Macrostructural and Microstructural White Matter Alterations Are Associated with Apathy across the Clinical Alzheimer’s Disease Spectrum
Abstract
1. Introduction
2. Materials and Methods
2.1. Participant Sample
2.2. MRI Pre-Processing
2.3. Statistical Analyses
3. Results
3.1. Demographic and Clinical Variables
3.2. Macrostructural and Microstructural WM Damage
3.3. Cognitive Performance and Regional GM Volume
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Le Heron, C.; Apps, M.A.J.; Husain, M. The anatomy of apathy: A neurocognitive framework for amotivated behaviour. Neuropsychologia 2018, 118, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Tan, L.; Wang, H.; Jiang, T.; Tan, M.; Tan, L.; Xu, W.; Li, J.; Wang, J.; Lai, T.; et al. The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: Systematic review and meta-analysis. J. Affect. Disord. 2016, 190, 264–271. [Google Scholar] [CrossRef]
- Sherman, C.; Liu, C.S.; Herrmann, N.; Lanctôt, K.L. Prevalence, neurobiology, and treatments for apathy in prodromal dementia. Int. Psychogeriatr. 2018, 30, 177–184. [Google Scholar] [CrossRef]
- Leung, D.K.Y.; Chan, W.C.; Spector, A.; Wong, G.H.Y. Prevalence of depression, anxiety, and apathy symptoms across dementia stages: A systematic review and meta-analysis. Int. J. Geriatr. Psychiatry 2021, 36, 1330–1344. [Google Scholar] [CrossRef] [PubMed]
- Landes, A.M.; Sperry, S.D.; Strauss, M.E. Prevalence of apathy, dysphoria, and depression in relation to dementia severity in Alzheimer’s disease. J. Neuropsychiatry Clin. Neurosci. 2005, 17, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.S.; Robert, P.; Ereshefsky, L.; Adler, L.; Bateman, D.; Cummings, J.; DeKosky, S.T.; Fischer, C.E.; Husain, M.; Ismail, Z.; et al. Diagnostic criteria for apathy in neurocognitive disorders. Alzheimer’s Dement. 2021, 17, 1892–1904. [Google Scholar] [CrossRef] [PubMed]
- Spalletta, G.; Long, J.D.; Robinson, R.G.; Trequattrini, A.; Pizzoli, S.; Caltagirone, C.; Orfei, M.D. Longitudinal Neuropsychiatric Predictors of Death in Alzheimer’s Disease. J. Alzheimer’s Dis. 2015, 48, 627–636. [Google Scholar] [CrossRef]
- You, S.C.; Walsh, C.M.; Chiodo, L.A.; Ketelle, R.; Miller, B.L.; Kramer, J.H. Neuropsychiatric Symptoms Predict Functional Status in Alzheimer’s Disease. J. Alzheimer’s Dis. 2015, 48, 863–869. [Google Scholar] [CrossRef]
- van der Linde, R.M.; Matthews, F.E.; Dening, T.; Brayne, C. Patterns and persistence of behavioural and psychological symptoms in those with cognitive impairment: The importance of apathy. Int. J. Geriatr. Psychiatry 2017, 32, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, W.; Li, G.; Xiao, S. Prefrontal Aβ pathology influencing the pathway from apathy to cognitive decline in non-dementia elderly. Transl. Psychiatry 2021, 11, 534. [Google Scholar] [CrossRef]
- Palmer, K.; Di Iulio, F.; Varsi, A.E.; Gianni, W.; Sancesario, G.; Caltagirone, C.; Spalletta, G. Neuropsychiatric predictors of progression from amnestic-mild cognitive impairment to Alzheimer’s disease: The role of depression and apathy. J. Alzheimer’s Dis. 2010, 20, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Perri, R.; Turchetta, C.S.; Caruso, G.; Fadda, L.; Caltagirone, C.; Carlesimo, G.A. Neuropsychological correlates of cognitive, emotional-affective and auto-activation apathy in Alzheimer’s disease. Neuropsychologia 2018, 118, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Drijgers, R.L.; Verhey, F.R.J.; Leentjens, A.F.G.; Köhler, S.; Aalten, P. Neuropsychological correlates of apathy in mild cognitive impairment and Alzheimer’s disease: The role of executive functioning. Int. Psychogeriatr. 