Towards Conceptual Clarification of Proactive Inhibitory Control: A Review
Abstract
:1. Introduction
2. Reactive Inhibition
2.1. Reactive Inhibition as Immediate Stopping Control over Actions
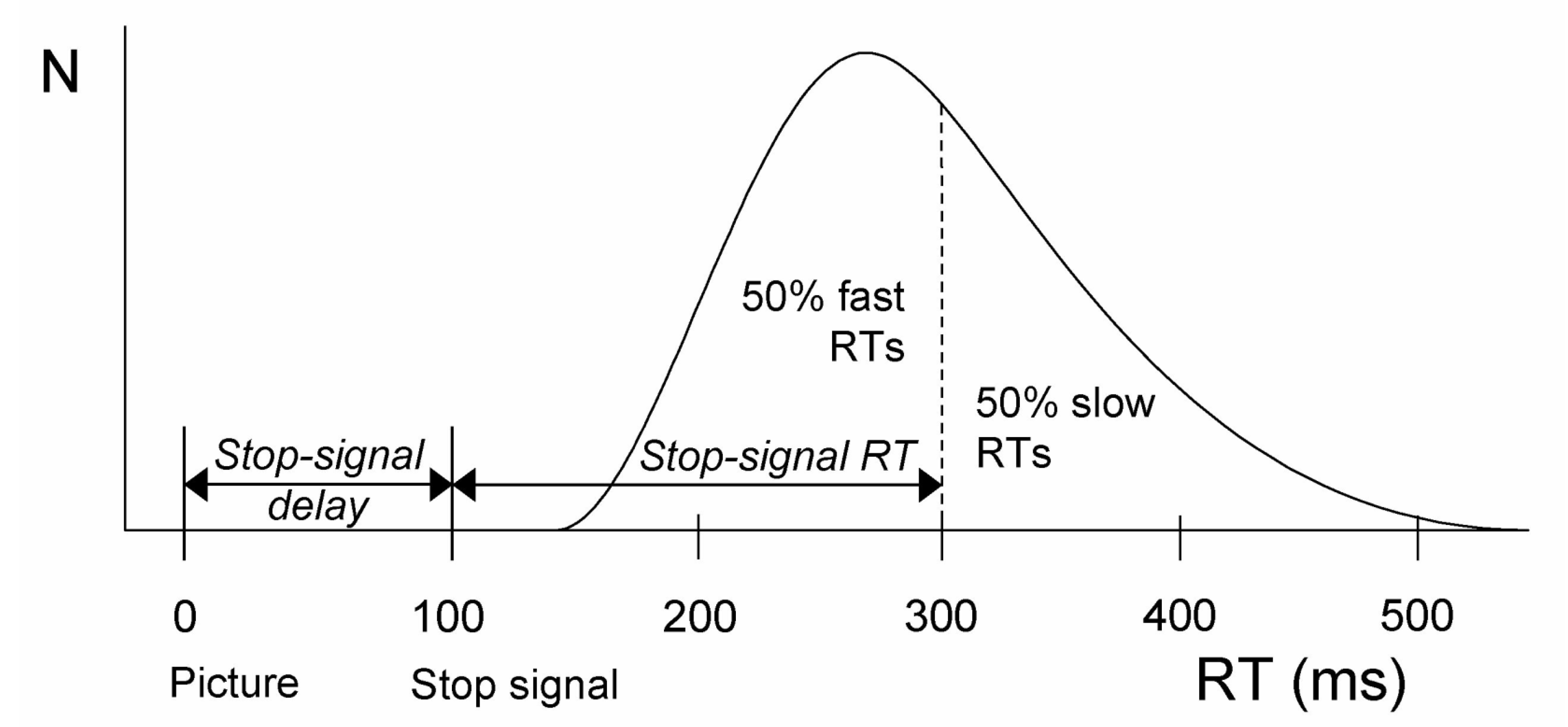
2.2. The Stop-Signal Task Measures Reactive Inhibition
3. Proactive Inhibition: Pre-Amping Reactive Inhibition
3.1. Amping Up Reactive Inhibition
3.2. Amping Down Reactive Inhibition
4. Proactive Inhibition: Pre-Setting the Action Control System
Presetting Action-Related Process to Facilitate Future Inhibition Success
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADHD | attention deficit/hyperactivity disorder |
| ms | milliseconds |
| MT | movement time |
| OCD | obsessive compulsive disorder |
| PD | Parkinson’s disease |
| RT | reaction time |
| SSRT | stop-signal reaction time |
References
- Logan, G.D. On the ability to inhibit though and action: A user’s guide to the stop-signal paradigm. In Inhibitory Processes in Attention, Memory, and Language; Dagenbach, D., Carr, T.H., Eds.; Academic Press: San Diego, CA, USA, 1994; pp. 189–239. [Google Scholar]
- Logan, G.D.; Cowan, W.B. On the ability to inhibit thought and action: A theory of an act of control. Psychol. Rev. 1984, 91, 291–327. [Google Scholar] [CrossRef]
- Posner, M.I.; Cohen, Y. Components of visual orienting. In Attention and Performance X: Control of Language Processes; Bouma, H., Bouwhuis, D.G., Eds.; Erlbaum: Hillsdale, MI, USA, 1984; pp. 531–556. [Google Scholar]
- Logan, G.D. On the ability to inhibit complex movements: A stop-signal study of typewriting. J. Exp. Psychol. Hum. Percept. Perform. 1982, 8, 778–792. [Google Scholar] [CrossRef]
- Logan, G.D. On the ability to inhibit simple thoughts and actions: I. Stop signal studies of decision and memory. J. Exp. Psychol. Learn. Mem. Cogn. 1983, 9, 585–606. [Google Scholar] [CrossRef]
- Lappin, J.S.; Eriksen, C.W. Use of a delayed signal to stop a visual reaction time response. J. Exp. Psychol. 1966, 72, 805–811. [Google Scholar] [CrossRef] [Green Version]
- Vince, M.A. The intermittency of control movements and the psychological refractory period. Br. J. Psychol. Gen. Sect. 1948, 38, 149–157. [Google Scholar] [CrossRef]
- Verbruggen, F.; Logan, G.D. Response inhibition in the stop-signal paradigm. Trends Cogn. Sci. 2008, 12, 418–424. [Google Scholar] [CrossRef] [Green Version]
- Verbruggen, F.; Aron, A.R.; Band, G.P.H.; Beste, C.; Bissett, P.G.; Brockett, A.T.; Brown, J.W.; Chamberlain, S.R.; Chambers, C.D.; Colonius, H.; et al. A consensus guide to capturing the ability to inhibit actions and impulsive behaviors in the stop-signal task. eLife 2019, 8, e46323. [Google Scholar] [CrossRef]
