1. Introduction
Neurodegenerative disorders such as Alzheimer’s disease (AD), frontotemporal dementia (FTD), amyotrophic lateral sclerosis (ALS), and Parkinson’s disease (PD), are characterized by the progressive deposition of misfolded and aggregated proteins that lose their physiological functions and acquire neurotoxic effects [
1]. In recent years, great scientific and economic efforts have been made towards the identification of more accurate and predictive biomarkers of neurodegeneration in vivo [
2]. Although the underlying pathogenic mechanisms vary among different diseases, an alteration of autophagic flux is now considered a common mechanism of neurodegeneration [
3].
Autophagy is characterized by a complex cellular process that leads to the clearance of misfolded or damaged proteins and dysfunctional organelles [
4]. This complex machinery is induced by different signaling pathways such as oxidative stress and neuronal excitotoxicity. In healthy neurons, autophagy is observed at low basal levels [
5] while activation of autophagy is well described in different neurodegenerative disorders [
6]. Increasing evidence suggests that in both AD and FTD, there is an upregulation of autophagic machinery. Several studies have shown that neuronal autophagy is significantly impaired in AD [
7,
8]. In FTD, disease-causing genes such as
SQSTM1,
OPTN, and
VCP are directly involved in protein degradation pathways as their products contribute to recruitment of ubiquitinated proteins to the autophagosome, a key component of autophagy [
9].
Human p62 is a scaffold protein of 440 amino acids, coded by the
SQSTM1 gene and involved in several functions, including autophagy, apoptosis, inflammation, and cell survival, suggesting a role as a signaling hub that regulates cell viability in response to cytotoxic stress [
10,
11]. Mutations of
SQSTM1 gene were initially described in Paget’s disease of bone [
12], a chronic progressive skeletal disorder; more recently, gene mutations were reported in ALS [
13] and FTD patients [
14,
15]. In neuropathological findings, p62 is present in cytoplasmic aggregates in both ALS and FTD samples, and represents a common component of neurofibrillary tangles in AD [
16].
Cerebrospinal fluid (CSF) is enriched in brain-derived substances, and CSF biomarkers might represent a valid instrument to assess neuropathology in vivo. At present, however, there are no established CSF biomarkers for monitoring brain autophagy in vivo. In 2017, Au et al. examined p62 concentrations in the CSF of 30 children with severe traumatic brain injury (TBI) and found that p62 concentrations were significantly increased, suggesting an impairment of the autophagic flux [
17]. In addition, increased p62 concentrations were associated with an unfavorable outcome. More recently, several CSF autophagic proteins, including p62, were analyzed in a cohort of 32 patients with early PD, showing a dysregulation of the lysosomal autophagy pathway [
18].
To date, a few studies have investigated CSF p62 concentrations across different neurodegenerative disorders. Thus, the primary aim of this study was to evaluate whether p62 concentrations were detectable in the cerebrospinal fluid of patients with AD or FTD, comparing them with a control population. In addition, we investigated correlations between this autophagy biomarker and clinical or radiological characteristics of AD and FTD.
2. Materials and Methods
Seventy-six consecutive patients referred to the “Rita Levi Montalcini” Department of Neuroscience of the University Hospital Città della Salute e della Scienza di Torino (Italy) were selected for the study. Twenty-two patients with AD (10 males and 12 females, mean age: 66.41 ± 8.01 years; age range: 47–80 years) and 28 patients with FTD (18 males and 10 females, mean age: 64 ± 7.98 years; age range: 50–78 years) were enrolled.
Clinical diagnosis of probable AD was established according to joint Alzheimer’s Association Workgroup and National Institute of Aging guidelines [
19] and the ATN classification scheme [
20]. The diagnosis for the behavioral variant of FTD was made according to the Rascovsky criteria [
21], and the different forms of language variant were diagnosed according to specific established criteria [
22]. Based on these criteria, 25 of the FTD patients were classified as behavioral variant FTD (bvFTD), 2 as nonfluent/agrammatic variant PPA (nfvPPA), and 1 as semantic variant primary progressive aphasia (svPPA). For each demented patient, neuroimaging findings (brain MRI, 18FDG-PET), neuropsychological evaluations, and CSF levels of AD core biomarkers Aβ42, total tau (t-Tau), and phosphorylated tau at threonine 181 (p-Tau) data were obtained. Patients with FTD were also sequenced for
GRN,
MAPT,
SQSTM1 (that encodes p62), and
C9orf72 genes.
Twenty-six cognitive-spared patients (10 males and 16 females, mean age: 56.18 ± 15.02 years; age range: 40–75 years) with neurological conditions other than neurodegenerative disorders were included as a control group, including patients with suspected acute polyneuropathy (n. 7), muscular dystrophy (n. 3), multiple sclerosis (n. 3), and normal pressure hydrocephalus (n. 13). The demographic and clinical features are summarized in
Table 1. The local Ethics Committee approved the protocol of the study.
CSF sampling and collection were performed according to previously published protocols and guidelines [
23]. After overnight fasting, CSF samples were collected in sterile polypropylene tubes via a lumbar puncture performed between 9 a.m. and 12 a.m. CSF samples were centrifuged at 1500×
g for 10 min at room temperature, subsequently transferred into aliquot tubes, and stored in a −80 °C freezer.