2011, 23, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- McPherson, S.; Fairbanks, L.; Tiken, S.; Cummings, J.L.; Back-Madruga, C. Apathy and executive function in Alzheimer’s disease. J. Int. Neuropsychol. Soc. 2002, 8, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Grossi, D.; de Lucia, N.; Trojano, L. Closing-in is related to apathy in Alzheimer’s disease patients. J. Alzheimer’s Dis. 2015, 43, 849–855. [Google Scholar] [CrossRef]
- Banning, L.C.P.; Ramakers, I.H.G.B.; Köhler, S.; Bron, E.E.; Verhey, F.R.J.; de Deyn, P.P.; Claassen, J.A.H.R.; Koek, H.L.; Middelkoop, H.A.M.; van der Flier, W.M.; et al. The Association between Biomarkers and Neuropsychiatric Symptoms across the Alzheimer’s Disease Spectrum. Am. J. Geriatr. Psychiatry 2020, 28, 735–744. [Google Scholar] [CrossRef]
- Vergallo, A.; Giampietri, L.; Pagni, C.; Giorgi, F.S.; Nicoletti, V.; Miccoli, M.; Libertini, P.; Petrozzi, L.; Bonuccelli, U.; Tognoni, G. Association Between CSF Beta-Amyloid and Apathy in Early-Stage Alzheimer Disease. J. Geriatr. Psychiatry Neurol. 2019, 32, 164–169. [Google Scholar] [CrossRef]
- Banning, L.C.P.; Ramakers, I.H.G.B.; Rosenberg, P.B.; Lyketsos, C.G.; Leoutsakos, J.S. Alzheimer’s disease biomarkers as predictors of trajectories of depression and apathy in cognitively normal individuals, mild cognitive impairment, and Alzheimer’s disease dementia. Int. J. Geriatr. Psychiatry 2021, 36, 224–234. [Google Scholar] [CrossRef]
- Mori, T.; Shimada, H.; Shinotoh, H.; Hirano, S.; Eguchi, Y.; Yamada, M.; Fukuhara, R.; Tanimukai, S.; Zhang, M.; Kuwabara, S.; et al. Apathy correlates with prefrontal amyloid β deposition in Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2014, 85, 449–455. [Google Scholar] [CrossRef]
- Tissot, C.; Therriault, J.; Pascoal, T.A.; Chamoun, M.; Lussier, F.Z.; Savard, M.; Mathotaarachchi, S.S.; Benedet, A.L.; Thomas, E.M.; Parsons, M.; et al. Association between regional tau pathology and neuropsychiatric symptoms in aging and dementia due to Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2021, 7, e12154. [Google Scholar] [CrossRef]
- Marshall, G.A.; Gatchel, J.R.; Donovan, N.J.; Muniz, M.C.; Schultz, A.P.; Becker, J.A.; Chhatwal, J.P.; Hanseeuw, B.J.; Papp, K.V.; Amariglio, R.E.; et al. Regional Tau Correlates of Instrumental Activities of Daily Living and Apathy in Mild Cognitive Impairment and Alzheimer’s Disease Dementia. J. Alzheimer’s Dis. 2019, 67, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Perin, S.; Harrington, K.D.; Lim, Y.Y.; Ellis, K.; Ames, D.; Pietrzak, R.H.; Schembri, A.; Rainey-Smith, S.; Salvado, O.; Laws, S.M.; et al. Amyloid burden and incident depressive symptoms in preclinical Alzheimer’s disease. J. Affect. Disord. 2018, 229, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.; Stomrud, E.; Lindberg, O.; Westman, E.; Johansson, P.M.; van Westen, D.; Mattsson, N.; Hansson, O. Apathy and anxiety are early markers of Alzheimer’s disease. Neurobiol. Aging 2020, 85, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Tekin, S.; Mega, M.S.; Masterman, D.M.; Chow, T.; Garakian, J.; Vinters, H.V.; Cummings, J.L. Orbitofrontal and anterior cingulate cortex neurofibrillary tangle burden is associated with agitation in Alzheimer disease. Ann. Neurol. 2001, 49, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Marshall, G.A.; Fairbanks, L.A.; Tekin, S.; Vinters, H.V.; Cummings, J.L. Neuropathologic correlates of apathy in Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2006, 21, 144–147. [Google Scholar] [CrossRef]
