The Split-Half Reliability and Construct Validity of the Virtual Reality-Based Path Integration Task in the Healthy Population
Abstract
1. Introduction
2. Methods
2.1. Participants
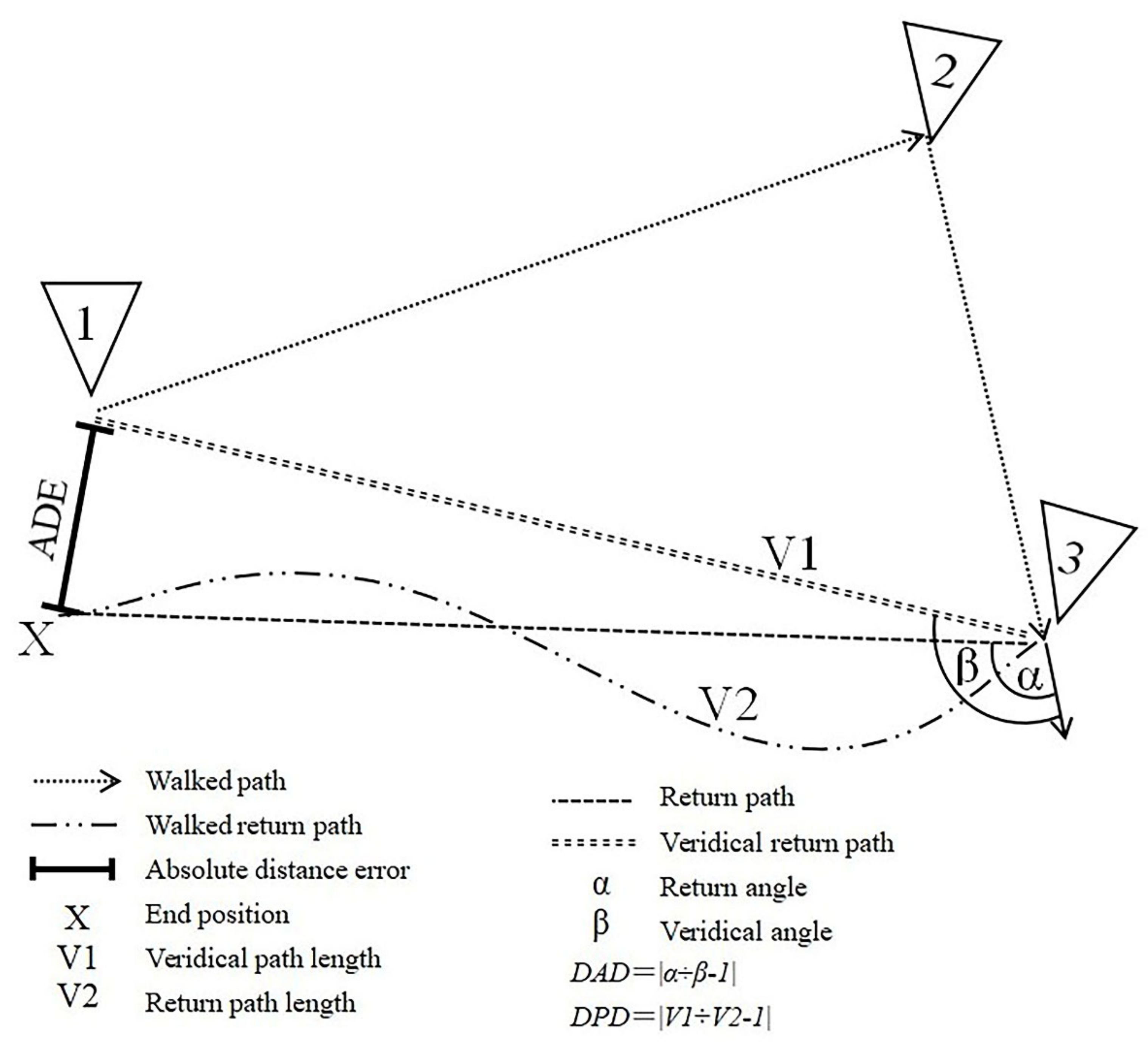
2.2. VR-Based Path Integration Task
2.3. DST
2.4. MR Scanning and Processing
2.5. Statistical Analysis
3. Results
3.1. Reliability of VR-Based Path Integration Task Measures
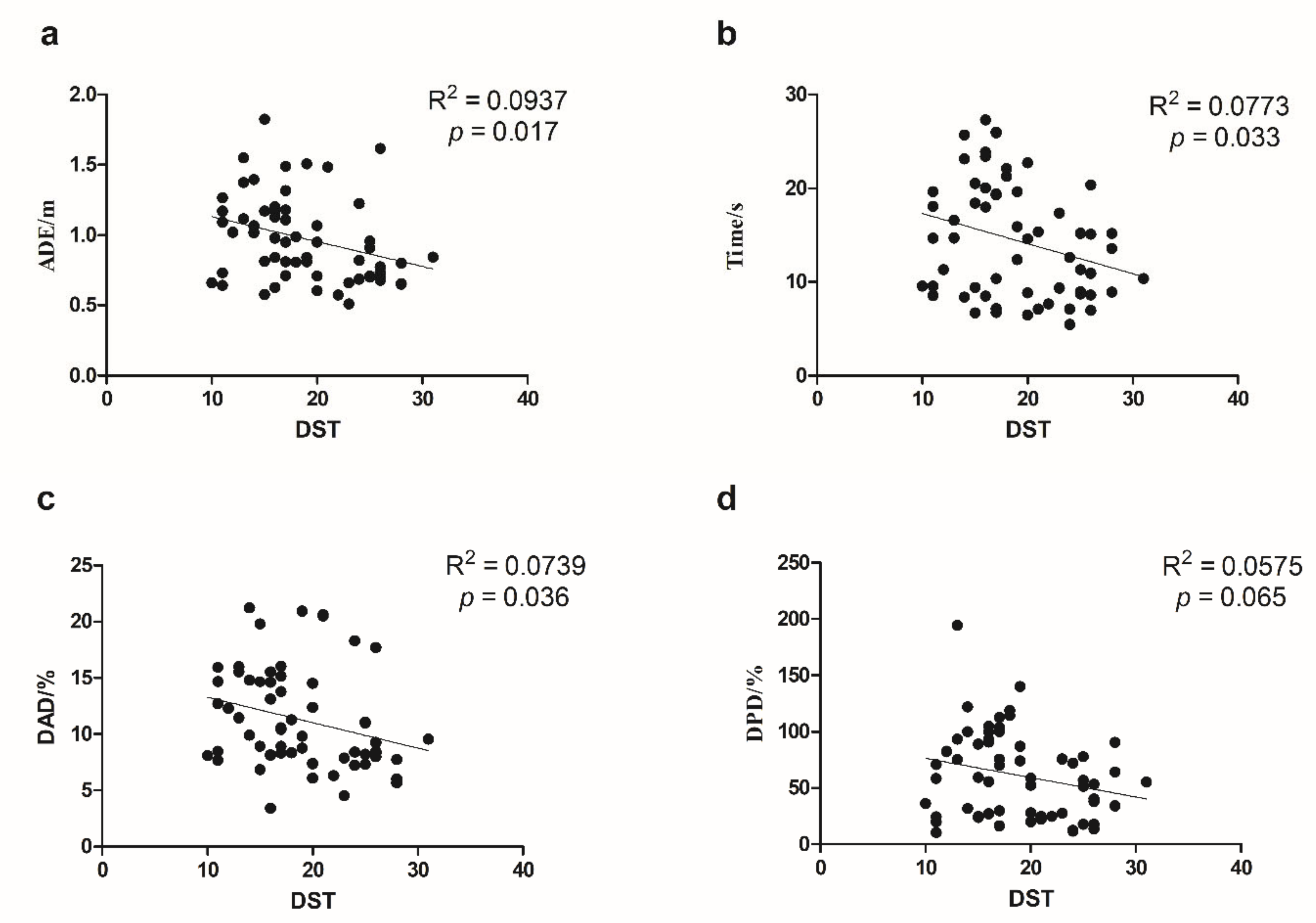
3.2. Association between Path Integration Measures and Age
3.3. Association between Path Integration Measures and General Cognitive Ability
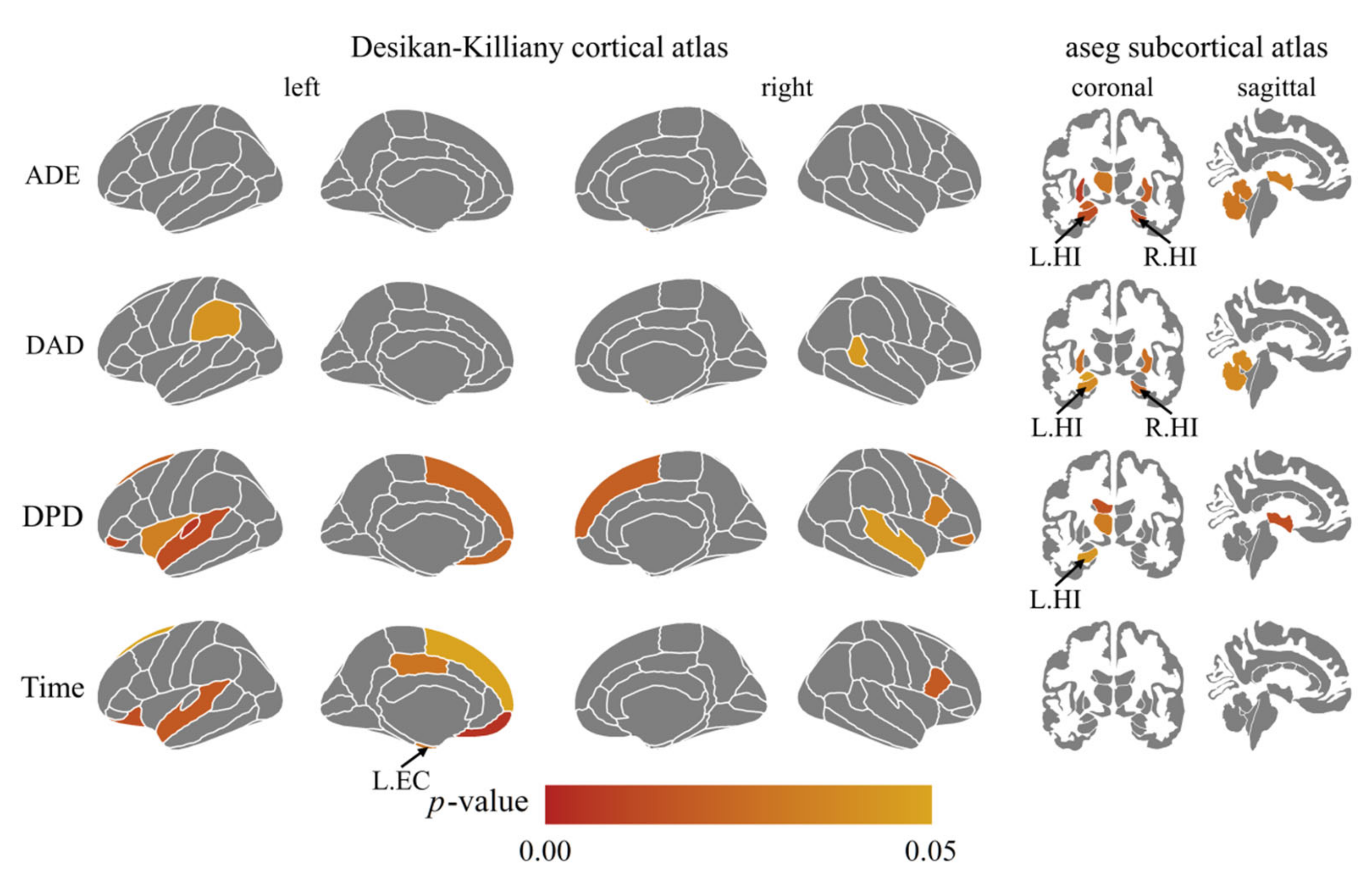
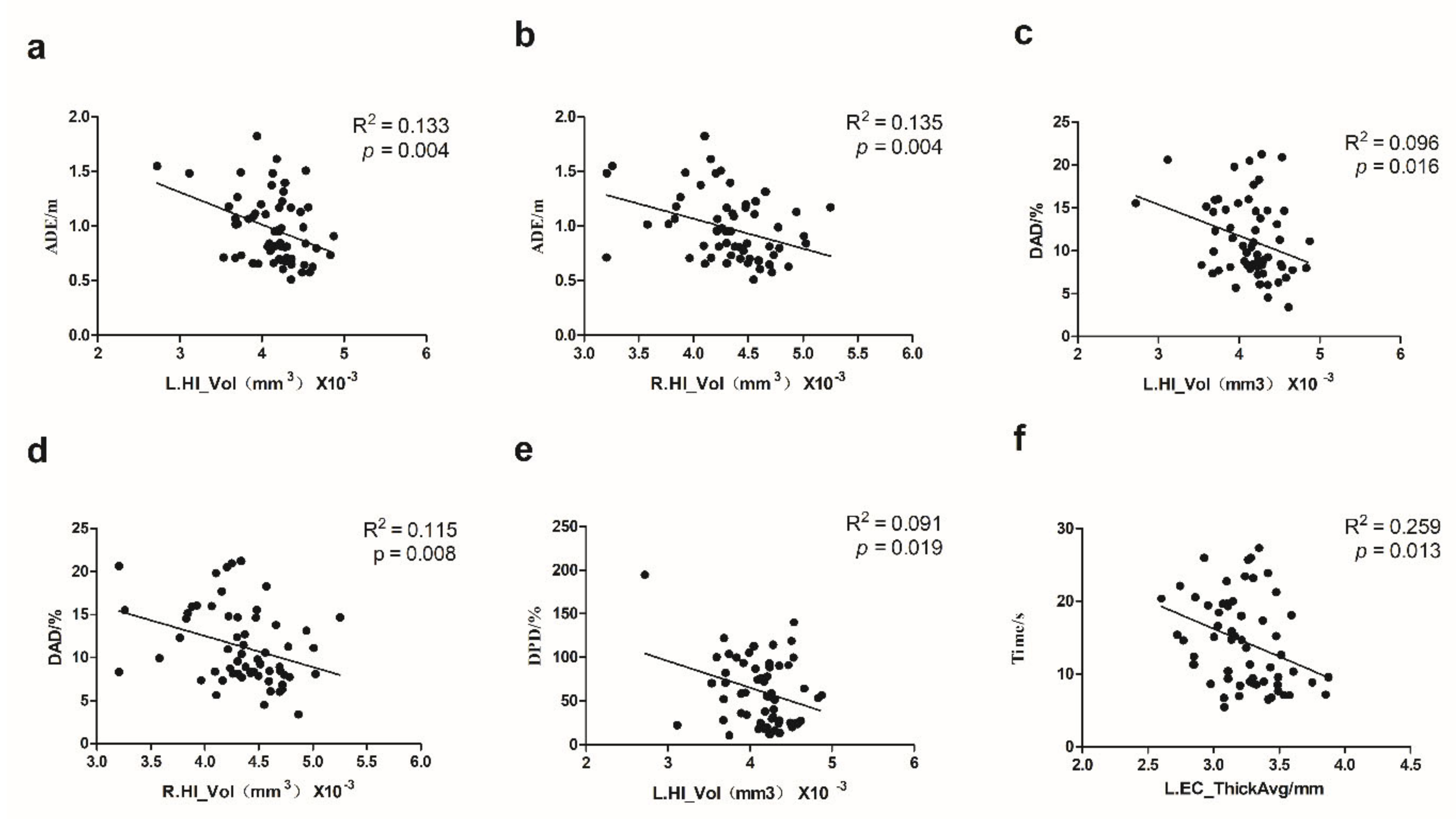
3.4. The Association between VR Task Indicators and Brain Structure Measures
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Ageing, 1950–2050; United Nations: New York, NY, USA, 2002; 483p. [Google Scholar]
- Rudnicka, E.; Napierala, P.; Podfigurna, A.; Meczekalski, B.; Smolarczyk, R.; Grymowicz, M. The World Health Organization (WHO) approach to healthy ageing. Maturitas 2020, 139, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Loewenstein, D.A.; Curiel, R.E.; Duara, R.; Buschke, H. Novel Cognitive Paradigms for the Detection of Memory Impairment in Preclinical Alzheimer’s Disease. Assessment 2018, 25, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Jagust, W.; Gitcho, A.; Sun, F.; Kuczynski, B.; Mungas, D.; Haan, M. Brain imaging evidence of preclinical Alzheimer’s disease in normal aging. Ann. Neurol. 2006, 59, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Small, S.A.; Tsai, W.Y.; DeLaPaz, R.; Mayeux, R.; Stern, Y. Imaging hippocampal function across the human life span: Is memory decline normal or not? Ann. Neurol. 2002, 51, 290–295. [Google Scholar] [CrossRef]