The CSF p62 levels were evaluated using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Cloud-Clone Corp). The limit of detection was 0.1 ng/mL for p62, and samples below the level of detection were assigned a value of 0 ng/mL. All samples were assayed in duplicate. CSF concentrations of Aβ42, t-Tau, and p-Tau181 were also evaluated using the ELISA method with available commercially Innotest Kits (Fujirebio), according to the manufacturer’s instructions.
All statistical analyses were performed using SPSS version 20 (SPSS Inc, Chicago, IL, USA). Continuous variables were presented as means ± standard deviations or medians and range, and categorical ones as count and percentage. We compared diagnostic groups (AD, FTD and controls) using ANOVA for normally distributed variables and Kruskal–Wallis and Mann–Whitney U tests for non-normally distributed variables, as appropriate. Correlations between p62 and CSF biomarkers were also evaluated using Pearson’s test, also adjusted for age. The statistical models were adjusted for possible confounders. A two-tailed p value of <0.05 was considered significant in biochemical and clinical comparisons.
3. Results
3.1. Overall Population
No difference in education, comorbidities, or smoking habits was found between cases and controls. The gender distribution was comparable in the control and FTD/AD cohorts. Patients with AD and FTD did not differ in age, whereas control subjects were younger than patients with dementia (
Table 1).
3.2. Analysis of p62 in AD and FTD Patients
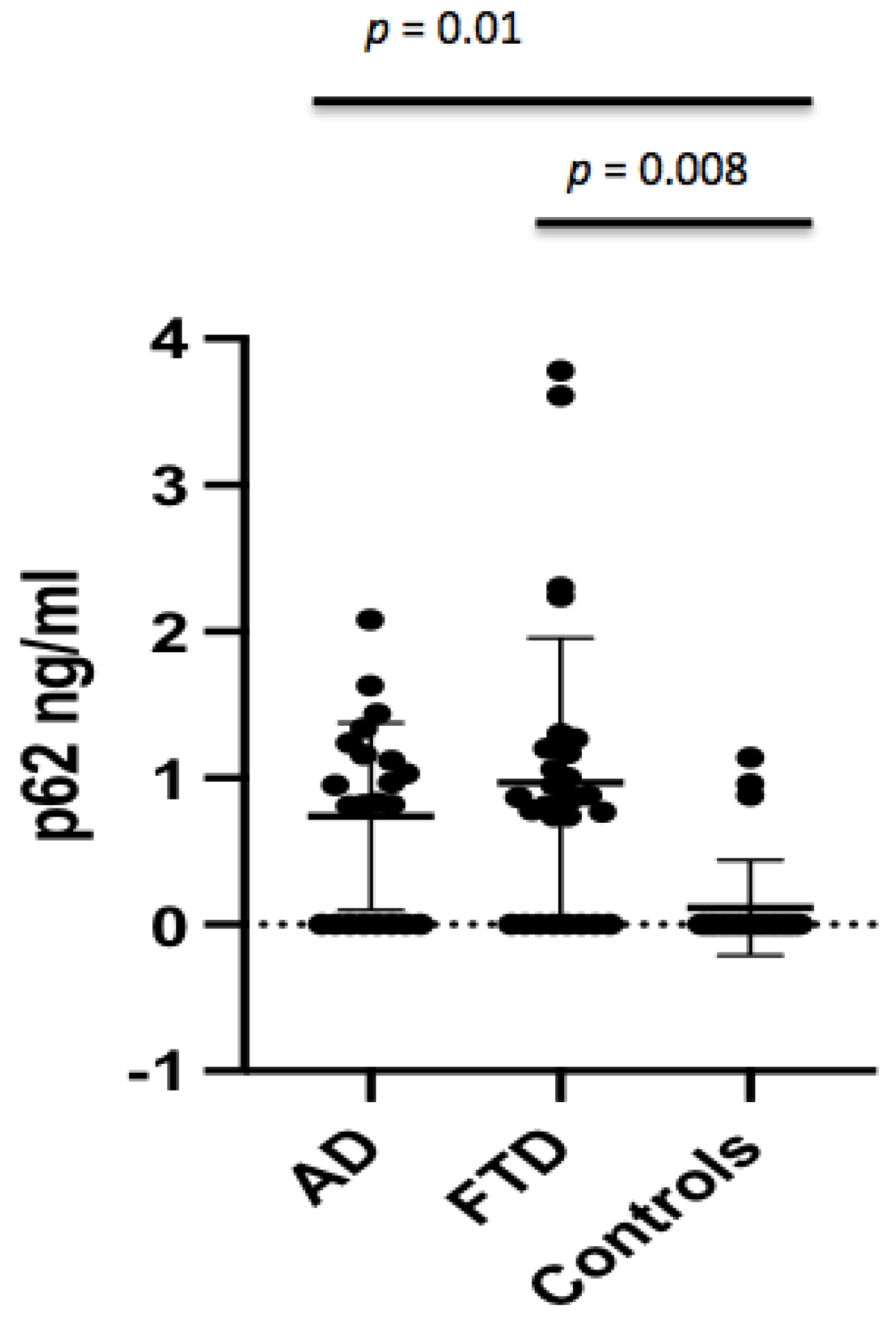
The p62 concentrations were found to be significantly different between AD patients and controls (0.74 ± 0.64 ng/mL vs. 0.15 ± 0.33 ng/mL,
p = 0.011) (
Figure 1), which persisted after adjusting for age (
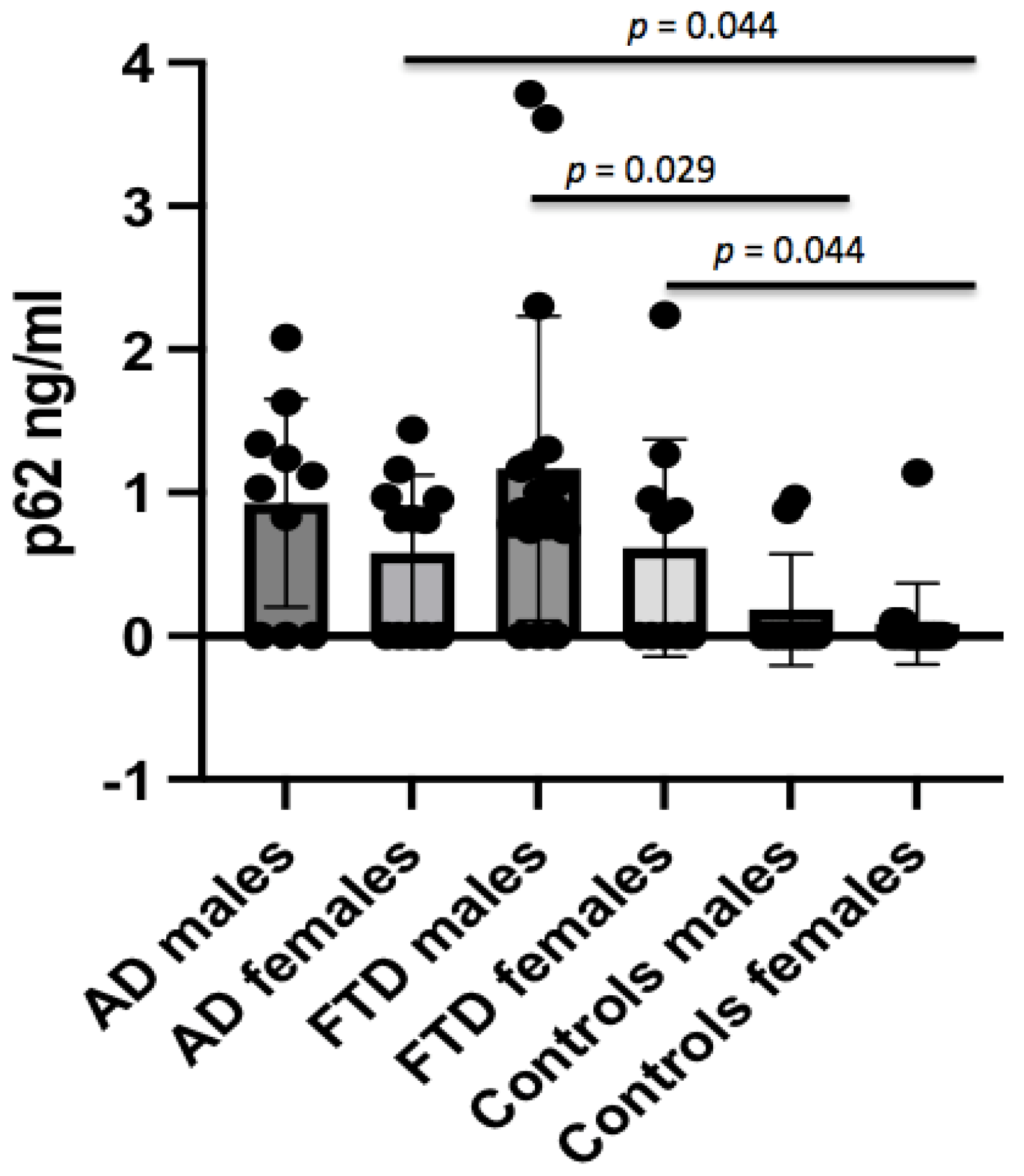
p = 0.01). The p62 concentrations were not different in AD males with respect to male controls, whereas female AD patients showed higher p62 levels when compared to female controls (0.58 ± 0.54 ng/mL vs. 0.07 ± 0.29 ng/mL,
p = 0.044) (
Figure 2). No significant difference in CSF p62 concentration was found between FTD and AD patients (
p = 0.779).
Crude p62 levels were significantly increased in FTD patients when compared to controls (0.97 ± 0.99 ng/mL vs. 0.15 ± 0.33 ng/mL,
p < 0.001) (
Figure 1), which persisted after adjusting for age (
p = 0.008). When analyzing the gender effect, p62 concentrations were found to be significantly different between male FTD patients and male controls (1.17 ± 1.06 ng/mL vs. 0.20 ± 0.41 ng/mL,
p = 0.029), and between female FTD patients and female controls (0.61 ± 0.76 ng/mL vs. 0.07 ± 0.29 ng/mL,
p = 0.044).