- Förstl, H.; Burns, A.; Levy, R.; Cairns, N.; Luthert, P.; Lantos, P. Neuropathological correlates of behavioural disturbance in confirmed Alzheimer’s disease. Br. J. Psychiatry 1993, 163, 364–368. [Google Scholar] [CrossRef]
- Guimarães, H.C.; Levy, R.; Teixeira, A.L.; Beato, R.G.; Caramelli, P. Neurobiology of apathy in Alzheimer’s disease. Arq. Neuro-Psiquiatr. 2008, 66, 436–443. [Google Scholar] [CrossRef]
- Marshall, G.A.; Monserratt, L.; Harwood, D.; Mandelkern, M.; Cummings, J.L.; Sultzer, D.L. Positron emission tomography metabolic correlates of apathy in Alzheimer disease. Arch. Neurol. 2007, 64, 1015–1020. [Google Scholar] [CrossRef]
- Apostolova, L.G.; Akopyan, G.G.; Partiali, N.; Steiner, C.A.; Dutton, R.A.; Hayashi, K.M.; Dinov, I.D.; Toga, A.W.; Cummings, J.L.; Thompson, P.M. Structural correlates of apathy in Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2007, 24, 91–97. [Google Scholar] [CrossRef]
- Raimo, S.; Santangelo, G.; D’Iorio, A.; Trojano, L.; Grossi, D. Neural correlates of apathy in patients with neurodegenerative disorders: An activation likelihood estimation (ALE) meta-analysis. Brain Imaging Behav. 2019, 13, 1815–1834. [Google Scholar] [CrossRef]
- Gatchel, J.R.; Donovan, N.J.; Locascio, J.J.; Becker, J.A.; Rentz, D.M.; Sperling, R.A.; Johnson, K.A.; Marshall, G.A. Regional 18F-Fluorodeoxyglucose Hypometabolism is Associated with Higher Apathy Scores Over Time in Early Alzheimer Disease. Am. J. Geriatr. Psychiatry 2017, 25, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Stanton, B.R.; Leigh, P.N.; Howard, R.J.; Barker, G.J.; Brown, R.G. Behavioural and emotional symptoms of apathy are associated with distinct patterns of brain atrophy in neurodegenerative disorders. J. Neurol. 2013, 260, 2481–2490. [Google Scholar] [CrossRef] [PubMed]
- Eggins, P.; Wong, S.; Wei, G.; Hodges, J.R.; Husain, M.; Piguet, O.; Irish, M.; Kumfor, F. A shared cognitive and neural basis underpinning cognitive apathy and planning in behavioural-variant frontotemporal dementia and Alzheimer’s disease. Cortex 2022, 154, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Tumati, S.; Marsman, J.C.; De Deyn, P.P.; Martens, S.; Aleman, A. Functional network topology associated with apathy in Alzheimer’s disease. J. Affect. Disord. 2020, 266, 473–481. [Google Scholar] [CrossRef]
- Jones, S.A.; De Marco, M.; Manca, R.; Bell, S.M.; Blackburn, D.J.; Wilkinson, I.D.; Soininen, H.; Venneri, A. Altered frontal and insular functional connectivity as pivotal mechanisms for apathy in Alzheimer’s disease. Cortex 2019, 119, 100–110. [Google Scholar] [CrossRef]
- Jang, J.Y.; Han, S.D.; Yew, B.; Blanken, A.E.; Dutt, S.; Li, Y.; Ho, J.K.; Gaubert, A.; Nation, D.A. Resting-State Functional Connectivity Signatures of Apathy in Community-Living Older Adults. Front. Aging Neurosci. 2021, 13, 691710. [Google Scholar] [CrossRef]