- Sherrill, K.R.; Erdem, U.M.; Ross, R.S.; Brown, T.I.; Hasselmo, M.E.; Stern, C.E. Hippocampus and retrosplenial cortex combine path integration signals for successful navigation. J. Neurosci. 2013, 33, 19304–19313. [Google Scholar] [CrossRef]
- McNaughton, B.L.; Battaglia, F.P.; Jensen, O.; Moser, E.I.; Moser, M.B. Path integration and the neural basis of the ‘cognitive map’. Nat. Rev. Neurosci. 2006, 7, 663–678. [Google Scholar] [CrossRef]
- Buzsaki, G.; Moser, E.I. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat. Neurosci. 2013, 16, 130–138. [Google Scholar] [CrossRef]
- Lombardi, G.; Crescioli, G.; Cavedo, E.; Lucenteforte, E.; Casazza, G.; Bellatorre, A.G.; Lista, C.; Costantino, G.; Frisoni, G.; Virgili, G.; et al. Structural magnetic resonance imaging for the early diagnosis of dementia due to Alzheimer’s disease in people with mild cognitive impairment. Cochrane Database Syst. Rev. 2020, 3, CD009628. [Google Scholar] [CrossRef]
- Cogne, M.; Taillade, M.; N’Kaoua, B.; Tarruella, A.; Klinger, E.; Larrue, F.; Sauzeon, H.; Joseph, P.A.; Sorita, E. The contribution of virtual reality to the diagnosis of spatial navigation disorders and to the study of the role of navigational aids: A systematic literature review. Ann. Phys. Rehabil. Med. 2017, 60, 164–176. [Google Scholar] [CrossRef]
- Harris, M.A.; Wolbers, T. Ageing effects on path integration and landmark navigation. Hippocampus 2012, 22, 1770–1780. [Google Scholar] [CrossRef]