- Boucher, L.; Palmeri, T.J.; Logan, G.D.; Schall, J.D. Inhibitory control in mind and brain: An interactive race model of countermanding saccades. Psychol. Rev. 2007, 114, 376–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Logan, G.D.; Yamaguchi, M.; Schall, J.D.; Palmeri, T.J. Inhibitory control in mind and brain 2.0: Blocked-input models of saccadic countermanding. Psychol. Rev. 2015, 122, 115–147. [Google Scholar] [CrossRef]
- Schall, J.D.; Palmeri, T.J.; Logan, G.D. Models of inhibitory control. Philos. Trans. R. Soc. B 2017, 372, 20160193. [Google Scholar] [CrossRef]
- Colonius, H.; Diederich, A. Paradox resolved: Stop signal race model with negative dependence. Psychol. Rev. 2018, 125, 1051–1058. [Google Scholar] [CrossRef]
- Colonius, H.A. Note on the stop signal paradigm, or how to observe the unobservable. Psychol. Rev. 1990, 97, 309–312. [Google Scholar] [CrossRef]
- Matzke, D.; Dolan, C.V.; Logan, G.D.; Brown, S.D.; Wagenmakers, E.J. Bayesian parametric estimation of stop-signal reaction time distributions. J. Exp. Psychol. Gen. 2013, 142, 1047–1073. [Google Scholar] [CrossRef]
- Soltanifar, M.; Dupuis, A.; Schachar, R.; Escobar, M.A. Frequentist mixture modelling of stop-signal reaction times. Biostat. Epidemiol. 2019, 3, 90–108. [Google Scholar] [CrossRef]
- Soltanifar, M.; Escobar, M.A.; Dupuis, A.; Schachar, R.A. Bayesian mixture modelling of stop-signal reaction time distributions: The second contextual solution for the problem of aftereffects of inhibition on SSRT estimations. Brain Sci. 2021, 11, 1102. [Google Scholar] [CrossRef]
- Williams, B.R.; Ponesse, J.S.; Schachar, R.J.; Logan, G.D.; Tannock, R. Development of inhibitory control across the life span. Dev. Psychol. 1999, 35, 205–213. [Google Scholar] [CrossRef]
- Lipszyc, J.; Schachar, R. Inhibitory control and psychopathology: A meta-analysis of studies using the stop signal task. Int. Neuropsychol. Soc. 2010, 16, 1064–1076. [Google Scholar] [CrossRef]
- Mancini, C.; Cardona, F.; Baglioni, V.; Panunzi, S.; Pantano, P.; Suppa, A.; Mirabella, G. Inhibition is impaired in children with obsessive-compulsive symptoms but not in those with tics. Mov. Disord. 2018, 33, 950–959. [Google Scholar] [CrossRef] [PubMed]
- Mirabella, G.; Mancini, C.; Valente, F.; Cardona, F. Children with primary complex motor stereotypies show impaired reactive but not proactive inhibition. Cortex 2020, 124, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Gauggel, S.; Rieger, M.; Feghoff, T.A. Inhibition of ongoing responses in patients with Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2004, 75, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Van den Wildenberg, W.P.M.; Ridderinkhof, K.R.; van Wouwe, N.C.; Neimat, J.S.; Bashore, T.R.; Wylie, S.A. Overriding actions in Parkinson’s disease: Impaired stopping and changing of motor responses. Behav. Neurosci. 2017, 131, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; van den Wildenberg, W.P.M.; de Graaf, Y.; Ames, S.L.; Baldacchino, A.; Bø, R.; Cadaveira, F.; Campanella, S.; Christiansen, P.; Claus, E.D.; et al. Is (poly-) substance use associated with impaired inhibitory control? A mega-analysis controlling for confounders. Neurosci. Biobehav. Rev. 2019, 105, 288–304. [Google Scholar] [CrossRef]