- Hahn, C.; Lim, H.; Won, W.Y.; Ahn, K.J.; Jung, W.; Lee, C.U. Apathy and white matter integrity in Alzheimer’s disease: A whole brain analysis with tract-based spatial statistics. PLoS ONE 2013, 8, e53493. [Google Scholar] [CrossRef]
- Cacciari, C.; Moraschi, M.; Di Paola, M.; Cherubini, A.; Orfei, M.D.; Giove, F.; Maraviglia, B.; Caltagirone, C.; Spalletta, G. White matter microstructure and apathy level in amnestic mild cognitive impairment. J. Alzheimer’s Dis. 2010, 20, 501–507. [Google Scholar] [CrossRef]
- Tighe, S.K.; Oishi, K.; Mori, S.; Smith, G.S.; Albert, M.; Lyketsos, C.G.; Mielke, M.M. Diffusion tensor imaging of neuropsychiatric symptoms in mild cognitive impairment and Alzheimer’s dementia. J. Neuropsychiatry Clin. Neurosci. 2012, 24, 484–488. [Google Scholar] [CrossRef]
- Ota, M.; Sato, N.; Nakata, Y.; Arima, K.; Uno, M. Relationship between apathy and diffusion tensor imaging metrics of the brain in Alzheimer’s disease. Int. J. Geriatr. Psychiatry 2012, 27, 722–726. [Google Scholar] [CrossRef]
- Kim, J.W.; Lee, D.Y.; Choo, I.H.; Seo, E.H.; Kim, S.G.; Park, S.Y.; Woo, J.I. Microstructural alteration of the anterior cingulum is associated with apathy in Alzheimer disease. Am. J. Geriatr. Psychiatry 2011, 19, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Setiadi, T.M.; Martens, S.; Opmeer, E.M.; Marsman, J.C.; Tumati, S.; Reesink, F.E.; De Deyn, P.P.; Aleman, A.; Ćurčić-Blake, B. Widespread white matter aberration is associated with the severity of apathy in amnestic Mild Cognitive Impairment: Tract-based spatial statistics analysis. Neuroimage Clin. 2021, 29, 102567. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.; Huang, W.; Hsu, Y.; Lo, C.; Deng, J.F.; Huang, C. Comparison of neuropsychiatric symptoms and diffusion tensor imaging correlates among patients with subcortical ischemic vascular disease and Alzheimer’s disease. BMC Neurol. 2017, 17, 144. [Google Scholar]
- Agüera-Ortiz, L.; Hernandez-Tamames, J.A.; Martinez-Martin, P.; Cruz-Orduña, I.; Pajares, G.; López-Alvarez, J.; Osorio, R.S.; Sanz, M.; Olazarán, J. Structural correlates of apathy in Alzheimer’s disease: A multimodal MRI study. Int. J. Geriatr. Psychiatry 2017, 32, 922–930. [Google Scholar] [CrossRef]
- Jonsson, M.; Edman, A.; Lind, K.; Rolstad, S.; Sjögren, M.; Wallin, A. Apathy is a prominent neuropsychiatric feature of radiological white-matter changes in patients with dementia. Int. J. Geriatr. Psychiatry 2010, 25, 588–595. [Google Scholar] [CrossRef]
- Starkstein, S.E.; Sabe, L.; Vázquez, S.; Di Lorenzo, G.; Martínez, A.; Petracca, G.; Tesón, A.; Chemerinski, E.; Leiguarda, R. Neuropsychological, psychiatric, and cerebral perfusion correlates of leukoaraiosis in Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 1997, 63, 66–73. [Google Scholar] [CrossRef][Green Version]
- Misquitta, K.; Dadar, M.; Louis Collins, D.; Tartaglia, M.C. White matter hyperintensities and neuropsychiatric symptoms in mild cognitive impairment and Alzheimer’s disease. Neuroimage Clin. 2020, 28, 102367. [Google Scholar] [CrossRef]
- Starkstein, S.E.; Mizrahi, R.; Capizzano, A.A.; Acion, L.; Brockman, S.; Power, B.D. Neuroimaging correlates of apathy and depression in Alzheimer’s disease. J. Neuropsychiatry Clin. Neurosci. 2009, 21, 259–265. [Google Scholar] [CrossRef]