- Stangl, M.; Kanitscheider, I.; Riemer, M.; Fiete, I.; Wolbers, T. Sources of path integration error in young and aging humans. Nat. Commun. 2020, 11, 2626. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, Y.; Smith, P.F.; Rosenberg, P.B. Vestibular impairment, cognitive decline and Alzheimer’s disease: Balancing the evidence. Aging Ment. Health 2020, 24, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Lowry, E.; Puthusseryppady, V.; Coughlan, G.; Jeffs, S.; Hornberger, M. Path Integration Changes as a Cognitive Marker for Vascular Cognitive Impairment?-A Pilot Study. Front. Hum. Neurosci. 2020, 14, 131. [Google Scholar] [CrossRef] [PubMed]
- Howett, D.; Castegnaro, A.; Krzywicka, K.; Hagman, J.; Marchment, D.; Henson, R.; Rio, M.; King, J.A.; Burgess, N.; Chan, D. Differentiation of mild cognitive impairment using an entorhinal cortex-based test of virtual reality navigation. Brain 2019, 142, 1751–1766. [Google Scholar] [CrossRef] [PubMed]
- Loomis, J.M.; Klatzky, R.L.; Golledge, R.G.; Cicinelli, J.G.; Pellegrino, J.W.; Fry, P.A. Nonvisual navigation by blind and sighted: Assessment of path integration ability. J. Exp. Psychol. Gen. 1993, 122, 73–91. [Google Scholar] [CrossRef]
- Ahmed, I.; Ishtiaq, S. Reliability and validity: Importance in Medical Research. J. Pak. Med. Assoc. 2021, 71, 2401–2406. [Google Scholar] [CrossRef]
- Taherdoost, H. Validity and Reliability of the Research Instrument: How to test the balidation of a Questionnaire survey in a research. Int. J. Acad. Res. Manag. 2016, 2016, 28–36. [Google Scholar] [CrossRef]
- Bettio, L.E.B.; Rajendran, L.; Gil-Mohapel, J. The effects of aging in the hippocampus and cognitive decline. Neurosci. Biobehav. Rev. 2017, 79, 66–86. [Google Scholar] [CrossRef]