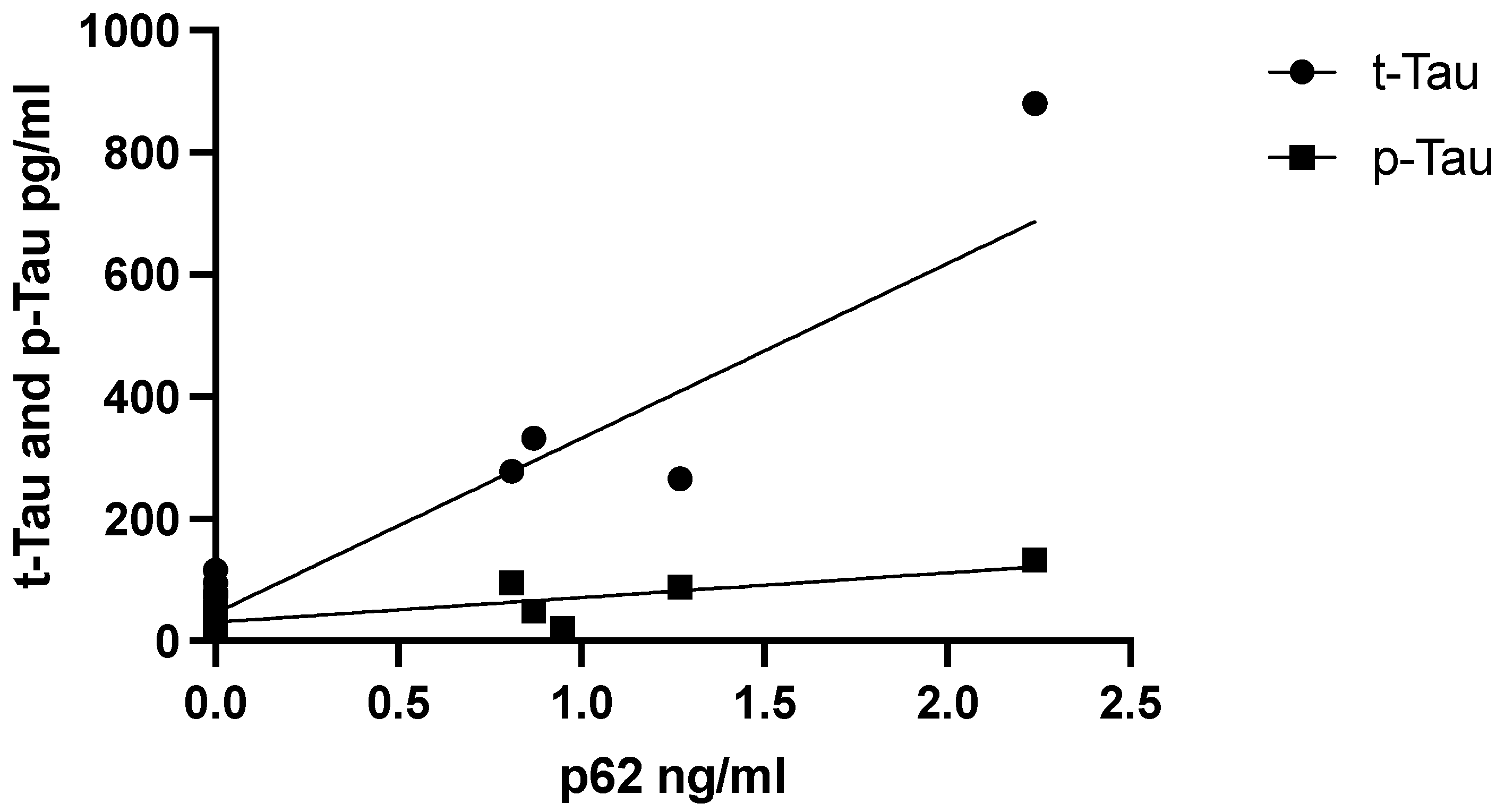
3.3. Relationship between p62 Levels and AD CSF Core Biomarker Levels, Aβ42, t-Tau, and p-Tau
We hypothesized that the levels of p62 would also affect neurodegenerative processes. Therefore, we examined the relationship between p62 levels and CSF AD core biomarkers Aβ42, t-Tau, and p-Tau. CSF p62 levels did not correlate with biomarkers of neurodegeneration in the AD group, which persisted when adjusted for age. In contrast, CSF p62 concentrations correlated positively with the neurodegenerative biomarkers t-Tau (r = 0.853,
p = 0.002) and p-Tau in the female FTD subgroup alone (r = 0.807,
p = 0.005) (
Figure 3).
