Adaptive Hybrid Surgery Experiences in Benign Skull Base Tumors
Abstract
1. Introduction
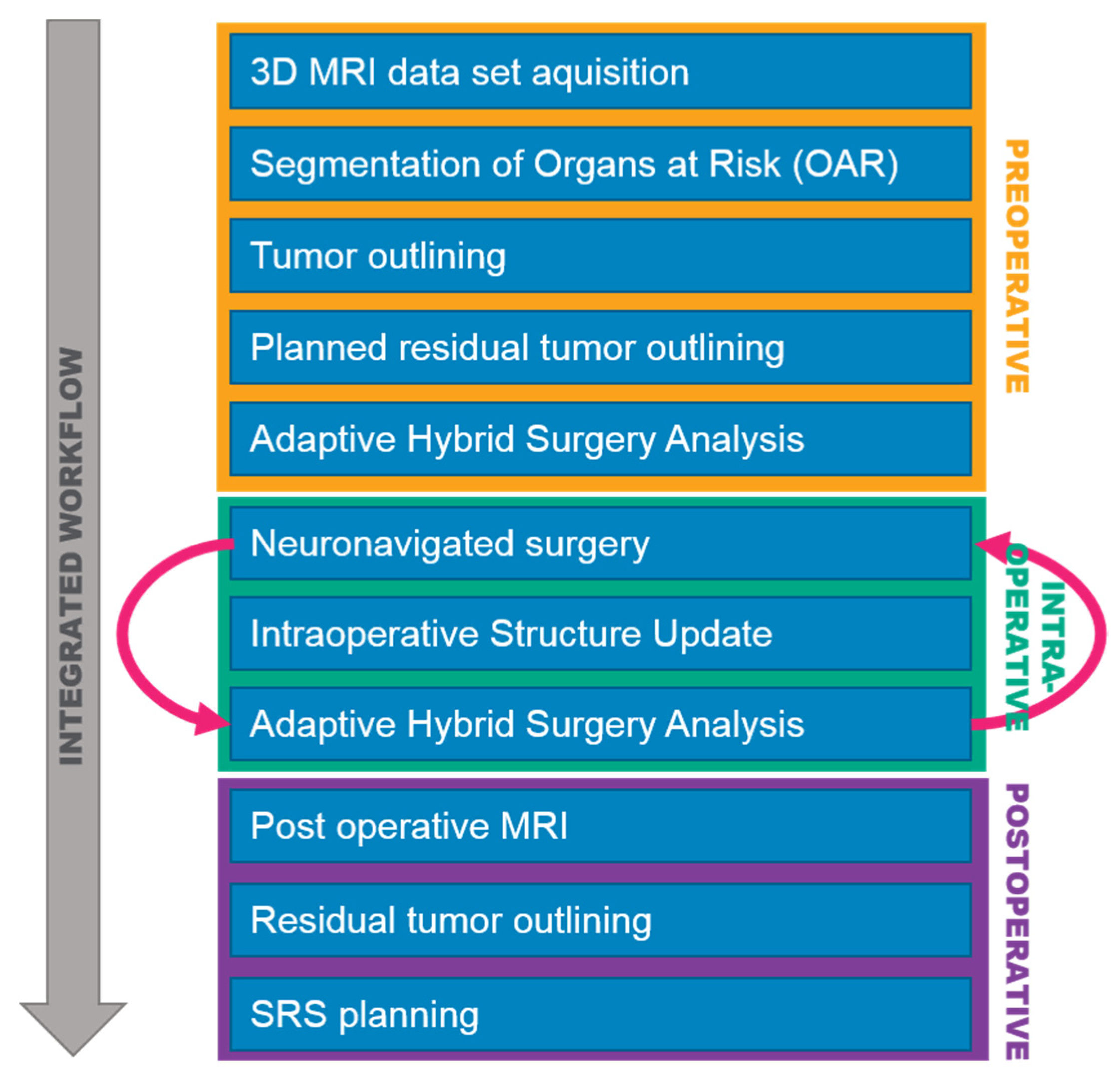
2. Materials and Methods
- Single fraction stereotactic radiosurgery (sf-SRS);
- Hypofractionated stereotactic radiosurgery (hf-SRS); and
- Conventional fractionated stereotactic radiotherapy (cf-SRT).
3. Results
3.1. Clinical Outcome
3.2. Postoperative Stereotactic Radiosurgery
3.3. Illustrative Cases
3.3.1. Case 1
3.3.2. Case 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethics Approval and Consent to Participate
Abbreviations
| AHSA | Adaptive Hybrid Surgery Analysis |
| ISU | Intraoperative Structure Update |
References
- Al-Mefty, O.; Fox, J.L.; Smith, R.R. Petrosal approach for petroclival meningiomas. Neurosurgery 1988, 22, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Sekhar, L.N.; Babu, R.P.; Wright, D.C. Surgical resection of cranial base meningiomas. Neurosurg. Clin. N. Am. 1994, 5, 299–330. [Google Scholar] [CrossRef]
- Sekhar, L.N.; Burgess, J.; Akin, O. Anatomical study of the cavernous sinus emphasizing operative approaches and related vascular and neural reconstruction. Neurosurgery 1987, 21, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Sekhar, L.N.; Swamy, N.K.; Jaiswal, V.; Rubinstein, E.; Hirsch, W.E., Jr.; Wright, D.C. Surgical excision of meningiomas involving the clivus: Preoperative and intraoperative features as predictors of postoperative functional deterioration. J. Neurosurg. 1994, 81, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Zentner, J.; Meyer, B.; Vieweg, U.; Herberhold, C.; Schramm, J. Petroclival meningiomas: Is radical resection always the best option? J. Neurol. Neurosurg. Psychiatry 1997, 62, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Lunsford, L.D. Contemporary management of meningiomas: Radiation therapy as an adjuvant and radiosurgery as an alternative to surgical removal? J. Neurosurg. 