- Garcia, A.D.; Buffalo, E.A. Anatomy and Function of the Primate Entorhinal Cortex. Annu. Rev. Vis. Sci. 2020, 6, 411–432. [Google Scholar] [CrossRef]
- Parsons, S.; Kruijt, A.-W.; Fox, E. Psychological Science Needs a Standard Practice of Reporting the Reliability of Cognitive-Behavioral Measurements. Adv. Methods Pract. Psychol. Sci. 2019, 2, 378–395. [Google Scholar] [CrossRef]
- Kirova, A.M.; Bays, R.B.; Lagalwar, S. Working memory and executive function decline across normal aging, mild cognitive impairment, and Alzheimer’s disease. Biomed. Res. Int. 2015, 2015, 748212. [Google Scholar] [CrossRef] [PubMed]
- Kessels, R.P.; Molleman, P.W.; Oosterman, J.M. Assessment of working-memory deficits in patients with mild cognitive impairment and Alzheimer’s dementia using Wechsler’s Working Memory Index. Aging Clin. Exp. Res. 2011, 23, 487–490. [Google Scholar] [CrossRef] [PubMed]
- Emrani, S.; Libon, D.J.; Lamar, M.; Price, C.C.; Jefferson, A.L.; Gifford, K.A.; Hohman, T.J.; Nation, D.A.; Delano-Wood, L.; Jak, A.; et al. Assessing Working Memory in Mild Cognitive Impairment with Serial Order Recall. J. Alzheimers Dis. 2018, 61, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Ruchinskas, R. Wechsler adult intelligence scale-4th edition digit span performance in subjective cognitive complaints, amnestic mild cognitive impairment, and probable dementia of the Alzheimer type. Clin. Neuropsychol. 2019, 33, 1436–1444. [Google Scholar] [CrossRef]
