.; Rajji, T.K.; Fitzgerald, P.; Chen, R.; Daskalakis, Z.J.; Blumberger, D.M. Characterization of Glutamatergic and GABAA-Mediated Neurotransmission in Motor and Dorsolateral Prefrontal Cortex Using Paired-Pulse TMS-EEG. Neuropsychopharmacology 2017, 42, 502–511. [Google Scholar] [CrossRef]
- Peek, A.L.; Rebbeck, T.; Puts, N.A.; Watson, J.; Aguila, M.R.; Leaver, A.M. Brain GABA and glutamate levels across pain conditions: A systematic literature review and meta-analysis of 1H-MRS studies using the MRS-Q quality assessment tool. NeuroImage 2020, 210, 116532. [Google Scholar] [CrossRef]
- Kandić, M.; Moliadze, V.; Andoh, J.; Flor, H.; Nees, F. Brain circuits involved in the development of chronic musculoskeletal pain: Evidence from non-invasive brain stimulation. Front. Neurol. 2021, 12, 732034. [Google Scholar] [CrossRef]
- Tamura, Y.; Hoshiyama, M.; Inui, K.; Nakata, H.; Qiu, Y.; Ugawa, Y.; Inoue, K.; Kakigi, R. Facilitation of A[delta]-fiber-mediated acute pain by repetitive transcranial magnetic stimulation. Neurology 2004, 62, 2176–2181. [Google Scholar] [CrossRef] [PubMed]
- Tracey, I.; Mantyh, P.W. The cerebral signature for pain perception and its modulation. Neuron 2007, 55, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Freigang, S.; Lehner, C.; Fresnoza, S.; Ali, K.M.; Hlavka, E.; Eitler, A.; Szilagyi, I.; Bornemann-Cimenti, H.; Deutschmann, H.; Reishofer, G.; et al. Comparing the impact of multi-session left dorsolateral prefrontal and primary motor cortex neuronavigated repetitive transcranial magnetic stimulation (nrTMS) on chronic pain patients. Brain Sci. 2021, 11, 961. [Google Scholar] [CrossRef]
- Price, D.D.; Finniss, D.G.; Benedetti, F. A comprehensive review of the placebo effect: Recent advances and Current thought. Annu. Rev. Psychol. 2008, 59, 565–590. [Google Scholar] [CrossRef]
- Hsieh, C.-A.; Maier, K.S. A preliminary Bayesian analysis of incomplete longitudinal data from a small sample: Methodological advances in an international comparative study of educational inequality. Int. J. Res. Method Educ. 2009, 32, 103–125. [Google Scholar] [CrossRef]
- McNeish, D. On using Bayesian methods to address small sample problems. Struct. Equ. Model. A Multidiscip. J. 2016, 23, 750–773. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Pilato, F.; Oliviero, A.; Dileone, M.; Saturno, E.; Mazzone, P.; Insola, A.; Profice, P.; Ranieri, F.; Capone, F.; et al. Origin of facilitation of motor-evoked potentials after paired magnetic stimulation: Direct recording of epidural activity in conscious humans. J. Neurophysiol. 2006, 96, 1765–1771. [Google Scholar] [CrossRef]
- Schabrun, S.M.; Elgueta-Cancino, E.L.; Hodges, P.W. Smudging of the motor cortex is related to the severity of low back pain. Spine 2017, 42, 1172–1178. [Google Scholar] [CrossRef]

| Total (n = 19) | Males (n = 10) | Females (n = 9) | Anodal-tDCS | Sham-tDCS | |
|---|---|---|---|---|---|
| Age | 53.16 (14.80; 21–76) | 57.80 (14.52) | 48.00 (13.32) | 49.69 (14.34) | 60.67 (12.87) |
| Years of Education | 12.65 (3.48) | 12.55 (3.25) | 12.77 (3.72) | 12.53 (3.59) | 12.92 (3.22) |
| Duration of Diagnosis (years) | 13.38 (12.41) | 17.93 (14.11) | 8.33 (7.47) | 12.31 (9.50) | 15.71 (16.87) |
| Resting Motor Threshold | 49.79 (8.75) | 47.80 (5.90) | 52.00 (11.07) | 49.31 (9.12) | 50.83 (8.59) |
| CLBP Classification | |||||
| Non-Specific | 84% | 80% | 89% | 85% | 83% |
| Specific | 16% | 20% | 11% | 15% | 17% |
| Percentage taking Pain Medication | 74% | 50% | 100% | 69% | 83% |
| Anti-Inflammatory (Celebrex) a | 42% | 40% | 44% | 54% | 17% |
| Pain Killer (Tremadol) a | 53% | 20% | 89% | 46% | 67% |
| Benzodiazepine (Valium) a | 21% | 10% | 33% | 23% | 17% |
| Anti-Depressants (Endep) a | 5% | 10% | - | 8% | - |
| Engaging in Physiotherapy | 47% | 30% | 67% | 54% | 33% |
| Past Surgery | 21% | 20% | 22% | 77% | 17% |
| Other Pain Management | 79% | 80% | 78% | 15% | 67% |
| Depression and Anxiety Disorder | 16% | 10% | 22% | 15% | 17% |
| Anti-Anxiety Medication a | 33% | - | 50% | 50% | - |
| Anodal-tDCS | Sham-tDCS | |||
|---|---|---|---|---|
| Pre-Intervention | Post-Intervention | Pre-Intervention | Post-Intervention | |
| VAS | 5.07 (1.87) | 3.59 (1.72) a | 4.85 (2.66) | 4.75 (3.14) |
| RMDQ | 11.85 (6.08) | 8.54 (6.84) b | 11.50 (3.39) | 11.50 (4.89) |
| PCS | 21.92 (12.93) | 16.23 (10.67) c | 16.67 (7.94) | 7.67 (4.68) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corti, E.J.; Nguyen, A.T.; Marinovic, W.; Gasson, N.; Loftus, A.M. Anodal-TDCS over Left-DLPFC Modulates Motor Cortex Excitability in Chronic Lower Back Pain. Brain Sci. 2022, 12, 1654. https://doi.org/10.3390/brainsci12121654
Corti EJ, Nguyen AT, Marinovic W, Gasson N, Loftus AM. Anodal-TDCS over Left-DLPFC Modulates Motor Cortex Excitability in Chronic Lower Back Pain. Brain Sciences. 2022; 12(12):1654. https://doi.org/10.3390/brainsci12121654
Chicago/Turabian StyleCorti, Emily J., An T. Nguyen, Welber Marinovic, Natalie Gasson, and Andrea M. Loftus. 2022. "Anodal-TDCS over Left-DLPFC Modulates Motor Cortex Excitability in Chronic Lower Back Pain" Brain Sciences 12, no. 12: 1654. https://doi.org/10.3390/brainsci12121654
APA StyleCorti, E. J., Nguyen, A. T., Marinovic, W., Gasson, N., & Loftus, A. M. (2022). Anodal-TDCS over Left-DLPFC Modulates Motor Cortex Excitability in Chronic Lower Back Pain. Brain Sciences, 12(12), 1654. https://doi.org/10.3390/brainsci12121654