- Colzato, L.S.; van den Wildenberg, W.P.M.; Hommel, B. Impaired inhibitory control in recreational cocaine users. PLoS ONE 2007, 2, e1143. [Google Scholar] [CrossRef]
- Elchlepp, H.; Lavric, A.; Chambers, C.D.; Verbruggen, F. Proactive inhibitory control: A general biasing account. Cogn. Psychol. 2016, 86, 27–61. [Google Scholar] [CrossRef] [Green Version]
- Zandbelt, B.B.; Bloemendaal, M.; Neggers, S.F.; Kahn, R.S.; Vink, M. Expectations and violations: Delineating the neural network of proactive inhibitory control. Hum. Brain Mapp. 2013, 34, 2015–2024. [Google Scholar] [CrossRef] [PubMed]
- Castro-Meneses, L.J.; Johnson, B.W.; Sowman, P.F. The effects of impulsivity and proactive inhibition on reactive inhibition and the go process: Insights from vocal and manual stop signal tasks. Front. Hum. Neurosci. 2015, 9, 529. [Google Scholar] [CrossRef] [Green Version]
- Ramautar, J.R.; Kok, A.; Ridderinkhof, K.R. Effects of stop-signal probability in the stop-signal paradigm: The N2/P3 complex further validated. Brain Cogn. 2004, 56, 234–252. [Google Scholar] [CrossRef]
- Federico, P.; Mirabella, G. Effects of probability bias in response readiness and response inhibition on reaching movements. Exp. Brain Res. 2014, 232, 1293–1307. [Google Scholar] [CrossRef] [PubMed]
- Slater-Hammel, A.T. Reliability, accuracy, and refractoriness of a transit reaction. Res. Q. Am. Assoc. Health Phys. Educ. 1960, 31, 217–228. [Google Scholar] [CrossRef]
- Van Belle, J.; Vink, M.; Durston, S.; Zandbelt, B.B. Common and unique neural networks for proactive and reactive response inhibition revealed by independent component analysis of functional MRI data. NeuroImage 2014, 103, 65–74. [Google Scholar] [CrossRef]
- Zandbelt, B.B.; Vink, M. On the role of the striatum in response inhibition. PLoS ONE 2010, 5, e13848. [Google Scholar] [CrossRef] [PubMed]
- Chikazoe, J.; Jimura, K.; Hirose, S.; Yamashita, K.; Miyashita, Y.; Konishi, S. Preparation to inhibit a response complements response inhibition during performance of a stop-signal task. J. Neurosci. 2009, 29, 15870–15877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karsdorp, P.A.; Geenen, R.; Vlaeyen, J.W. Response inhibition predicts painful task duration and performance in healthy individuals performing a cold pressor task in a motivational context. Eur. J. Pain 2014, 18, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Patros, C.H.G.; Sweeney, K.L.; Mahone, E.M.; Mostofsky, S.H.; Rosch, K.S. Greater delay discounting among girls, but not boys, with ADHD correlates with cognitive control. Child Neuropsychol. 2018, 24, 1026–1046. [Google Scholar] [CrossRef]