- Li, A.W.Y.; King, J. Spatial memory and navigation in ageing: A systematic review of MRI and fMRI studies in healthy participants. Neurosci. Biobehav. Rev. 2019, 103, 33–49. [Google Scholar] [CrossRef]
- Adamo, D.E.; Briceno, E.M.; Sindone, J.A.; Alexander, N.B.; Moffat, S.D. Age differences in virtual environment and real world path integration. Front. Aging Neurosci. 2012, 4, 26. [Google Scholar] [CrossRef]
- Skolimowska, J.; Wesierska, M.; Lewandowska, M.; Szymaszek, A.; Szelag, E. Divergent effects of age on performance in spatial associative learning and real idiothetic memory in humans. Behav. Brain Res. 2011, 218, 87–93. [Google Scholar] [CrossRef]
- Xie, Y.; Bigelow, R.T.; Frankenthaler, S.F.; Studenski, S.A.; Moffat, S.D.; Agrawal, Y. Vestibular Loss in Older Adults Is Associated with Impaired Spatial Navigation: Data from the Triangle Completion Task. Front. Neurol. 2017, 8, 173. [Google Scholar] [CrossRef]
- Allen, G.L.; Kirasic, K.C.; Rashotte, M.A.; Haun, D.B. Aging and path integration skill: Kinesthetic and vestibular contributions to wayfinding. Percept. Psychophys. 2004, 66, 170–179. [Google Scholar] [CrossRef]
- Konishi, K.; McKenzie, S.; Etchamendy, N.; Roy, S.; Bohbot, V.D. Hippocampus-dependent spatial learning is associated with higher global cognition among healthy older adults. Neuropsychologia 2017, 106, 310–321. [Google Scholar] [CrossRef]
- Burke, S.N.; Barnes, C.A. Neural plasticity in the ageing brain. Nat. Rev. Neurosci. 2006, 7, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Lister, J.P.; Barnes, C.A. Neurobiological changes in the hippocampus during normative aging. Arch. Neurol. 2009, 66, 829–833. [Google Scholar] [CrossRef] [PubMed]