1994, 80, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Ichinose, T.; Goto, T.; Ishibashi, K.; Takami, T.; Ohata, K. The role of radical microsurgical resection in multimodal treatment for skull base meningioma. J. Neurosurg. 2010, 113, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, J.P.; Starke, R.M.; Kano, H.; Barnett, G.H.; Mathieu, D.; Chiang, V.; Yu, J.B.; Hess, J.; McBride, H.L.; Honea, N.; et al. Gamma Knife radiosurgery for posterior fossa meningiomas: A multicenter study. J. Neurosurg. 2015, 122, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
- Park, C.K.; Jung, H.W.; Kim, J.E.; Paek, S.H.; Kim, D.G. The selection of the optimal therapeutic strategy for petroclival meningiomas. Surg. Neurol. 2006, 66, 160–165, discussion in 165–166. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Inbar, O.; Sviri, G.E. Adaptive Hybrid Surgery: Paradigm Shift for Patient-centered Neurosurgery. Rambam Maimonides Med. J. 2018, 9, e0025. [Google Scholar] [CrossRef] [PubMed]
- Schwyzer, L.; Kienzler, J.C.; Coluccia, D.; Fandino, J. Integrating Surgery and Radiosurgery: Our first experiences with adaptive hybrid surgery analysis software in benign skull base tumors. J. Neurol. Surg. Part A Cent. Eur. Neurosurg. 2017, 78, S1–S22. [Google Scholar]
- Seifert, V. Clinical management of petroclival meningiomas and the eternal quest for preservation of quality of life: Personal experiences over a period of 20 years. Acta Neurochir. 2010, 152, 1099–1116. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, O.; Han, Y.Y.; Talbott, E.O.; Iyer, A.K.; Kano, H.; Kondziolka, D.; Brown, M.A.; Lunsford, L.D. Gamma Knife Radiosurgery for Vestibular Schwannomas and Quality of Life Evaluation. Stereotact. Funct. Neurosurg. 2017, 95, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Zumofen, D.W.; Guffi, T.; Epple, C.; Westermann, B.; Krahenbuhl, A.K.; Zabka, S.; Taub, E.; Bodmer, D.; Mariani, L. Intended Near-Total Removal of Koos Grade IV Vestibular Schwannomas: Reconsidering the Treatment Paradigm. Neurosurgery 2017, 82, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Lipski, S.M.; Hayashi, M.; Chernov, M.; Levivier, M.; Okada, Y. Modern Gamma Knife radiosurgery of vestibular schwannomas: Treatment concept, volumetric tumor response, and functional results. Neurosurg. Rev. 2015, 38, 309–318, discussion in 318. [Google Scholar] [CrossRef] [PubMed]
- Daniel, R.T.; Tuleasca, C.; George, M.; Pralong, E.; Schiappacasse, L.; Zeverino, M.; Maire, R.; Levivier, M. Preserving normal facial nerve function and improving hearing outcome in large vestibular schwannomas with a combined approach: Planned subtotal resection followed by gamma knife radiosurgery. Acta Neurochir. 2017, 159, 1197–1211. [Google Scholar] [CrossRef] [PubMed]
- Bartek, J., Jr.; Wangerid, T.; Pettersson-Segerlind, J.; Benmakhlouf, H.; Forander, P. Adaptive hybrid surgery analysis (AHSA) for adjuvant gamma knife radiosurgery treatment of vestibular schwannoma residuals. Clin. Neurol. Neurosurg. 2019, 185, 105487. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, J.P.; Lagman, C.; Prashant, G.N.; Alkhalid, Y.; Nguyen, T.; Duong, C.; Udawatta, M.; Gaonkar, B.; Tenn, S.E.; Bloch, O.; et al. Planned Subtotal Resection of Vestibular Schwannoma Differs from the Ideal Radiosurgical Target Defined by Adaptive Hybrid Surgery. World Neurosurg. 2018, 114, e441–e446. [Google Scholar] [CrossRef] [PubMed]
- Barani, I.J.; Parsa, A.T. Adaptive hybrid surgery: Feasibility of Planned Subtotal Resection of Benign Skull Base Tumors Followed by Radiosurgery to Minimize Morbidity Without Compromising Tumor Control. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, S278–S279. [Google Scholar] [CrossRef]

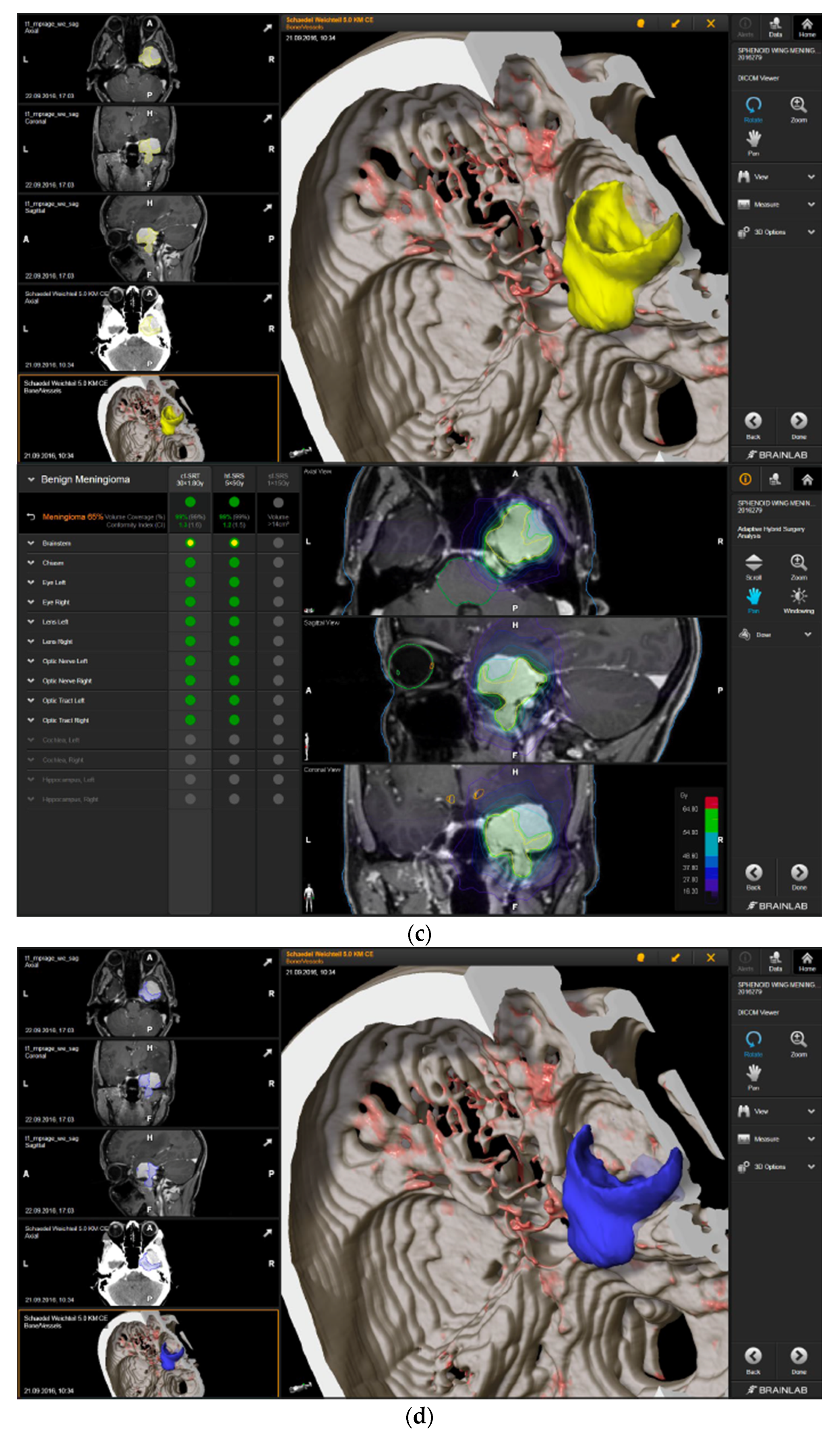
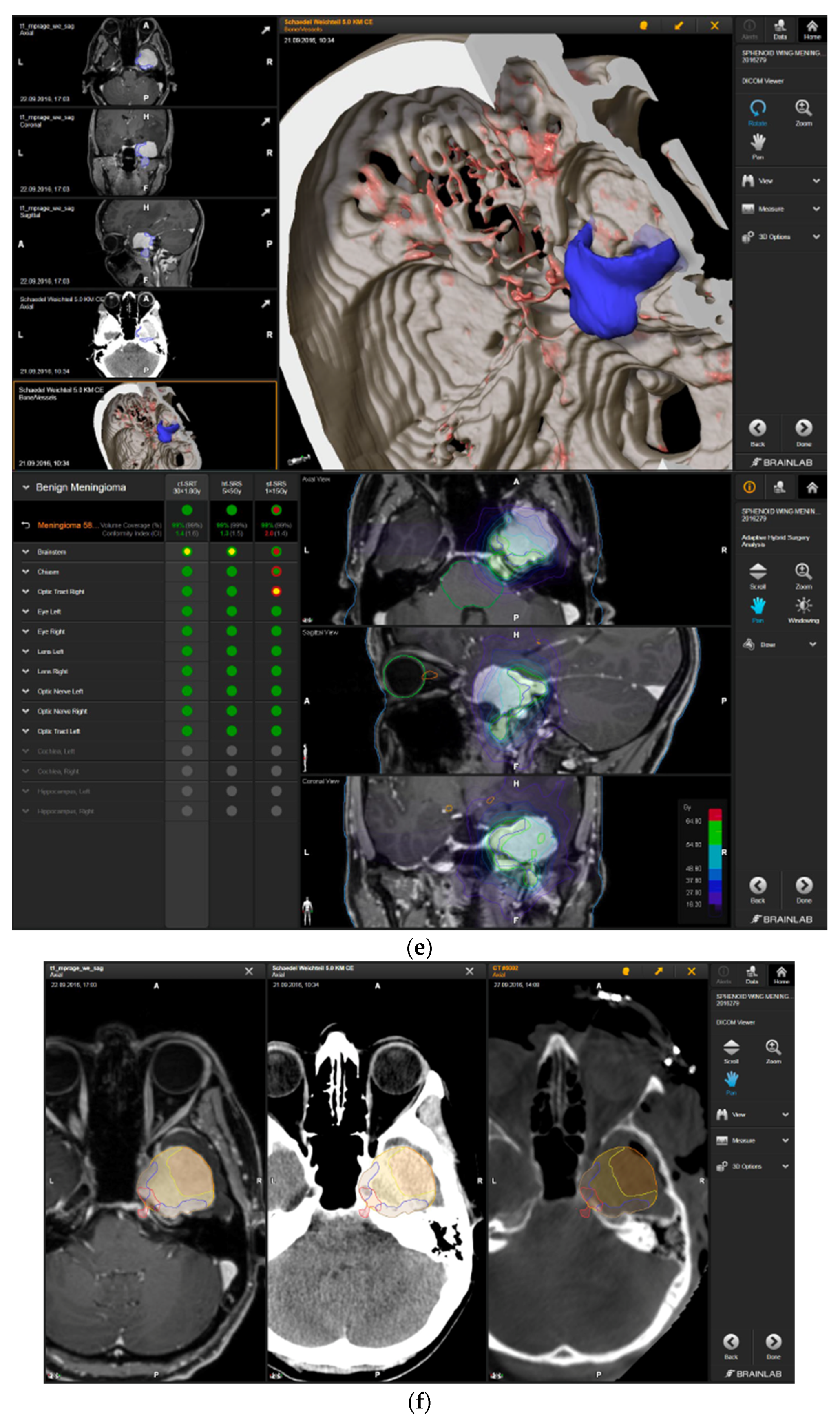
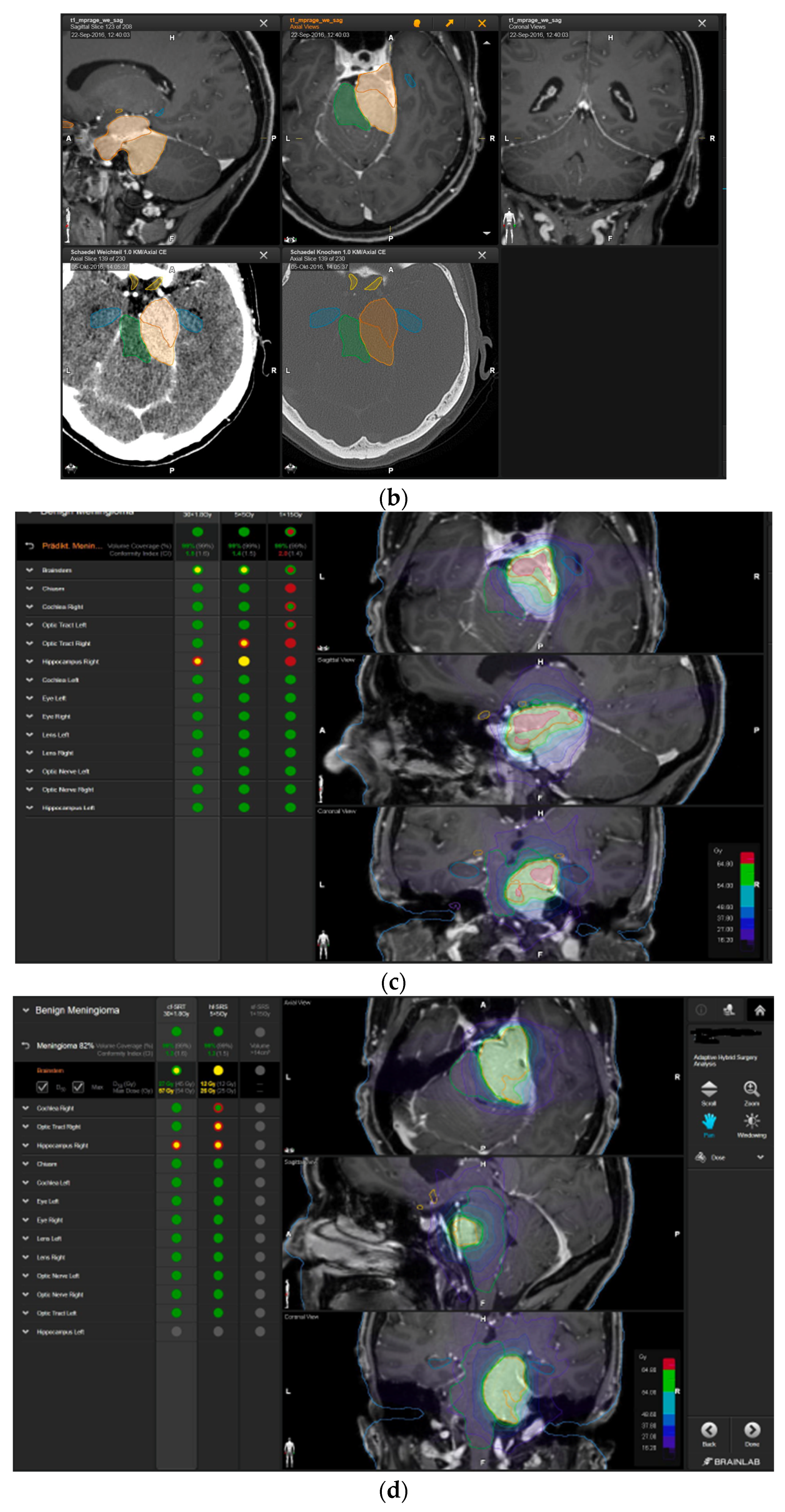
 —brainstem: max. dose is marginally safe, whereas mean dose is safe. For single fraction stereotactic radiosurgery, the tumor volume coverage seems effective, while the conformity index was indicating over-treatment.