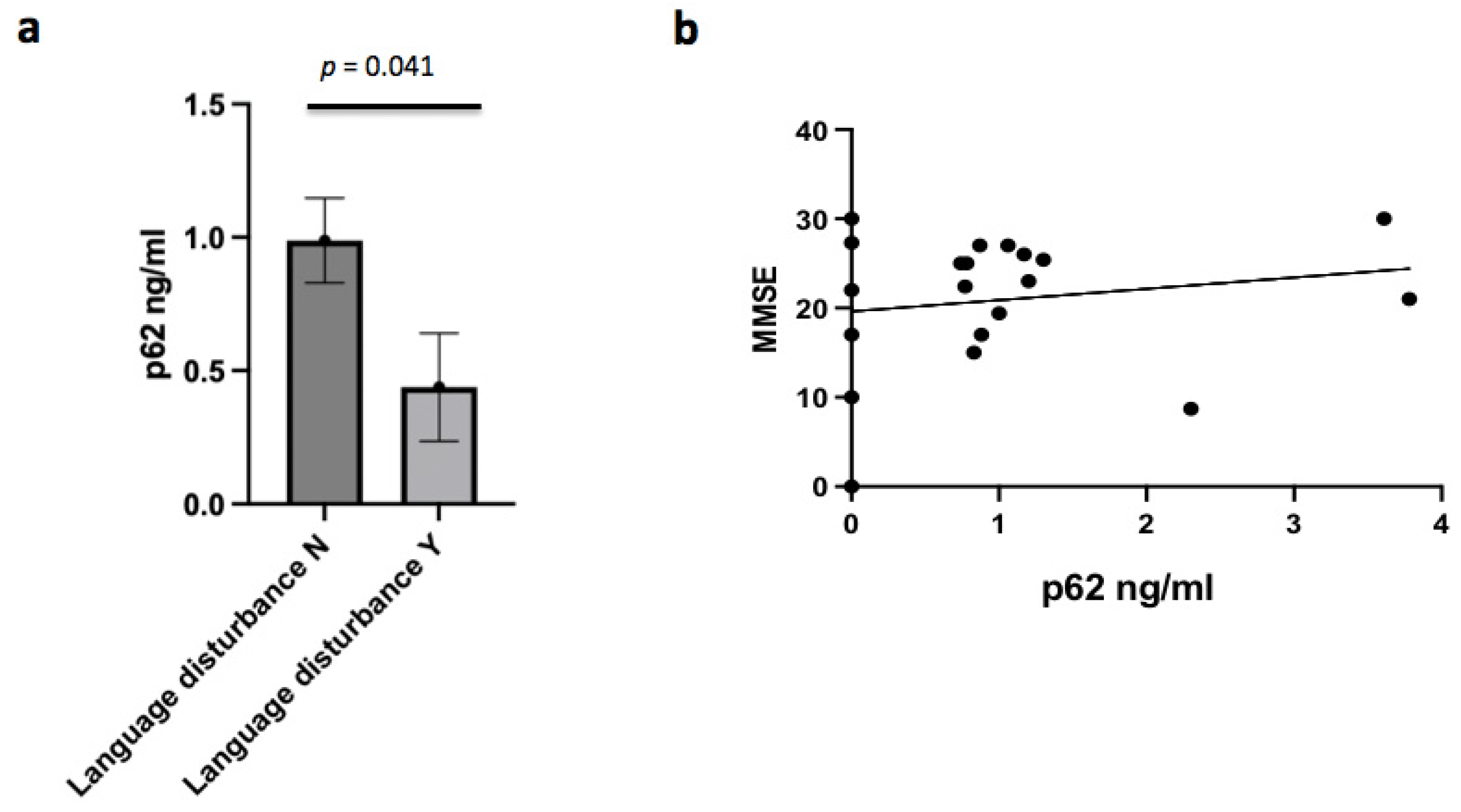
3.4. P62 and Clinical Characteristics
Several clinical features emerged as significantly linked to CSF p62 concentrations in the AD group. In AD patients, p62 levels were negatively associated and with the presence of language disturbance (r = −0.438;
p = 0.041) (
Figure 4a). In AD males, p62 concentrations were inversely associated with the presence of temporal and spatial disorientation (r = −0.674;
p = 0.033), and depression (r = −0.781;
p = 0.008). Furthermore, p62 concentrations were negatively correlated with temporal atrophy in the AD subgroup (r = −0.529;
p = 0.011), mainly in the male subgroup (r = −0.854;
p = 0.002). No correlation between p62 levels and MMSE was found (
Figure 4b). Finally, no correlation was found between p62 concentrations and the severity of disease.
No correlations between p62 levels and the collected clinical characteristics of FTD patients were found. In particular, no correlation between p62 CSF concentration and neuropsychological test results was found (including MMSE and Frontal Assessment Battery). In addition, we did not find any correlations between p62 levels and severity of the disease.
In this cohort of patients, we did not find any mutations in the SQSTM1 gene; we only found the single-nucleotide polymorphism rs186996560 in the SQSTM1 in a FTD patient. In our FTD group, p62 levels were elevated in patients both carrying and not carrying mutations in known causative genes in respect to controls. No significant correlation was found between p62 levels and the presence or absence of gene mutations (p = 0.317).
4. Discussion
To our knowledge, this is the first study to investigate CSF p62 concentrations as marker of autophagy in patients with dementia. We showed that CSF p62 concentrations were significantly increased both in AD and FTD patients in comparison to controls. Furthermore, in female patients with FTD, we found that CSF p62 concentrations correlated with markers of neurodegeneration like p-Tau and t-Tau. In patients with AD, several neuropsychological characteristics of the disease showed a significant correlation with the investigated biomarker of autophagy. Taken together, our data support the hypothesis that CSF p62 concentrations may be a useful in vivo biomarker of neuronal autophagy.