- Jacob, P.Y.; Poucet, B.; Liberge, M.; Save, E.; Sargolini, F. Vestibular control of entorhinal cortex activity in spatial navigation. Front. Integr. Neurosci. 2014, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Mokrisova, I.; Laczo, J.; Andel, R.; Gazova, I.; Vyhnalek, M.; Nedelska, Z.; Levcik, D.; Cerman, J.; Vlcek, K.; Hort, J. Real-space path integration is impaired in Alzheimer’s disease and mild cognitive impairment. Behav. Brain Res. 2016, 307, 150–158. [Google Scholar] [CrossRef]
- Brown, E.M.; Pierce, M.E.; Clark, D.C.; Fischl, B.R.; Iglesias, J.E.; Milberg, W.P.; McGlinchey, R.E.; Salat, D.H. Test-retest reliability of FreeSurfer automated hippocampal subfield segmentation within and across scanners. Neuroimage 2020, 210, 116563. [Google Scholar] [CrossRef]
- Moffat, S.D.; Elkins, W.; Resnick, S.M. Age differences in the neural systems supporting human allocentric spatial navigation. Neurobiol. Aging 2006, 27, 965–972. [Google Scholar] [CrossRef]
- Ino, T.; Inoue, Y.; Kage, M.; Hirose, S.; Kimura, T.; Fukuyama, H. Mental navigation in humans is processed in the anterior bank of the parieto-occipital sulcus. Neurosci. Lett. 2002, 322, 182–186. [Google Scholar] [CrossRef]
- Rochefort, C.; Lefort, J.M.; Rondi-Reig, L. The cerebellum: A new key structure in the navigation system. Front. Neural Circuits 2013, 7, 35. [Google Scholar] [CrossRef]
- Fletcher, L.N.; Williams, S.R. Neocortical Topology Governs the Dendritic Integrative Capacity of Layer 5 Pyramidal Neurons. Neuron 2019, 101, 76–90. [Google Scholar] [CrossRef]