- Desmarais, P.; Gao, A.F.; Lanctôt, K.; Rogaeva, E.; Ramirez, J.; Herrmann, N.; Stuss, D.T.; Black, S.E.; Keith, J.; Masellis, M. White matter hyperintensities in autopsy-confirmed frontotemporal lobar degeneration and Alzheimer’s disease. Alzheimer’s Res. Ther. 2021, 13, 129. [Google Scholar] [CrossRef]
- Staekenborg, S.S.; Gillissen, F.; Romkes, R.; Pijnenburg, Y.A.L.; Barkhof, F.; Scheltens, P.; van der Flier, W.M. Behavioural and psychological symptoms are not related to white matter hyperintensities and medial temporal lobe atrophy in Alzheimer’s disease. Int. J. Geriatr. Psychiatry 2008, 23, 387–392. [Google Scholar] [CrossRef]
- Torso, M.; Serra, L.; Giulietti, G.; Spanò, B.; Tuzzi, E.; Koch, G.; Caltagirone, C.; Cercignani, M.; Bozzali, M. Strategic lesions in the anterior thalamic radiation and apathy in early Alzheimer’s disease. PLoS ONE 2015, 10, e0124998. [Google Scholar] [CrossRef]
- Kaufer, D.I.; Cummings, J.L.; Ketchel, P.; Smith, V.; MacMillan, A.; Shelley, T.; Lopez, O.L.; DeKosky, S.T. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J. Neuropsychiatry Clin. Neurosci. 2000, 12, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.C. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology 1993, 43, 2412–2414. [Google Scholar] [CrossRef] [PubMed]
- Hansson, O.; Seibyl, J.; Stomrud, E.; Zetterberg, H.; Trojanowski, J.Q.; Bittner, T.; Lifke, V.; Corradini, V.; Eichenlaub, U.; Batrla, R.; et al. CSF biomarkers of Alzheimer’s disease concord with amyloid-β PET and predict clinical progression: A study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimer’s Dement. 2018, 14, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Nobis, L.; Husain, M. Apathy in Alzheimer’s disease. Curr. Opin. Behav. Sci. 2018, 22, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Malone, I.B.; Leung, K.K.; Clegg, S.; Barnes, J.; Whitwell, J.L.; Ashburner, J.; Fox, N.C.; Ridgway, G.R. Accurate automatic estimation of total intracranial volume: A nuisance variable with less nuisance. Neuroimage 2015, 104, 366–372. [Google Scholar] [CrossRef]
- Schmidt, P.; Gaser, C.; Arsic, M.; Buck, D.; Förschler, A.; Berthele, A.; Hoshi, M.; Ilg, R.; Schmid, V.J.; Zimmer, C.; et al. An automated tool for detection of FLAIR-hyperintense white-matter lesions in Multiple Sclerosis. Neuroimage 2012, 59, 3774–3783. [Google Scholar] [CrossRef] [PubMed]
- Manca, R.; Stabile, M.R.; Bevilacqua, F.; Cadorin, C.; Piccione, F.; Sharrack, B.; Venneri, A. Cognitive speed and white matter integrity in secondary progressive multiple sclerosis. Mult. Scler. Relat. Disord. 2019, 30, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Mühlau, M.; Buck, D.; Förschler, A.; Boucard, C.C.; Arsic, M.; Schmidt, P.; Gaser, C.; Berthele, A.; Hoshi, M.; Jochim, A.; et al. White-matter lesions drive deep gray-matter atrophy in early multiple sclerosis: Support from structural MRI. Mult. Scler. J. 2013, 19, 1485–1492. [Google Scholar] [CrossRef]
- Smith, S.M.; Nichols, T.E. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 2009, 44, 83–98. [Google Scholar] [CrossRef]
- Hollocks, M.J.; Lawrence, A.J.; Brookes, R.L.; Barrick, T.R.; Morris, R.G.; Husain, M.; Markus, H.S. Differential relationships between apathy and depression with white matter microstructural changes and functional outcomes. Brain 2015, 138, 3803–3815. [Google Scholar] [CrossRef] [PubMed]