—brainstem: max. dose is marginally safe, whereas mean dose is safe. For single fraction stereotactic radiosurgery, the tumor volume coverage seems effective, while the conformity index was indicating over-treatment.  —brainstem: mean dose is safe, while max. dose is unsafe;
—brainstem: mean dose is safe, while max. dose is unsafe;  —chiasm: mean dose is unsafe, while max. dose is safe;
—chiasm: mean dose is unsafe, while max. dose is safe;  —right optic tract: mean dose is unsafe, while max. dose is safe. (c) First intraoperative structure update (ISU) at the time point of 65% residual tumor volume, showing the tumor coverage and organ at risk constraints. At this stage of the resection, AHSA demonstrated that only conventional and hypofractionated radiotherapy were feasible.
—right optic tract: mean dose is unsafe, while max. dose is safe. (c) First intraoperative structure update (ISU) at the time point of 65% residual tumor volume, showing the tumor coverage and organ at risk constraints. At this stage of the resection, AHSA demonstrated that only conventional and hypofractionated radiotherapy were feasible.  —brainstem: max. dose is marginally safe, whereas mean dose is safe. (d) Second ISU acquisition and residual tumor reduction to 58%. (e) Third ISU acquisition with a reduction of residual tumor volume to 41%. Dose constraints for conventional, hypofractionated radiotherapy, and radiosurgery are demonstrated. At the final stage of the resection, the conventional and hypofractionated radiotherapy organ risk constraints were unchanged.
—brainstem: max. dose is marginally safe, whereas mean dose is safe. (d) Second ISU acquisition and residual tumor reduction to 58%. (e) Third ISU acquisition with a reduction of residual tumor volume to 41%. Dose constraints for conventional, hypofractionated radiotherapy, and radiosurgery are demonstrated. At the final stage of the resection, the conventional and hypofractionated radiotherapy organ risk constraints were unchanged.  —brainstem shows that max. dose is marginally safe, whereas mean dose is safe. The single-dose stereotactic radiosurgery constraints show that:
—brainstem shows that max. dose is marginally safe, whereas mean dose is safe. The single-dose stereotactic radiosurgery constraints show that:  —brainstem: mean dose safe, max. dose unsafe;
—brainstem: mean dose safe, max. dose unsafe;  —chiasm: mean dose unsafe, max. dose safe;
—chiasm: mean dose unsafe, max. dose safe;  —right optic tract: mean dose marginally safe, max. dose unsafe. (f) Fusion of intraoperative CT to final intraoperative ISU. (g) Preoperative and 3 months postoperative MRI imaging for planning of radiosurgery (5 × 5 Gy).
—right optic tract: mean dose marginally safe, max. dose unsafe. (f) Fusion of intraoperative CT to final intraoperative ISU. (g) Preoperative and 3 months postoperative MRI imaging for planning of radiosurgery (5 × 5 Gy).
 —brainstem: max. dose is marginally safe, whereas mean dose is safe. For single fraction stereotactic radiosurgery, the tumor volume coverage seems effective, while the conformity index was indicating over-treatment.
—brainstem: max. dose is marginally safe, whereas mean dose is safe. For single fraction stereotactic radiosurgery, the tumor volume coverage seems effective, while the conformity index was indicating over-treatment.  —brainstem: mean dose is safe, while max. dose is unsafe;
—brainstem: mean dose is safe, while max. dose is unsafe;  —chiasm: mean dose is unsafe, while max. dose is safe;
—chiasm: mean dose is unsafe, while max. dose is safe;  —right optic tract: mean dose is unsafe, while max. dose is safe. (c) First intraoperative structure update (ISU) at the time point of 65% residual tumor volume, showing the tumor coverage and organ at risk constraints. At this stage of the resection, AHSA demonstrated that only conventional and hypofractionated radiotherapy were feasible.
—right optic tract: mean dose is unsafe, while max. dose is safe. (c) First intraoperative structure update (ISU) at the time point of 65% residual tumor volume, showing the tumor coverage and organ at risk constraints. At this stage of the resection, AHSA demonstrated that only conventional and hypofractionated radiotherapy were feasible.  —brainstem: max. dose is marginally safe, whereas mean dose is safe. (d) Second ISU acquisition and residual tumor reduction to 58%. (e) Third ISU acquisition with a reduction of residual tumor volume to 41%. Dose constraints for conventional, hypofractionated radiotherapy, and radiosurgery are demonstrated. At the final stage of the resection, the conventional and hypofractionated radiotherapy organ risk constraints were unchanged.