- Debey, E.; Ridderinkhof, K.R.; De Houwer, J.; De Schryver, M.; Verschuere, B.J. Suppressing the truth as a mechanism of deception: Delta plots reveal the role of response inhibition in lying. Conscious Cogn. 2015, 37, 148–159. [Google Scholar] [CrossRef]
- Bissett, P.G.; Logan, G.D. Post-stop-signal adjustments: Inhibition improves subsequent inhibition. J. Exp. Psychol. Learn Mem. Cogn. 2012, 38, 955–966. [Google Scholar] [CrossRef] [Green Version]
- Leotti, A.L.; Wager, T.D. Motivational influences on response inhibition measures. J. Exp. Psychol. Hum. Percept. Perform. 2010, 36, 430–447. [Google Scholar] [CrossRef] [Green Version]
- Doekemeijer, R.A.; Verbruggen, F.; Boehler, C.N. Face the (trigger) failure: Trigger failures strongly drive the effect of reward on response inhibition. Cortex 2001, 139, 166–177. [Google Scholar] [CrossRef]
- Smittenaar, P.; Rutledge, R.B.; Zeidman, P.; Adams, R.A.; Brown, H.; Lewis, G.; Dolan, R.J. Proactive and reactive response inhibition across the lifespan. PLoS ONE 2015, 10, e0140383. [Google Scholar] [CrossRef]
- Manza, P.; Schwartz, G.; Masson, M.; Kann, S.; Volkow, N.D.; Li, C.R.; Leung, H.C. Levodopa improves response inhibition and enhances striatal activation in early-stage Parkinson’s disease. Neurobiol. Aging 2018, 66, 12–22. [Google Scholar] [CrossRef]
- Wylie, S.A.; van Wouwe, N.C.; Godfrey, S.G.; Bissett, P.G.; Logan, G.D.; Kanoff, K.E.; Claassen, D.O.; Neimat, J.S.; van den Wildenberg, W.P.M. Dopaminergic medication shifts the balance between going and stopping in Parkinson’s disease. Neuropsychologia 2018, 109, 262–269. [Google Scholar] [CrossRef]
- Mancini, C.; Modugno, N.; Santilli, M.; Pavone, L.; Grillea, G.; Morace, R.; Mirabella, G. Unilateral stimulation of subthalamic nucleus does not affect inhibitory control. Front. Neurol. 2018, 9, 1149. [Google Scholar] [CrossRef]
- Mirabella, G.; Iaconelli, S.; Romanelli, P.; Modugno, N.; Lena, F.; Manfredi, M.; Cantore, G. Deep-brain stimulation of subthalamic nuclei affects arm response inhibition in Parkinson’s patients. Cereb. Cortex 2012, 22, 1124–1132. [Google Scholar] [CrossRef] [Green Version]
- Mirabella, G.; Iaconelli, S.; Modugno, N.; Giannini, G.; Lena, F.; Cantore, G. Stimulation of subthalamic nuclei restores a near normal planning strategy in Parkinson’s patients. PLoS ONE 2013, 8, e62793. [Google Scholar] [CrossRef] [Green Version]
- Van den Wildenberg, W.P.M.; van Boxtel, G.J.M.; van der Molen, M.W.; Bosch, D.A.; Speelman, J.D.; Brunia, C.H.M. Stimulation of the subthalamic region facilitates the selection and inhibition of motor responses in Parkinson’s disease. J. Cogn. Neurosci. 2006, 18, 626–636. [Google Scholar] [CrossRef] [Green Version]
- Van Wouwe, N.C.; Neimat, J.S.; van den Wildenberg, W.P.M.; Hughes, S.B.; Lopez, A.M.; Phibbs, F.T.; Schall, J.D.; Rodriguez, W.J.; Bradley, E.B.; Dawant, B.M.; et al. Subthalamic nucleus subregion stimulation modulates inhibitory control. Cereb. Cortex Commun. 2020, 1, tgaa083. [Google Scholar] [CrossRef]