- Klimiec-Moskal, E.; Karcz, P.; Kowalska, K.; Slowik, A.; Herman-Sucharska, I.; Dziedzic, T. Magnetisation transfer imaging revealed microstructural changes related to apathy symptoms after ischaemic stroke. Int. J. Geriatr. Psychiatry 2021, 36, 1264–1273. [Google Scholar] [CrossRef]
- Pinto, E.; Peters, R. Literature review of the Clock Drawing Test as a tool for cognitive screening. Dement. Geriatr. Cogn. Disord. 2009, 27, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Supasitthumrong, T.; Herrmann, N.; Tunvirachaisakul, C.; Shulman, K. Clock drawing and neuroanatomical correlates: A systematic review. Int. J. Geriatr. Psychiatry 2019, 34, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Hirjak, D.; Sambataro, F.; Remmele, B.; Kubera, K.M.; Schröder, J.; Seidl, U.; Thomann, A.K.; Maier-Hein, K.H.; Wolf, R.C.; Thomann, P.A. The relevance of hippocampal subfield integrity and clock drawing test performance for the diagnosis of Alzheimer’s disease and mild cognitive impairment. World J. Biol. Psychiatry 2019, 20, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Bubb, E.J.; Metzler-Baddeley, C.; Aggleton, J.P. The cingulum bundle: Anatomy, function, and dysfunction. Neurosci. Biobehav. Rev. 2018, 92, 104–127. [Google Scholar] [CrossRef] [PubMed]
- Puglisi, G.; Howells, H.; Sciortino, T.; Leonetti, A.; Rossi, M.; Conti Nibali, M.; Gabriel Gay, L.; Fornia, L.; Bellacicca, A.; Viganò, L.; et al. Frontal pathways in cognitive control: Direct evidence from intraoperative stimulation and diffusion tractography. Brain 2019, 142, 2451–2465. [Google Scholar]
- Thiebaut de Schotten, M.; Dell’Acqua, F.; Forkel, S.J.; Simmons, A.; Vergani, F.; Murphy, D.G.M.; Catani, M. A lateralized brain network for visuospatial attention. Nat. Neurosci. 2011, 14, 1245–1246. [Google Scholar] [CrossRef]
- Catani, M.; Thiebaut de Schotten, M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex 2008, 44, 1105–1132. [Google Scholar] [CrossRef] [PubMed]
- Nomi, J.S.; Schettini, E.; Broce, I.; Dick, A.S.; Uddin, L.Q. Structural Connections of Functionally Defined Human Insular Subdivisions. Cereb. Cortex 2018, 28, 3445–3456. [Google Scholar] [CrossRef]
- Mortby, M.E.; Adler, L.; Agüera-Ortiz, L.; Bateman, D.R.; Brodaty, H.; Cantillon, M.; Geda, Y.E.; Ismail, Z.; Lanctôt, K.L.; Marshall, G.A.; et al. Apathy as a Treatment Target in Alzheimer’s Disease: Implications for Clinical Trials. Am. J. Geriatr. Psychiatry 2022, 30, 119–147. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | AP-PT (n = 61) | NA-PT (n = 61) | CU (n = 61) | F | p |
|---|---|---|---|---|---|
| Age (years) | 73.33 (6.97) | 73.93 (8.47) | 73.11 (6.26) | 0.21 | 0.813 |
| Education (years) a | 16.00 (4) | 16.00 (5) | 16.00 (4) | 1.27 b | 0.529 |
| Sex (F/M) c | 17/44 * | 29/32 | 33/28 | 9.27 d | 0.010 |
| CDR a | 0.00 (0.5) * | 0.50 (0) * | 0.00 (0) | 149.60 b | <0.001 |
| MMSE a | 26.00 (4) * | 26.00 (3) * | 29.00 (2) | 50.02 b | <0.001 |
| NPI-Q (total) a | 6.00 (6) * | 1.00 (2) * | 0.00 (0) | 132.16 b | <0.001 |
| NPI-Q total—Apathy score a | 5.00 (6) * | 1.00 (2) * | 0.00 (0) | 106.38 b | <0.001 |
| TIV (ml) | 1500.58 (140.57) * | 1452.82 (151.20) | 1439.58 (123.10) | 3.26 | 0.041 |
| GMV/TIV a | 0.39 (0.05) * | 0.41 (0.07) * | 0.43 (0.05) | 20.34 b | <0.001 |
| WMV/TIV a | 0.27 (0.03) | 0.27 (0.02) | 0.27 (0.02) | 1.06 b | 0.589 |
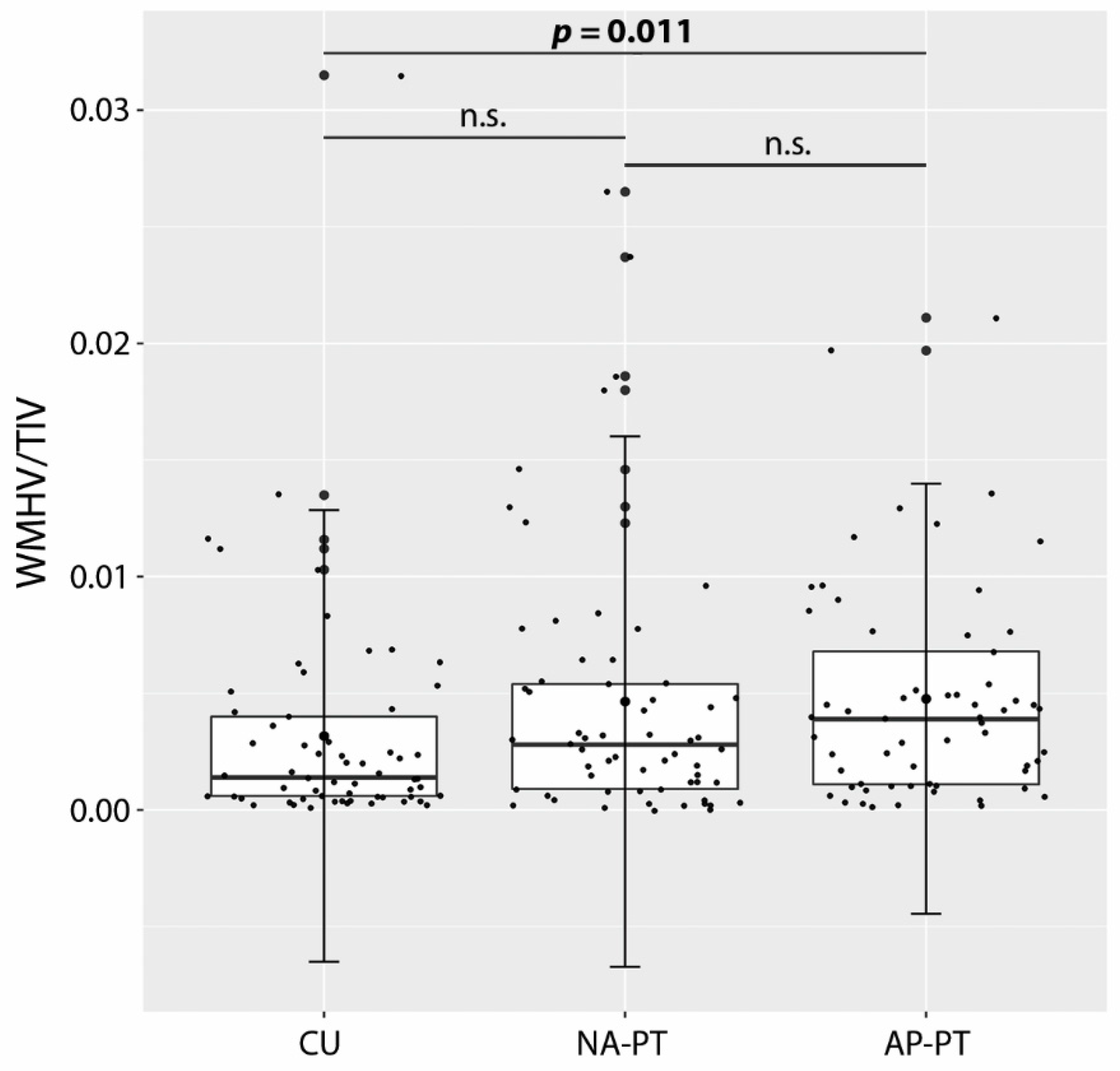
| WMHV/TIV a | 0.004 (0.006) * | 0.003 (0.004) | 0.001 (0.003) | 8.27 b | 0.016 |