—brainstem: max. dose is marginally safe, whereas mean dose is safe. (d) Second ISU acquisition and residual tumor reduction to 58%. (e) Third ISU acquisition with a reduction of residual tumor volume to 41%. Dose constraints for conventional, hypofractionated radiotherapy, and radiosurgery are demonstrated. At the final stage of the resection, the conventional and hypofractionated radiotherapy organ risk constraints were unchanged.  —brainstem shows that max. dose is marginally safe, whereas mean dose is safe. The single-dose stereotactic radiosurgery constraints show that:
—brainstem shows that max. dose is marginally safe, whereas mean dose is safe. The single-dose stereotactic radiosurgery constraints show that:  —brainstem: mean dose safe, max. dose unsafe;
—brainstem: mean dose safe, max. dose unsafe;  —chiasm: mean dose unsafe, max. dose safe;
—chiasm: mean dose unsafe, max. dose safe;  —right optic tract: mean dose marginally safe, max. dose unsafe. (f) Fusion of intraoperative CT to final intraoperative ISU. (g) Preoperative and 3 months postoperative MRI imaging for planning of radiosurgery (5 × 5 Gy).
—right optic tract: mean dose marginally safe, max. dose unsafe. (f) Fusion of intraoperative CT to final intraoperative ISU. (g) Preoperative and 3 months postoperative MRI imaging for planning of radiosurgery (5 × 5 Gy).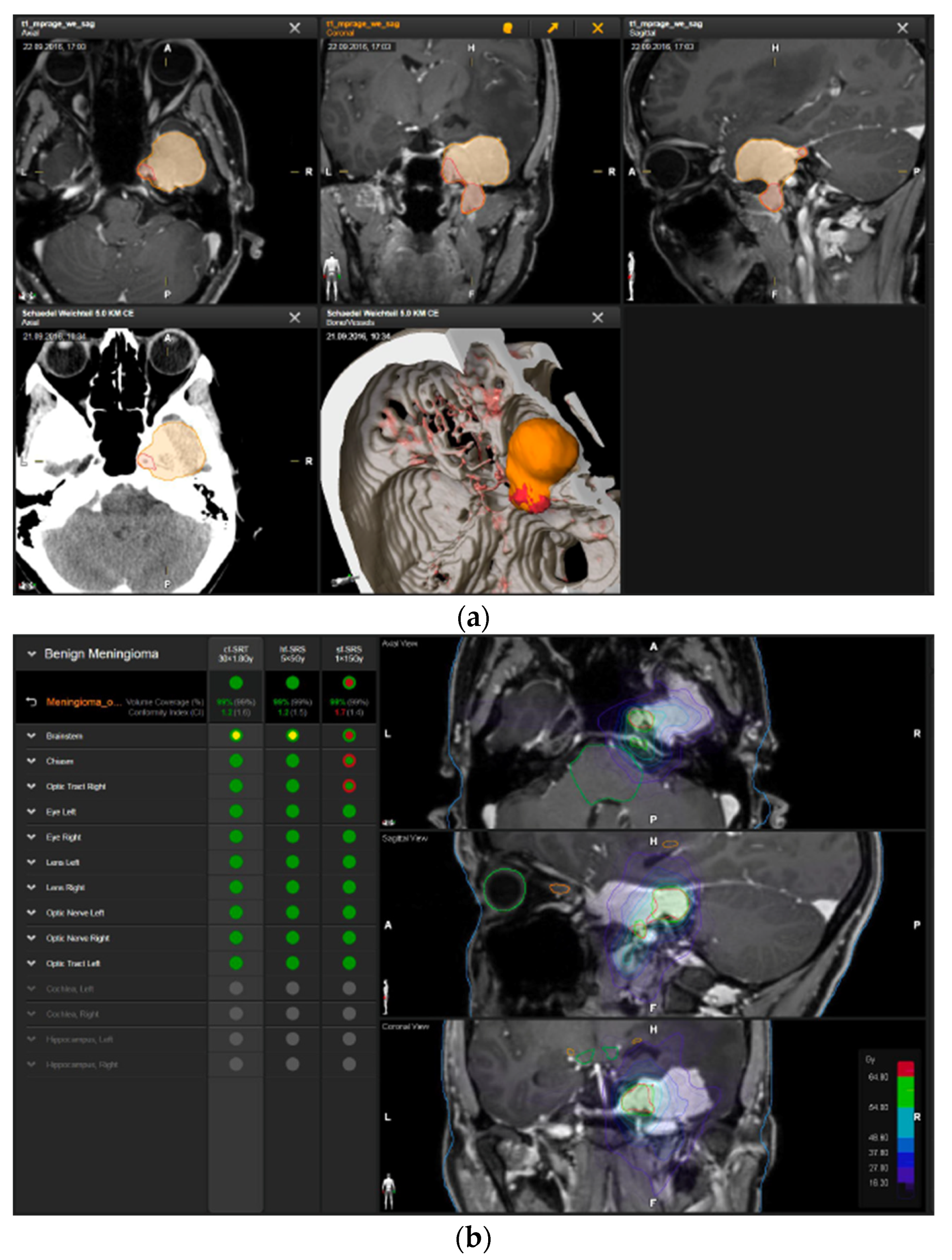

 —brainstem: mean dose is safe, while the max. dose is marginally safe;
—brainstem: mean dose is safe, while the max. dose is marginally safe;  —right optic tract: mean dose unsafe, max. dose is marginally safe;
—right optic tract: mean dose unsafe, max. dose is marginally safe; 
 —right hippocampus: marginally safe, and mean dose unsafe, max. dose is marginally safe. (d) First intraoperative structure update (ISU) with a residual tumor volume of 82% with the calculated dose constraints for conventional and hypofractionated radiotherapy. Single dose stereotactic radiosurgery was not feasible with this degree of remaining tumor. At this point, the dose constraints for conventional radiotherapy were:
—right hippocampus: marginally safe, and mean dose unsafe, max. dose is marginally safe. (d) First intraoperative structure update (ISU) with a residual tumor volume of 82% with the calculated dose constraints for conventional and hypofractionated radiotherapy. Single dose stereotactic radiosurgery was not feasible with this degree of remaining tumor. At this point, the dose constraints for conventional radiotherapy were:  —brainstem: mean dose is safe, max. dose is marginally safe;
—brainstem: mean dose is safe, max. dose is marginally safe;  —right hippocampus: mean dose unsafe, max. dose is marginally safe. Hypofractionated radiotherapy:
—right hippocampus: mean dose unsafe, max. dose is marginally safe. Hypofractionated radiotherapy:  —brainstem: marginally safe;
—brainstem: marginally safe;  —right cochlea: mean dose unsafe, max. dose safe;
—right cochlea: mean dose unsafe, max. dose safe;  —right optic tract: mean dose unsafe, max. dose is marginally safe;