- Atkinson-Clement, C.; Tarrano, C.; Porte, C.A.; Wattiez, N.; Delorme, C.; McGovern, E.M.; Brochard, V.; Thobois, S.; Tranchant, C.; Grabli, D.; et al. Dissociation in reactive and proactive inhibitory control in myoclonus dystonia. Sci. Rep. 2020, 18, 13933. [Google Scholar] [CrossRef]
- Logan, G.D.; Burkell, J. Dependence and independence in responding to double stimulation: A comparison of stop, change, and dual-task paradigms. J. Exp. Psychol. Hum. Percept. Perform. 1986, 12, 549–563. [Google Scholar] [CrossRef]
- Bekker, E.M.; Overtoom, C.C.; Kenemans, J.L.; Kooij, J.J.; De Noord, I.; Buitelaar, J.K.; Verbaten, M.N. Stopping and changing in adults with ADHD. Psychol. Med. 2005, 35, 807–816. [Google Scholar] [CrossRef] [Green Version]
- Boecker, M.; Buecheler, M.M.; Schroeter, M.L.; Gauggel, S. Prefrontal brain activation during stop-signal response inhibition: An event-related functional near-infrared spectroscopy study. Behav. Brain Res. 2007, 176, 259–266. [Google Scholar] [CrossRef]
- Boecker, M.; Drueke, B.; Vorhold, V.; Knops, A.; Philippen, B.; Gauggel, S. When response inhibition is followed by response reengagement: An event-related fMRI study. Hum. Brain. Mapp. 2011, 32, 94–106. [Google Scholar] [CrossRef]
- De Jong, R.; Coles, M.G.; Logan, G.D. Strategies and mechanisms in nonselective and selective inhibitory motor control. J. Exp. Psychol. Hum. Percept. Perform. 1995, 21, 498–511. [Google Scholar] [CrossRef]
- Verbruggen, F.; Schneider, D.W.; Logan, G.D. How to stop and change a response: The role of goal activation in multitasking. J. Exp. Psychol. Hum. Percept. Perform. 2008, 34, 1212–1228. [Google Scholar] [CrossRef] [Green Version]
- Jahfari, S.; Stinear, C.M.; Claffey, M.; Verbruggen, F.; Aron, A.R. Responding with restraint: What are the neurocognitive mechanisms? J. Cogn. Neurosci. 2010, 22, 1479–1492. [Google Scholar] [CrossRef] [Green Version]
- Verbruggen, F.; Logan, G.D.; Liefooghe, B.; Vandierendonck, A. Short-term aftereffects of response inhibition: Repetition priming or between-trial control adjustments? J. Exp. Psychol. Hum. Percept. Perform. 2008, 34, 413–426. [Google Scholar] [CrossRef] [Green Version]
- Bedard, A.; Nichols, S.; Barbosa, J.A.; Schachar, R.; Logan, G.D.; Tannock, R. The development of selective inhibitory control across the life span. Dev. Neuropsychol. 2002, 21, 93–111. [Google Scholar] [CrossRef]
- Bissett, P.G.; Logan, G.D. Selective stopping? Maybe not. J. Exp. Psychol. Gen. 2014, 143, 455–472. [Google Scholar] [CrossRef] [Green Version]
- Dimoska, A.; Johnstone, S.J.; Barry, R.J. The auditory-evoked N2 and P3 components in the stop-signal task: Indices of inhibition, response-conflict or error-detection. Brain Cogn. 2006, 62, 98–112. [Google Scholar] [CrossRef]