- Buckner, R.L.; Andrews-Hanna, J.R.; Schacter, D.L. The brain’s default network: Anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008, 1124, 1–38. [Google Scholar] [CrossRef]
- Laird, A.R.; Fox, P.M.; Eickhoff, S.B.; Turner, J.A.; Ray, K.L.; McKay, D.R.; Glahn, D.C.; Beckmann, C.F.; Smith, S.M.; Fox, P.T. Behavioral interpretations of intrinsic connectivity networks. J. Cogn. Neurosci. 2011, 23, 4022–4037. [Google Scholar] [CrossRef] [PubMed]
- Dosenbach, N.U.; Visscher, K.M.; Palmer, E.D.; Miezin, F.M.; Wenger, K.K.; Kang, H.C.; Burgund, E.D.; Grimes, A.L.; Schlaggar, B.L.; Petersen, S.E. A core system for the implementation of task sets. Neuron 2006, 50, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.W.; Reynolds, J.R.; Power, J.D.; Repovs, G.; Anticevic, A.; Braver, T.S. Multi-task connectivity reveals flexible hubs for adaptive task control. Nat. Neurosci. 2013, 16, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Nomi, J.S.; Farrant, K.; Damaraju, E.; Rachakonda, S.; Calhoun, V.D.; Uddin, L.Q. Dynamic functional network connectivity reveals unique and overlapping profiles of insula subdivisions. Hum. Brain Mapp. 2016, 37, 1770–1787. [Google Scholar] [CrossRef] [PubMed]
- Cereda, C.; Ghika, J.; Maeder, P.; Bogousslavsky, J. Strokes restricted to the insular cortex. Neurology 2002, 59, 1950–1955. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, K.; Wen, W.; An, Q.; Hamasaki, S.; Yamakawa, H.; Tamura, Y.; Yamashita, A.; Asama, H. Modified sensory feedback enhances the sense of agency during continuous body movements in virtual reality. Sci. Rep. 2021, 11, 2553. [Google Scholar] [CrossRef]