—right optic tract: mean dose unsafe, max. dose is marginally safe;  —right hippocampus: mean dose unsafe, max. dose is marginally safe. (e) Second intraoperative ISU with residual tumor volume of 74% and calculated dose constraints for hypofractionated radiotherapy. Single dose stereotactic radiosurgery was still not considered feasible with this residual tumor volume. The dose constraints for organs at risk for conventional and hypofractionated radiotherapy were unchanged compared to the first ISU. (f) Third intraoperative ISU with residual tumor volume of 47% and calculated dose constraints for conventional, hypofractionated radiotherapy, and radiosurgery. The current dose constraints for organs at risk were the following for conventional radiation:
—right hippocampus: mean dose unsafe, max. dose is marginally safe. (e) Second intraoperative ISU with residual tumor volume of 74% and calculated dose constraints for hypofractionated radiotherapy. Single dose stereotactic radiosurgery was still not considered feasible with this residual tumor volume. The dose constraints for organs at risk for conventional and hypofractionated radiotherapy were unchanged compared to the first ISU. (f) Third intraoperative ISU with residual tumor volume of 47% and calculated dose constraints for conventional, hypofractionated radiotherapy, and radiosurgery. The current dose constraints for organs at risk were the following for conventional radiation:  —brainstem: mean dose is safe, max. dose is marginally safe;
—brainstem: mean dose is safe, max. dose is marginally safe;  —right hippocampus: mean dose unsafe, max. dose is marginally safe. Hypofractionated radiation, which was unchanged for the first and second ISU:
—right hippocampus: mean dose unsafe, max. dose is marginally safe. Hypofractionated radiation, which was unchanged for the first and second ISU:  —brainstem: marginally safe;
—brainstem: marginally safe;  —right cochlea: mean dose unsafe, max. dose safe;
—right cochlea: mean dose unsafe, max. dose safe;  —right optic tract: mean dose unsafe, max. dose is marginally safe;
—right optic tract: mean dose unsafe, max. dose is marginally safe;  —right hippocampus: mean dose unsafe, max. dose is marginally safe. For single fraction radiosurgery, the OAR dose constraints were available but considered to be unsafe.
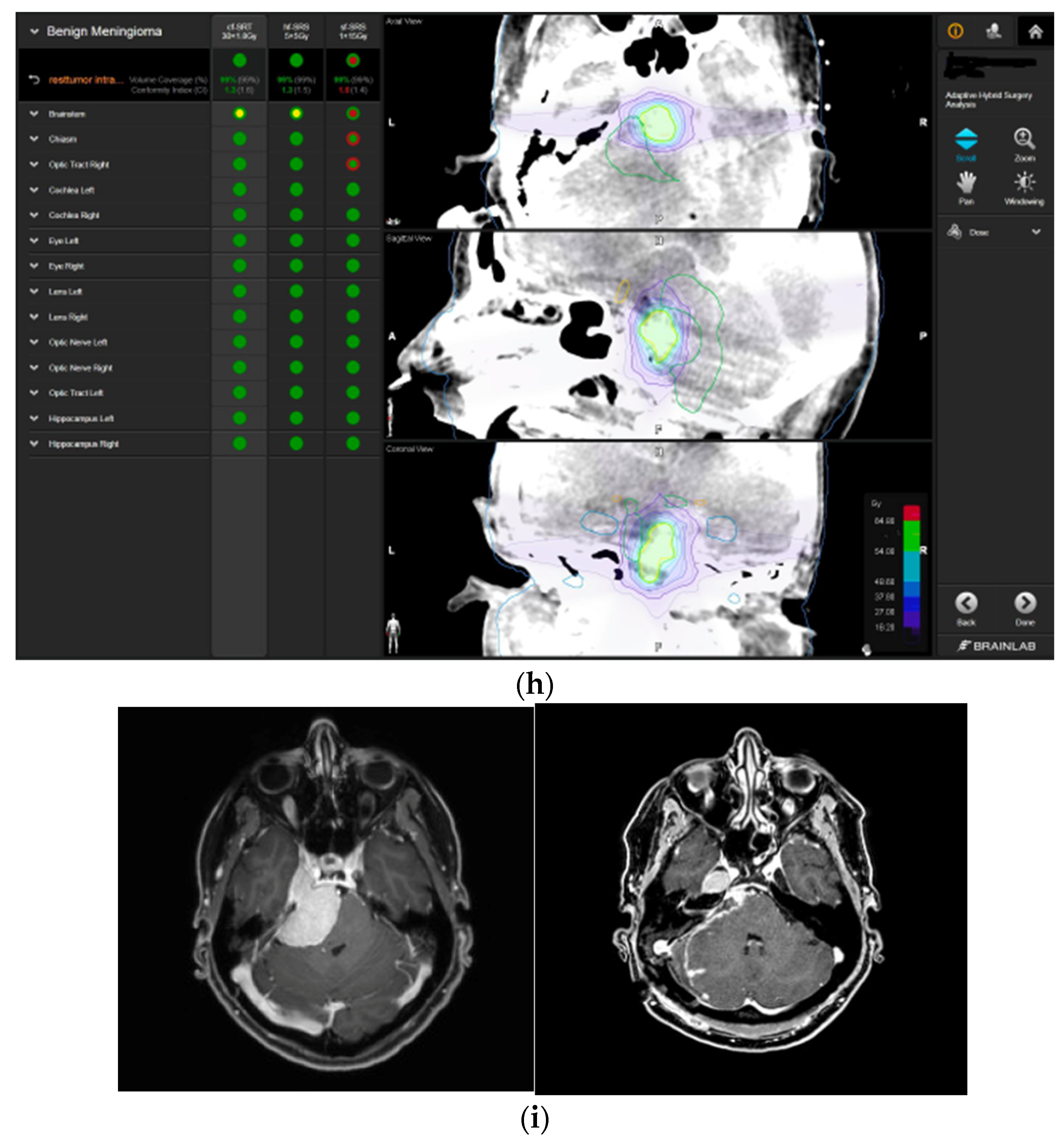
—right hippocampus: mean dose unsafe, max. dose is marginally safe. For single fraction radiosurgery, the OAR dose constraints were available but considered to be unsafe.  —brainstem: mean dose unsafe, max. dose safe;
—brainstem: mean dose unsafe, max. dose safe;  —chiasma: unsafe;
—chiasma: unsafe;  —right cochlea: unsafe;
—right cochlea: unsafe;  —left optic tract: mean dose unsafe, max. dose safe;
—left optic tract: mean dose unsafe, max. dose safe;  —right optic tract: unsafe;
—right optic tract: unsafe;  —right hippocampus: unsafe. (g) Overlay of preoperatively estimated and intraoperative effective residual tumor volume in AHSA. (h) Final intraoperative dose constraints after last ISU and data fusion with intraoperative CT. The dose constraints for OARs appeared to improve and were as follows for conventional and hypofractionated radiotherapy:
—right hippocampus: unsafe. (g) Overlay of preoperatively estimated and intraoperative effective residual tumor volume in AHSA. (h) Final intraoperative dose constraints after last ISU and data fusion with intraoperative CT. The dose constraints for OARs appeared to improve and were as follows for conventional and hypofractionated radiotherapy:  —brainstem: mean dose is safe, max. dose is marginally safe.