- Bissett, P.G.; Logan, G.D. Balancing cognitive demands: Control adjustments in the stop-signal paradigm. J. Exp. Psychol. Learn. Mem. Cogn. 2011, 37, 392–404. [Google Scholar] [CrossRef] [Green Version]
- Emeric, E.E.; Brown, J.W.; Boucher, L.; Carpenter, R.H.; Hanes, D.P.; Harris, R.; Logan, G.D.; Mashru, R.N.; Paré, M.; Pouget, P.; et al. Influence of history on countermanding saccade performance in humans and macaque monkeys. Vision Res. 2007, 47, 35–49. [Google Scholar] [CrossRef]
- Rieger, M.; Gauggel, S. Inhibitory after-effects in the stop signal paradigm. Br. J. Psychol. 1999, 90, 509–518. [Google Scholar] [CrossRef]
- Logan, G.D. Attention, automaticity, and the ability to stop a speeded choice response. In Attention and Performance IX; Long, J., Baddeley, A.D., Eds.; Erlbaum: Hillsdale, MI, USA, 1981; pp. 205–222. [Google Scholar]
- Mirabella, G.; Pani, P.; Ferraina, S. Context influences on the preparation and execution of reaching movements. Cogn. Neuropsychol. 2008, 25, 996–1010. [Google Scholar] [CrossRef]
- Aron, A.R.; Behrens, T.E.; Smith, S.; Frank, M.J.; Poldrack, R.A. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J. Neurosci. 2007, 27, 3743–3752. [Google Scholar] [CrossRef] [Green Version]
- van Hulst, B.M.; de Zeeuw, P.; Vlaskamp, C.; Rijks, Y.; Zandbelt, B.B.; Durston, S. Children with ADHD symptoms show deficits in reactive but not proactive inhibition, irrespective of their formal diagnosis. Psychol. Med. 2018, 48, 2515–2521. [Google Scholar] [CrossRef] [Green Version]
- Mirabella, G. Inhibitory control and impulsive responses in neurodevelopmental disorders. Dev. Med. Child Neurol. 2021, 63, 520–526. [Google Scholar] [CrossRef]
- Suarez, I.; De Los Reyes Aragón, C.; Grandjean, A.; Barceló, E.; Mebarak, M.; Lewis, S.; Pineda-Alhucema, W.; Casini, L. Two sides of the same coin: ADHD affects reactive but not proactive inhibition in children. Cogn. Neuropsychol. 2021, 38, 349–363. [Google Scholar] [CrossRef]
- Schmitt, L.M.; White, S.P.; Cook, E.H.; Sweeney, J.A.; Mosconi, M.W. Cognitive mechanisms of inhibitory control deficits in autism spectrum disorder. J. Child Psychol. Psychiatry 2018, 59, 586–595. [Google Scholar] [CrossRef]
- Di Caprio, V.; Modugno, N.; Mancini, C.; Olivola, E.; Mirabella, G. Early-stage Parkinson’s patients show selective impairment in reactive but not proactive inhibition. Mov. Disord. 2020, 35, 409–418. [Google Scholar] [CrossRef]
- Mirabella, G.; Fragola, M.; Giannini, G.; Modugno, N.; Lakens, D. Inhibitory control is not lateralized in Parkinson’s patients. Neuropsychologia 2017, 102, 177–189. [Google Scholar] [CrossRef]
- Ridderinkhof, K.R. Micro- and macro-adjustments of task set: Activation and suppression in conflict tasks. Psychol. Res. 2002, 66, 312–323. [Google Scholar] [CrossRef]
- Osman, A.; Lou, L.; Muller-Gethmann, H.; Rinkenauer, G.; Mattes, S.; Ulrich, R. Mechanisms of speed-accuracy tradeoff: Evidence from covert motor processes. Biol. Psychol. 2000, 51, 173–199. [Google Scholar] [CrossRef] [PubMed]