- Di Plinio, S.; Perrucci, M.G.; Ebisch, S.J.H. The Prospective Sense of Agency is Rooted in Local and Global Properties of Intrinsic Functional Brain Networks. J. Cogn. Neurosci. 2020, 32, 1764–1779. [Google Scholar] [CrossRef]
- Haggard, P. Sense of agency in the human brain. Nat. Rev. Neurosci. 2017, 18, 196–207. [Google Scholar] [CrossRef]

| Characteristics | Mean ± SD |
|---|---|
| Age | 47.6 ± 16.8 (years) |
| DST | 18.9 ± 5.3 |
| ADE | DAD | DPD | |
|---|---|---|---|
| Spearman–Brown | 0.84 | 0.81 | 0.72 |
| Cronbach’s alpha | 0.90 | 0.86 | 0.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, X.; Zhang, Z.; Zhou, Y.; Chen, Q.; Yang, L.-Z.; Li, H. The Split-Half Reliability and Construct Validity of the Virtual Reality-Based Path Integration Task in the Healthy Population. Brain Sci. 2022, 12, 1635. https://doi.org/10.3390/brainsci12121635
Fu X, Zhang Z, Zhou Y, Chen Q, Yang L-Z, Li H. The Split-Half Reliability and Construct Validity of the Virtual Reality-Based Path Integration Task in the Healthy Population. Brain Sciences. 2022; 12(12):1635. https://doi.org/10.3390/brainsci12121635
Chicago/Turabian StyleFu, Xiao, Zhenglin Zhang, Yanfei Zhou, Qi Chen, Li-Zhuang Yang, and Hai Li. 2022. "The Split-Half Reliability and Construct Validity of the Virtual Reality-Based Path Integration Task in the Healthy Population" Brain Sciences 12, no. 12: 1635. https://doi.org/10.3390/brainsci12121635
APA StyleFu, X., Zhang, Z., Zhou, Y., Chen, Q., Yang, L.-Z., & Li, H. (2022). The Split-Half Reliability and Construct Validity of the Virtual Reality-Based Path Integration Task in the Healthy Population. Brain Sciences, 12(12), 1635. https://doi.org/10.3390/brainsci12121635