Increased CSF p62 concentrations in patients with neurodegenerative disorders may have several neurobiological explanations. First, p62 is involved in several cellular processes relevant to aging, including protein degradation [
24], mitophagy [
25], and inflammation. It also seems to be essential for aggregation and autophagic clearance of ubiquitinated proteins [
26,
27]. The increase in p62 in patients with neurodegenerative disorders could be the result of promoting autophagic clearance of protein aggregates (known as aggrephagy). Loss of function of p62 has been demonstrated to cause increased Abeta42, tau hyperphosphorylation and neurodegeneration in experimental models of Alzheimer’s disease [
28]. Notably, an overexpression of p62 has been shown to prevent cognitive decline in transgenic models of AD mice through autophagy-mediated increased neuronal survival and reduced amyloid plaque formation. In addition, p62 was proposed as a multitarget approach for the treatment of AD through the analysis of a recent experimental study with a mouse model of AD [
29].
The different forms of frontotemporal dementia are characterized by the aggregation of insoluble proteins within cells. The accumulation of aggregates is often due to genetic mutations in their coding genes. Most inclusions in FTD are characterized by immuno-reactivity, and the neurons and glia of FTD patients are characterized by p62 accumulations.
It has been shown that p62 represents a substrate for macroautophagy; hence, p62 levels increase when macroautophagy is inhibited, and its concentration decreases when macroautophagy is induced [
30]. In this regard, the aggregation of p62 may represent a stress response involving selective autophagy [
31] and serving a neuroprotective function. In our FTD group, p62 levels were elevated in patients both carrying and not carrying mutations in known causative genes. However, the highest level of p62 was found in one subject carrying a
MAPT gene mutation. In addition, we found that that CSF p62 levels correlate with biomarkers of neurodegeneration in FTD, and p62 concentrations were positively correlated with t-Tau and p-Tau. Many studies have shown that autophagy deficiency can cause tau accumulation [
32,
33]. Increasing evidence points to an important role of p62 in the degradation of tau protein by binding polyubiquitinated tau to facilitate its elimination. The spread of tau pathology has been accompanied by the accumulation of p62, and this has been noted in both in vivo and in vitro models [
34]. In mouse models, an alteration of p62 has been shown to lead to the accumulation of hyperphosphorylated tau, synaptic deficiencies, and tau tangle-like structures. In addition, increased tau secretion is associated with the accumulation of p62 and LC3, two different markers of autophagic vacuole intermediates [
35]. In a recent study, the overexpression of MAPT increased p62 level in primary cultured hippocampal neurons [
36]. P62 may be neuroprotective since it promotes autophagic clearance of accumulating tau, shuttling the protein tau to the proteasome for degradation, even if when overexpressed it may be in turn neurotoxic. Thus, an increase in CSF p62 concentration could represent an interesting biomarker for neurodegeneration.
This is the first study to demonstrate an increase in p62 concentrations in the CSF of patients with FTD and AD; in view of the limited samples, the results obtained and their conclusions should be interpreted with caution, and further studies are needed to confirm our data. Autophagy represents a dynamic process, and our results may change according to different stages of neurodegeneration, so studies involving subjects at different stages of disease are desirable. Finally, a further investigation of other different autophagic biomarkers may be crucial for a better understanding of the autophagic machinery in neurodegenerative disorders.
5. Conclusions
Our study showed a significant increase of CSF p62 concentrations in patients with Alzheimer’s disease and frontotemporal dementia, supporting an important role of autophagy alterations in these neurodegenerative conditions. In addition, a significant correlation between increased autophagy and clinical characteristics of dementia was found. However, due to the relatively small number of subjects examined, replication studies in larger series are warranted in order to confirm our data.
Author Contributions
E.R.: conceptualization, writing—original draft, writing—review and editing, analyses. S.B.: conceptualization, biochemical analyses, writing—original draft. F.R.: conceptualization, writing—review and editing. A.M.: patient selection, writing—review and editing. A.C.: neuropsychological evaluations, writing—review and editing. C.B.: biochemical analyses, writing—review and editing. M.C.V.: biochemical analyses, writing—review and editing. I.R.: conceptualization, writing—original draft, writing—review and editing, analyses. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by Ethics Committee of Città della Salute e della Scienza di Torino (protocol code NEU-2013-03).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data are not publicly available because they contain information that could compromise research participant privacy/consent. Clinical and biochemical data will be available upon request from any qualified investigator.