- Ullsperger, M.; Bylsma, L.M.; Botvinick, M.M. The conflict adaptation effect: It’s not just priming. Cogn. Affect. Behav. Neurosci. 2005, 5, 467–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Wouwe, N.C.; Kanoff, K.E.; Claassen, D.O.; Spears, C.A.; Neimat, J.; van den Wildenberg, W.P.M.; Wylie, S.A. Dissociable effects of dopamine on the initial capture and the reactive inhibition of impulsive actions in Parkinson’s disease. J. Cogn. Neurosci. 2016, 28, 710–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wylie, S.A.; Claassen, D.O.; Huizenga, H.M.; Schewel, K.D.; Ridderinkhof, K.R.; Bashore, T.R.; van den Wildenberg, W.P.M. Dopamine agonists and the suppression of impulsive motor actions in Parkinson disease. J. Cogn. Neurosci. 2012, 24, 1709–1724. [Google Scholar] [CrossRef] [Green Version]
- van Wouwe, N.C.; Pallavaram, S.; Phibbs, F.T.; Martinez-Ramirez, D.; Neimat, J.S.; Dawant, B.M.; D’Haese, P.F.; Kanoff, K.E.; van den Wildenberg, W.P.M.; Okun, M.S.; et al. Focused stimulation of dorsal subthalamic nucleus improves reactive inhibitory control of action impulses. Neuropsychologia 2017, 99, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Ridderinkhof, K.R.; Forstmann, B.U.; Wylie, S.A.; Burle, B.; van den Wildenberg, W.P.M. Neurocognitive mechanisms of action control: Resisting the call of the sirens. Wiley Interdiscip. Rev. Cogn. Sci. 2011, 2, 174–192. [Google Scholar] [CrossRef]
- Matzke, D.; Love, J.; Heathcote, A. A Bayesian approach for estimating the probability of trigger failures in the stop-signal paradigm. Behav. Res. Methods 2017, 49, 267–281. [Google Scholar] [CrossRef] [Green Version]
- Weigard, A.; Heathcote, A.; Matzke, D.; Huang-Pollock, C. Cognitive modeling suggests that attentional failures drive longer stop-signal reaction time estimates in attention deficit/hyperactivity disorder. Clin. Psychol. Sci. 2019, 7, 856–872. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van den Wildenberg, W.P.M.; Ridderinkhof, K.R.; Wylie, S.A. Towards Conceptual Clarification of Proactive Inhibitory Control: A Review. Brain Sci. 2022, 12, 1638. https://doi.org/10.3390/brainsci12121638
van den Wildenberg WPM, Ridderinkhof KR, Wylie SA. Towards Conceptual Clarification of Proactive Inhibitory Control: A Review. Brain Sciences. 2022; 12(12):1638. https://doi.org/10.3390/brainsci12121638
Chicago/Turabian Stylevan den Wildenberg, Wery P. M., K. Richard Ridderinkhof, and Scott A. Wylie. 2022. "Towards Conceptual Clarification of Proactive Inhibitory Control: A Review" Brain Sciences 12, no. 12: 1638. https://doi.org/10.3390/brainsci12121638
APA Stylevan den Wildenberg, W. P. M., Ridderinkhof, K. R., & Wylie, S. A. (2022). Towards Conceptual Clarification of Proactive Inhibitory Control: A Review. Brain Sciences, 12(12), 1638. https://doi.org/10.3390/brainsci12121638