—brainstem: mean dose is safe, max. dose is marginally safe.  —brainstem: mean dose safe, max. dose unsafe;
—brainstem: mean dose safe, max. dose unsafe;  —chiasm: mean dose unsafe, max. dose safe;
—chiasm: mean dose unsafe, max. dose safe;  —right optic tract: mean dose unsafe, max. dose safe. (i) Comparison of pre- and 3 months postoperative MRI for stereotactic radiation planning. The residual tumor was finally treated with hypofractionated radiotherapy (5 × 5 Gy).
—right optic tract: mean dose unsafe, max. dose safe. (i) Comparison of pre- and 3 months postoperative MRI for stereotactic radiation planning. The residual tumor was finally treated with hypofractionated radiotherapy (5 × 5 Gy).
 —brainstem: mean dose is safe, while the max. dose is marginally safe;
—brainstem: mean dose is safe, while the max. dose is marginally safe;  —right optic tract: mean dose unsafe, max. dose is marginally safe;
—right optic tract: mean dose unsafe, max. dose is marginally safe; 
 —right hippocampus: marginally safe, and mean dose unsafe, max. dose is marginally safe. (d) First intraoperative structure update (ISU) with a residual tumor volume of 82% with the calculated dose constraints for conventional and hypofractionated radiotherapy. Single dose stereotactic radiosurgery was not feasible with this degree of remaining tumor. At this point, the dose constraints for conventional radiotherapy were:
—right hippocampus: marginally safe, and mean dose unsafe, max. dose is marginally safe. (d) First intraoperative structure update (ISU) with a residual tumor volume of 82% with the calculated dose constraints for conventional and hypofractionated radiotherapy. Single dose stereotactic radiosurgery was not feasible with this degree of remaining tumor. At this point, the dose constraints for conventional radiotherapy were:  —brainstem: mean dose is safe, max. dose is marginally safe;
—brainstem: mean dose is safe, max. dose is marginally safe;  —right hippocampus: mean dose unsafe, max. dose is marginally safe. Hypofractionated radiotherapy:
—right hippocampus: mean dose unsafe, max. dose is marginally safe. Hypofractionated radiotherapy:  —brainstem: marginally safe;
—brainstem: marginally safe;  —right cochlea: mean dose unsafe, max. dose safe;
—right cochlea: mean dose unsafe, max. dose safe;  —right optic tract: mean dose unsafe, max. dose is marginally safe;
—right optic tract: mean dose unsafe, max. dose is marginally safe;  —right hippocampus: mean dose unsafe, max. dose is marginally safe. (e) Second intraoperative ISU with residual tumor volume of 74% and calculated dose constraints for hypofractionated radiotherapy. Single dose stereotactic radiosurgery was still not considered feasible with this residual tumor volume. The dose constraints for organs at risk for conventional and hypofractionated radiotherapy were unchanged compared to the first ISU. (f) Third intraoperative ISU with residual tumor volume of 47% and calculated dose constraints for conventional, hypofractionated radiotherapy, and radiosurgery. The current dose constraints for organs at risk were the following for conventional radiation:
—right hippocampus: mean dose unsafe, max. dose is marginally safe. (e) Second intraoperative ISU with residual tumor volume of 74% and calculated dose constraints for hypofractionated radiotherapy. Single dose stereotactic radiosurgery was still not considered feasible with this residual tumor volume. The dose constraints for organs at risk for conventional and hypofractionated radiotherapy were unchanged compared to the first ISU. (f) Third intraoperative ISU with residual tumor volume of 47% and calculated dose constraints for conventional, hypofractionated radiotherapy, and radiosurgery. The current dose constraints for organs at risk were the following for conventional radiation:  —brainstem: mean dose is safe, max. dose is marginally safe;
—brainstem: mean dose is safe, max. dose is marginally safe;  —right hippocampus: mean dose unsafe, max. dose is marginally safe. Hypofractionated radiation, which was unchanged for the first and second ISU:
—right hippocampus: mean dose unsafe, max. dose is marginally safe. Hypofractionated radiation, which was unchanged for the first and second ISU:  —brainstem: marginally safe;
—brainstem: marginally safe;  —right cochlea: mean dose unsafe, max. dose safe;
—right cochlea: mean dose unsafe, max. dose safe;  —right optic tract: mean dose unsafe, max. dose is marginally safe;
—right optic tract: mean dose unsafe, max. dose is marginally safe;  —right hippocampus: mean dose unsafe, max. dose is marginally safe. For single fraction radiosurgery, the OAR dose constraints were available but considered to be unsafe.
—right hippocampus: mean dose unsafe, max. dose is marginally safe. For single fraction radiosurgery, the OAR dose constraints were available but considered to be unsafe.  —brainstem: mean dose unsafe, max. dose safe;
—brainstem: mean dose unsafe, max. dose safe;  —chiasma: unsafe;
—chiasma: unsafe;  —right cochlea: unsafe;
—right cochlea: unsafe;  —left optic tract: mean dose unsafe, max. dose safe;
—left optic tract: mean dose unsafe, max. dose safe;  —right optic tract: unsafe;
—right optic tract: unsafe;  —right hippocampus: unsafe. (g) Overlay of preoperatively estimated and intraoperative effective residual tumor volume in AHSA. (h) Final intraoperative dose constraints after last ISU and data fusion with intraoperative CT. The dose constraints for OARs appeared to improve and were as follows for conventional and hypofractionated radiotherapy:
—right hippocampus: unsafe. (g) Overlay of preoperatively estimated and intraoperative effective residual tumor volume in AHSA. (h) Final intraoperative dose constraints after last ISU and data fusion with intraoperative CT. The dose constraints for OARs appeared to improve and were as follows for conventional and hypofractionated radiotherapy:  —brainstem: mean dose is safe, max. dose is marginally safe.
—brainstem: mean dose is safe, max. dose is marginally safe.  —brainstem: mean dose safe, max. dose unsafe;
—brainstem: mean dose safe, max. dose unsafe;  —chiasm: mean dose unsafe, max. dose safe;
—chiasm: mean dose unsafe, max. dose safe;  —right optic tract: mean dose unsafe, max. dose safe. (i) Comparison of pre- and 3 months postoperative MRI for stereotactic radiation planning. The residual tumor was finally treated with hypofractionated radiotherapy (5 × 5 Gy).