Acknowledgments
This study was supported by Ministero dell’Istruzione, dell’Università e della Ricerca e MIUR project “Dipartimenti di Eccellenza 2018 e 2022” to Department of Neuroscience “Rita Levi Montalcini”, University of Torino, and AIRAlzh Onlus-ANCC-COOP (S.B.).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Spires-Jones, T.L.; Attems, J.; Thal, D.R. Interactions of pathological proteins in neurodegenerative diseases. Acta Neuropathol. 2017, 134, 187–205. [Google Scholar] [CrossRef] [PubMed]
- Zetterberg, H. Biofluid-based biomarkers for Alzheimer’s disease-related pathologies: An update and synthesis of the literature. Alzheimers Dement. 2022, 18, 1687–1693. [Google Scholar] [CrossRef] [PubMed]
- Menzies, F.M.; Angeleen, F.; Caricasole, A.; Bento, C.F.; Andrews, S.P.; Ashkenazi, A.; Füllgrabe, J.; Jackson, A.; Sanchez, M.J.; Karabiyik, C.; et al. Autophagy and Neurodegeneration: Pathogenic Mechanisms and Therapeutic Opportunities. Neuron 2017, 93, 1015–1034. [Google Scholar] [CrossRef]
- Kraft, C.; Peter, M.; Hofmann, K. Selective autophagy: Ubiquitin-mediated recognition and beyond. Nat. Cell Biol. 2010, 12, 836–841. [Google Scholar] [CrossRef]
- Rubinsztein, D.C.; DiFiglia, M.; Heintz, N.; Nixon, R.A.; Qin, Z.H.; Ravikumar, B.; Stefanis, L.; Tolkovsky, A. Autophagy and its possible roles in nervous system diseases, damage and repair. Autophagy 2005, 1, 11–22. [Google Scholar] [CrossRef]
- Menzies, F.M.; Fleming, A.; Rubinsztein, D.C. Compromised autophagy and neurodegenerative diseases. Nat. Rev. Neurosci. 2005, 16, 345–357. [Google Scholar] [CrossRef]
- Nixon, R.A.; Wegiel, J.; Kumar, A.; Yu, W.H.; Peterhoff, C.; Cataldo, A.; Cuervo, A.M. Extensive involvement of autophagy in Alzheimer disease: An immuno-electron microscopy study. J. Neuropathol. Exp. Neurol. 2005, 64, 113–122. [Google Scholar] [CrossRef]
- Guan, X.; Iyaswamy, A.; Sreenivasmurthy, S.G.; Su, C.; Zhu, Z.; Liu, J.; Kan, Y.; Cheung, K.H.; Lu, J.; Tan, J.; et al. Mechanistic Insights into Selective Autophagy Subtypes in Alzheimer’s. Int. J. Mol. Sci. 2022, 23, 3609. [Google Scholar] [CrossRef]
- Root, J.; Merino, P.; Nuckols, A.; Johnson, M.; Kukar, T. Lysosome dysfunction as a cause of neurodegenerative diseases: Lessons from frontotemporal dementia and amyotrophic lateral sclerosis. Neurobiol. Dis. 2021, 154, 105360. [Google Scholar] [CrossRef]
- Lin, X.; Li, S.-; Zhao, Y.; Ma, X.; Zhang, K.; He, X.; Wang, Z. Interaction domains of p62: A bridge between p62 and selective autophagy. DNA Cell Biol. 2013, 32, 220–227. [Google Scholar] [CrossRef]
- Kumar, A.V.; Mills, J.; Lapierre, L.R. Selective Autophagy Receptor p62/SQSTM1, a Pivotal Player in Stress and Aging. Front. Cell Dev. Biol. 2022, 10, 793328. [Google Scholar] [CrossRef] [PubMed]
- Galson, D.L.; Roodman, G.D. Pathobiology of Paget’s Disease of Bone. J. Bone Metab. 2014, 21, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Fecto, F.; Yan, J.; Vemula, S.P.; Liu, E.; Yang, Y.; Chen, W.; Zheng, J.G.; Shi, Y.; Siddique, N.; Arrat, H.; et al. SQSTM1 mutations in familial and sporadic amyotrophic lateral sclerosis. Arch. Neurol. 2011, 68, 1440–1446. [Google Scholar] [CrossRef] [PubMed]
- Rubino, E.; Rainero, I.; Chiò, A.; Rogaeva, E.; Galimberti, D.; Fenoglio, P.; Grinberg, Y.; Isaia, G.; Calvo, A.; Gentile, S.; et al. SQSTM1 mutations in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Neurology 2012, 79, 1556–1562. [Google Scholar] [CrossRef]
- Le Ber, I.; Camuzat, A.; Guerreiro, R.; Bouya-Ahmed, K.; Bras, J.; Nicolas, G.; Gabelle, A.; Didic, M.; De Septenville, A.; Millecamps, S.; et al. SQSTM1 Mutations in French patients with frontotemporal dementia or frontotemporal dementia with amyotrophic lateral sclerosis. JAMA Neurol. 2013, 70, 1403–1410. [Google Scholar]
- Kuusisto, E.; Salminen, A.; Alafuzoff, I. Ubiquitin-binding protein p62 is present in neuronal and glial inclusions in human tauopathies and synucleinopathies. Neuroreport 2001, 12, 2085–2090. [Google Scholar] [CrossRef]
- Au, A.K.; Aneja, R.K.; Bayır, H.; Bell, M.J.; Janesko-Feldman, K.; Kochanek, P.M.; Clark, R.S.B. Autophagy Biomarkers Beclin 1 and p62 are Increased in Cerebrospinal Fluid after Traumatic Brain Injury. Neurocrit. Care 2017, 26, 348–355. [Google Scholar] [CrossRef]