| Aβ (+/−) | 41/17 * (n = 58) | 32/21 * (n = 53) | 12/39 (n = 51) | 26.17 d | <0.001 |
| p-tau (+/−) | 38/20 * (n = 58) | 31/22 * (n = 53) | 10/41 (n = 51) | 25.87 d | <0.001 |
| p | Cluster Extent | Side | White Matter Tract | t | MNI Coordinates | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
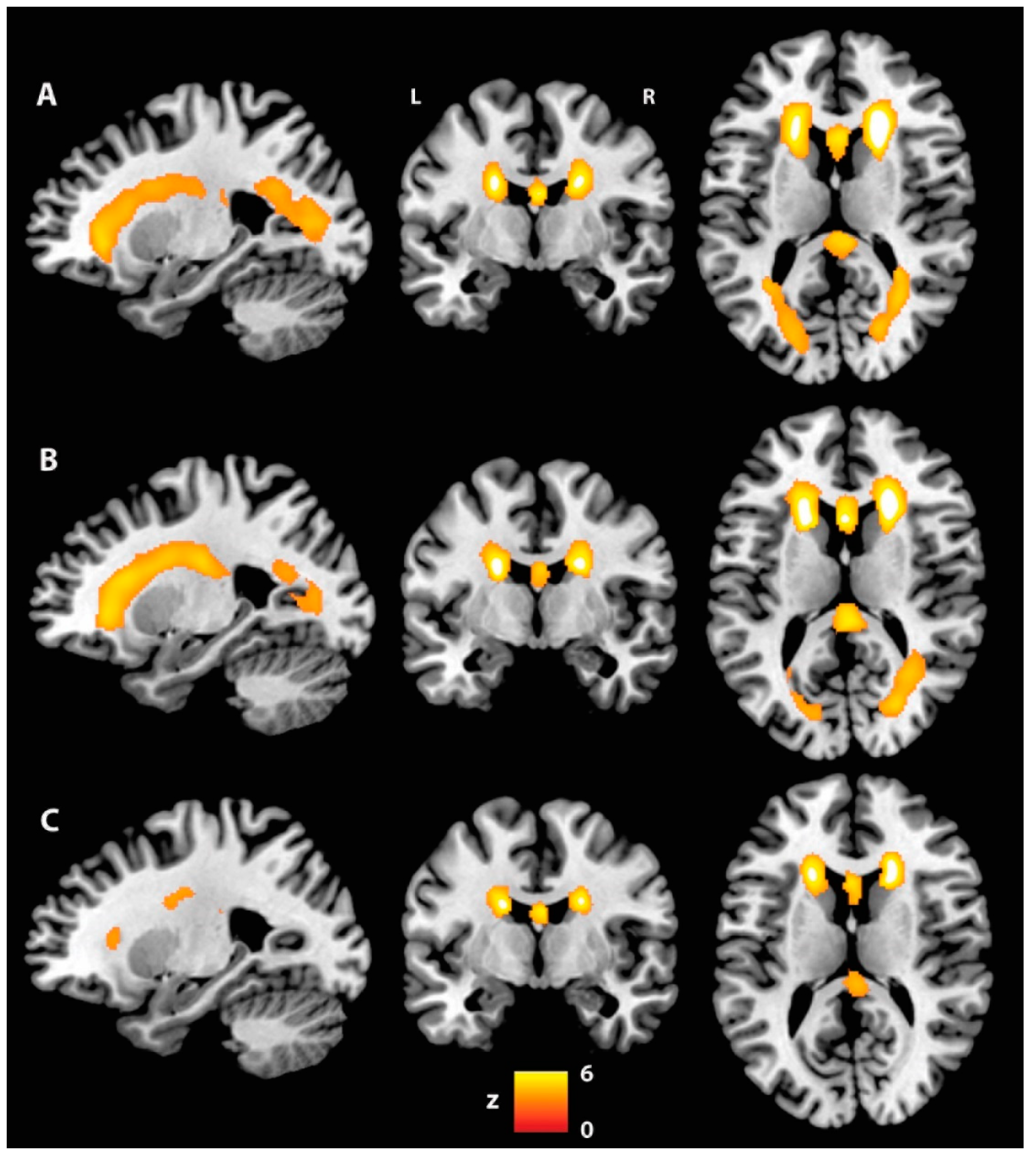
| Fractional anisotropy: AP-PT < CU | |||||||
| 0.035 | 3283 | - | Fornix | 4.65 | 0 | −4 | 13 |
| - | Fornix | 4.31 | 0 | 5 | 7 | ||
| - | Fornix | 4.28 | 0 | 2 | 10 | ||
| L | Uncinate fasciculus | 4.20 | −23 | −1 | −9 | ||
| R | ATR | 4.20 | 14 | −10 | 16 | ||
| L | ATR | 4.20 | −13 | −5 | 16 | ||
| 0.040 | 1546 | L | SLF | 3.91 | −38 | 21 | 16 |
| L | SLF | 3.84 | −35 | 2 | 30 | ||
| L | SLF | 3.82 | −32 | 5 | 29 | ||
| L | SLF | 3.60 | −34 | 6 | 23 | ||
| L | SLF | 3.52 | −33 | −17 | 38 | ||
| L | SLF | 3.49 | −33 | −33 | 37 | ||
| 0.041 | 929 | R | IFOF | 4.34 | 35 | −11 | −14 |
| R | IFOF | 4.12 | 38 | −9 | −15 | ||
| R | ILF | 3.42 | 37 | −6 | −22 | ||
| R | IFOF | 3.35 | 34 | −6 | −13 | ||
| R | Uncinate fasciculus | 3.24 | 30 | 7 | −12 | ||
| R | ILF | 3.20 | 41 | −7 | −36 | ||
| 0.045 | 177 | R | Cingulum (temporal) | 5.03 | 22 | −13 | −28 |
| R | Cingulum (temporal) | 4.21 | 29 | −29 | −17 | ||
| R | Cingulum (temporal) | 3.96 | 29 | −30 | −15 | ||
| R | Cingulum (temporal) | 3.71 | 30 | −26 | −19 | ||
| R | Cingulum (temporal) | 3.66 | 25 | −24 | −20 | ||
| R | Cingulum (temporal) | 3.66 | 26 | −26 | −20 | ||
| 0.050 | 117 | L | Forceps minor | 3.03 | −19 | 33 | 14 |
| L | Forceps minor | 2.93 | −15 | 26 | 20 | ||
| L | Forceps minor | 2.92 | −17 | 34 | 14 | ||
| L | Forceps minor | 2.53 | −16 | 32 | 21 | ||
| L | Forceps minor | 2.52 | −17 | 30 | 19 | ||
| L | Forceps minor | 2.48 | −16 | 30 | 22 | ||
| 0.047 | 82 | R | ILF | 4.04 | 33 | −1 | −30 |
| R | ILF | 3.56 | 31 | 3 | −31 | ||
| R | Cingulum (temporal) | 3.52 | 34 | −5 | −31 | ||
| R | ILF | 3.17 | 32 | 0 | −28 | ||
| R | ILF | 2.87 | 30 | 0 | −29 | ||
| R | Cingulum (temporal) | 2.78 | 34 | −3 | −34 | ||
| 0.049 | 76 | R | Cingulum (temporal) | 4.03 | 36 | −17 | −28 |
| R | Cingulum (temporal) | 3.42 | 34 | −19 | −26 | ||
| R | Cingulum (temporal) | 3.34 | 36 | −12 | −29 | ||
| R | Cingulum (temporal) | 2.86 | 36 | −20 | −25 | ||
| R | Cingulum (temporal) | 2.77 | 34 | −9 | −35 | ||
| R | Cingulum (temporal) | 2.72 | 34 | −9 | −33 | ||
| 0.050 | 64 | L | ATR | 3.06 | −21 | −50 | 36 |
| L | ATR | 3.04 | −22 | −47 | 39 | ||
| L | ATR | 3.01 | −20 | −52 | 36 | ||
| L | SLF | 2.76 | −23 | −50 | 35 | ||
| 0.050 | 52 | L | Forceps minor | 3.34 | −11 | 28 | −8 |
| L | Forceps minor | 3.06 | −12 | 28 | −6 | ||
| L | Forceps minor | 2.87 | −12 | 30 | −5 | ||
| L | Forceps minor | 2.34 | −15 | 35 | −6 | ||
| 0.050 | 50 | L | Forceps minor | 3.09 | −13 | 45 | −15 |
| L | Forceps minor | 3.02 | −14 | 43 | −14 | ||
| L | Forceps minor | 2.98 | −15 | 42 | −11 | ||
| 0.050 | 45 | L | SLF | 3.15 | −32 | 27 | 25 |
| L | SLF | 2.87 | −36 | 29 | 26 | ||
| L | SLF | 2.82 | −36 | 31 | 26 | ||
| L | SLF | 2.76 | −33 | 27 | 28 | ||
| L | SLF | 2.66 | −36 | 27 | 27 | ||
| 0.050 | 44 | R | Forceps minor | 2.79 | 18 | 39 | −4 |
| R | Forceps minor | 2.65 | 19 | 37 | −6 | ||
| R | Forceps minor | 2.62 | 17 | 41 | −8 | ||
| R | Forceps minor | 2.57 | 17 | 37 | −6 | ||