—right optic tract: mean dose unsafe, max. dose safe. (i) Comparison of pre- and 3 months postoperative MRI for stereotactic radiation planning. The residual tumor was finally treated with hypofractionated radiotherapy (5 × 5 Gy).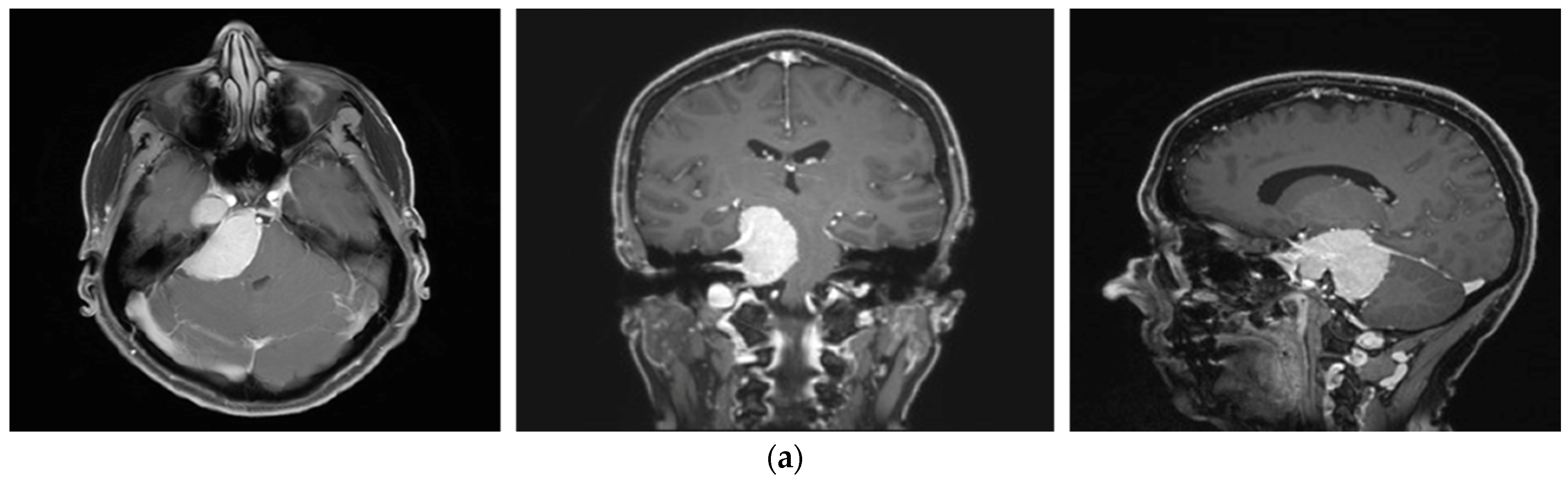

| Case No. | Diagnosis | Preop. TuVol (cm3) | Estim Resid TuVol (cm3) | AHSA Remnant TuVol (cm3) | Postop TuVol (MR) cm3 | EOR (Postop. MR) (cm3) | EOR (%) |
|---|---|---|---|---|---|---|---|
| 1 | Sphenoid wing meningioma WHO I | 22.54 | 5.00 | 8.89 | 4.82 | 17.72 | 78.6 |
| 2 | Petroclival meningioma WHO I | 25.5 | 10.63 | 12.17 | 7.98 | 17.52 | 68.7 |
| 3 | Cerebellopontine angle ependymoma WHO II | 20.8 | 8.05 | 6.46 | 8.56 | 12.24 | 58.8 |
| 4 | Vestibular schwannoma KOOS IV WHO I | 8.52 | 1.56 | 2.13 | 1.51 | 7.01 | 82.3 |
| 5 | Cerebellopontine angle meningioma WHO I | 11.8 | 6.49 | 6.19 | 7.01 | 4.79 | 40.7 |
| Diagnosis | Initial Symptoms | New Neurological Deficits after Tumor Resection | Postoperative Development of Preoperative Symptoms | Postoperative Follow Up Time | Complications from Stereotactic or Conventional Radiation Therapy |
|---|---|---|---|---|---|
| Sphenoid wing meningioma | Aggravating head and neck pain, fatigue, diagnosis of burnout, fronto-limbic cognitive dysfunction | Postoperative progressive edema and swelling with uncal herniation, requiring emergency craniectomy and prolonged recovery Homonymous superior hemianopsia Left facial nerve palsy House Brackmann II Right hyposensitivity V1–V3 | Reduced frequency of head and neck pain | 72 months | none |
| Petroclival meningioma | Progressive head pain, progressive swallowing difficulties, hypesthesia right side, double vision, hypesthesia V1–V3 right side, mild left hemiparesis, sleep disorder, impaired concentration | Right complete deafness and failure of vestibulocochlear nerve Right facial nerve palsy House Brackmann III | Reduced frequency of head pain, no more swallowing difficulties, complete remission of hemiparesis | 72 months | none |
| Cerebellopontine angle ependymoma | Gait instability, progressive right hypacusis, vertigo | Right complete deafness, right facial nerve palsy, House Brackmann II | Unchanged | 68 months | N/A |
| Vestibular schwannoma | Progressive gait instability, progressive left hypacusis, tinnitus, left hypesthesia V1–V2, facial palsy House Brackmann II | Progressive facial palsy House Brackmann III, slight swallowing issues | Improvement of gait ability, complete remission of tinnitus | 70 months | N/A |
| Cerebellopontine angle meningioma | Incidental finding with brain stem compression | Slight left hypoacusis | Unchanged | 66 months | none |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kienzler, J.C.; Fandino, J. Adaptive Hybrid Surgery Experiences in Benign Skull Base Tumors. Brain Sci. 2022, 12, 1326. https://doi.org/10.3390/brainsci12101326
Kienzler JC, Fandino J. Adaptive Hybrid Surgery Experiences in Benign Skull Base Tumors. Brain Sciences. 2022; 12(10):1326. https://doi.org/10.3390/brainsci12101326
Chicago/Turabian StyleKienzler, Jenny Christine, and Javier Fandino. 2022. "Adaptive Hybrid Surgery Experiences in Benign Skull Base Tumors" Brain Sciences 12, no. 10: 1326. https://doi.org/10.3390/brainsci12101326
APA StyleKienzler, J. C., & Fandino, J. (2022). Adaptive Hybrid Surgery Experiences in Benign Skull Base Tumors. Brain Sciences, 12(10), 1326. https://doi.org/10.3390/brainsci12101326