- Youn, J.; Lee, S.B.; Lee, H.S.; Yang, H.Y.; Park, J.; Kim, J.S.; Oh, E.; Park, S.; Jang, W. Cerebrospinal Fluid Levels of Autophagy-related Proteins Represent Potentially Novel Biomarkers of Early-Stage Parkinson’s Disease. Sci. Rep. 2018, 8, 16866. [Google Scholar] [CrossRef]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging and the Alzheimer’s Association Workgroup. Alzheimers Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Rascovsky, K.; Hodges, J.; Knopman, D.; Mendez, M.F.; Kramer, J.H.; Neuhaus, J.; van Swieten, J.C.; Seelaar, H.; Dopper, E.G.; Onyike, C.U.; et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011, 134, 2456–2477. [Google Scholar] [CrossRef] [PubMed]
- Gorno-Tempini, M.L.; Hillis, A.E.; Weintraub, S.; Kertesz, A.; Mendez, M.; Cappa, S.F.; Ogar, J.M.; Rohrer, J.D.; Black, S.; Boeve, B.F.; et al. Classification of primary progressive aphasia and its variant. Neurology 2011, 76, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Vanderstichele, H.; Demeyer, L.; Janelidze, S.; Coart, E.; Stoops, E.; Mauroo, K.; Herbst, V.; François, C.; Hansson, O. Recommendations for cerebrospinal fluid collection for the analysis by ELISA of neurogranin trunc P75, α-synuclein, and total tau in combination with Aβ(1–42)/Aβ(1–40). Alzheimers Res. Ther. 2017, 9, 40. [Google Scholar] [CrossRef]
- Pankiv, S.; Clausen, T.H.; Lamark, T.; Brech, A.; Bruun, J.A.; Outzen, H.; Øvervatn, A.; Bjørkøy, G.; Johansen, T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J. Biol. Chem. 2007, 282, 24131–24145. [Google Scholar] [CrossRef]
- Gureev, A.P.; Sadovnikova, I.S.; Starkov, N.N.; Starkov, A.A.; Popov, V.N. p62-Nrf2 p62 Mitophagy Regulatory Loop as a Target for Preventive Therapy of Neurodegenerative Diseases. Brain Sci. 2020, 10, 847. [Google Scholar] [CrossRef]
- Komatsu, M.; Waguri, S.; Koike, M.; Sou, Y.S.; Ueno, T.; Hara, T.; Mizushima, N.; Iwata, J.; Ezaki, J.; Murata, S.; et al. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell 2007, 131, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Nezis, I.P.; Simonsen, A.; Sagona, A.P.; Finley, K.; Gaumer, S.; Contamine, D.; Rusten, T.E.; Stenmark, H.; Brech, A. Ref(2)P, the Drosophila melanogaster homologue of mammalian p62, is required for the formation of protein aggregates in adult brain. J. Cell Biol. 2008, 180, 1065–1071. [Google Scholar] [CrossRef]
- Caccamo, A.; Ferreira, E.; Branca, C.; Oddo, S. p62 improves AD-like pathology by increasing autophagy. Mol. Psychiatry 2017, 22, 865–873. [Google Scholar] [CrossRef]
- Cecarini, V.; Bonfili, L.; Gogoi, O.; Lawrence, S.; Venanzi, F.M.; Azevedo, V.; Mancha-Agresti, P.; Drumond, M.M.; Rossi, G.; Berardi, S.; et al. Neuroprotective effects of p62(SQSTM1)-engineered lactic acid bacteria in Alzheimer's disease: A pre-clinical study. Aging 2020, 12, 15995–16020. [Google Scholar] [CrossRef]
- Xilouri, M.; Stefanis, L. Autophagic pathways in Parkinson disease and related disorders. Expert Rev. Mol. Med. 2011, 13, e8. [Google Scholar] [CrossRef]
- Lim, J.; Lachenmayer, M.L.; Wu, S.; Liu, W.; Kundu, M.; Wang, R.; Komatsu, M.; Oh, Y.J.; Zhao, Y.; Yue, Z. Proteotoxic stress induces phosphorylation of p62/SQSTM1 by ULK1 to regulate selective autophagic clearance of protein aggregates. PLoS Genet. 2015, 11, e1004987. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Harrison, J.; Deaton, C.A.; Johnson, G.V.W. Tau Clearance Mechanisms. Adv. Exp. Med. Biol. 2019, 1184, 57–68. [Google Scholar] [PubMed]
- Koopman, M.B.; Ferrari, L.; Rüdiger, S.G.D. How do protein aggregates escape quality control in neurodegeneration? Trends Neurosci. 2022, 45, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Blaudin de Thé, F.X.; Lassus, B.; Schaler, A.W.; Fowler, S.L.; Goulbourne, C.N.; Jeggo, R.; Mannoury la Cour, C.; Millan, M.J.; Duff, K.E. P62 accumulates through neuroanatomical circuits in response to tauopathy propagation. Acta Neuropathol. Commun. 2021, 9, 177. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, Y.; Wang, C.; Tang, Y.; Mok, S.A.; Tsai, R.M.; Rojas, J.C.; Karydas, A.; Miller, B.L.; Boxer, A.L.; et al. Promoting tau secretion and propagation by hyperactive p300/CBP via autophagy-lysosomal pathway in tauopathy. Mol. Neurodegener. 2020, 15, 2. [Google Scholar] [CrossRef]
- Feng, Q.; Luo, Y.; Zhang, X.N.; Yang, X.F.; Hong, X.Y.; Sun, D.S.; Li, X.C.; Hu, Y.; Li, X.G.; Zhang, J.F.; et al. MAPT/Tau accumulation represses autophagy flux by disrupting IST1-regulated ESCRT-III complex formation: A vicious cycle in Alzheimer neurodegeneration. Autophagy 2020, 16, 641–658. [Google Scholar] [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).