| 0.050 | 39 | L | Forceps minor | 3.46 | −28 | 39 | 17 |
| L | Forceps minor | 3.26 | −30 | 40 | 14 | ||
| L | Forceps minor | 2.93 | −30 | 39 | 18 | ||
| L | Forceps minor | 2.80 | −30 | 37 | 17 | ||
| L | Forceps minor | 2.77 | −30 | 37 | 15 | ||
| L | Forceps minor | 2.56 | −30 | 38 | 12 | ||
| 0.050 | 32 | L | SLF | 3.37 | −19 | −52 | 54 |
| L | SLF | 3.19 | −18 | −53 | 51 | ||
| L | SLF | 3.00 | −18 | −49 | 46 | ||
| L | SLF | 2.81 | −18 | −53 | 48 | ||
| 0.050 | 27 | L | Forceps major | 3.92 | −26 | −56 | 21 |
| L | Forceps major | 3.54 | −23 | −56 | 22 | ||
| 0.050 | 18 | R | Uncinate fasciculus | 2.59 | 20 | 20 | −13 |
| 0.050 | 15 | L | SLF | 2.36 | −31 | 10 | 43 |
| L | SLF | 2.19 | −31 | 6 | 44 | ||
| L | SLF | 2.16 | −31 | 8 | 43 | ||
| L | SLF | 2.13 | −31 | 7 | 46 | ||
| Axial diffusivity: AP-PT > CU | |||||||
| 0.040 | 661 | L | SLF | 4.43 | −25 | −6 | 25 |
| L | SLF | 4.03 | −25 | −6 | 19 | ||
| L | SLF | 3.88 | −28 | −3 | 23 | ||
| L | SLF | 3.82 | −28 | −7 | 24 | ||
| L | SLF | 3.78 | −24 | −15 | 13 | ||
| L | SLF | 3.48 | −26 | −13 | 16 | ||
| Radial diffusivity: AP-PT > CU | |||||||
| 0.040 | 16 | - | Fornix | 4.58 | 0 | −4 | 13 |
| - | Fornix | 4.14 | 0 | 0 | 12 | ||
| - | Fornix | 4.05 | 0 | 3 | 9 | ||
| Characteristics | AP-PT (n = 61) | NA-PT (n = 61) | CU (n = 61) | F | p |
|---|---|---|---|---|---|
| CDT—drawing a | 5.00 (1) * | 5.00 (1) | 5.00 (1) | 5.66 b | 0.059 |
| CDT—copy a | 5.00 (1) * | 5.00 (0) | 5.00 (0) | 9.47 b | 0.009 |
| LMT—IR | 7.69 (4.41) (n = 58) * | 7.90 (4.06) * | 13.90 (3.05) | 50.04 | <0.001 |
| LMT—DR a | 4.50 (9) * (n = 58) | 6.00 (8) * | 12.00 (5) | 71.57 b | <0.001 |
| AVLT—IR | 29.00 (10.37) * | 30.13 (8.04) * | 44.85 (10.03) | 52.44 | <0.001 |
| AVLT—DR a | 0.00 (4) * | 2.00 (4) * | 8.00 (5) | 59.99 b | <0.001 |
| CFT-A (total) | 14.31 (5.26) * | 15.26 (4.71) * | 20.90 (4.90) | 31.42 | <0.001 |
| CFT-A (perseverations) a | 0.00 (1) | 0.00 (1) | 0.00 (1) | 2.49 b | 0.288 |
| CFT-A (intrusions) a | 0.00 (0) | 0.00 (0) | 0.00 (0) | 0.52 b | 0.769 |
| TMT-A (sec) a | 41.00 (25) * | 40.00 (18) * | 34.00 (13) | 17.44 b | <0.001 |
| TMT-B (sec) a | 116.00 (137) * (n = 59) | 120 (114) * | 76.00 (32) | 30.33 b | <0.001 |
| BNT (total) a | 27.00 (6) * | 26.00 (4) * | 29.00 (3) | 17.94 b | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manca, R.; Jones, S.A.; Venneri, A. Macrostructural and Microstructural White Matter Alterations Are Associated with Apathy across the Clinical Alzheimer’s Disease Spectrum. Brain Sci. 2022, 12, 1383. https://doi.org/10.3390/brainsci12101383
Manca R, Jones SA, Venneri A. Macrostructural and Microstructural White Matter Alterations Are Associated with Apathy across the Clinical Alzheimer’s Disease Spectrum. Brain Sciences. 2022; 12(10):1383. https://doi.org/10.3390/brainsci12101383
Chicago/Turabian StyleManca, Riccardo, Sarah A. Jones, and Annalena Venneri. 2022. "Macrostructural and Microstructural White Matter Alterations Are Associated with Apathy across the Clinical Alzheimer’s Disease Spectrum" Brain Sciences 12, no. 10: 1383. https://doi.org/10.3390/brainsci12101383
APA StyleManca, R., Jones, S. A., & Venneri, A. (2022). Macrostructural and Microstructural White Matter Alterations Are Associated with Apathy across the Clinical Alzheimer’s Disease Spectrum. Brain Sciences, 12(10), 1383. https://doi.org/10.3390/brainsci12101383






