Central Feminization of Obese Male Mice Reduces Metabolic Syndrome
Abstract
1. Introduction
2. Materials and Methods
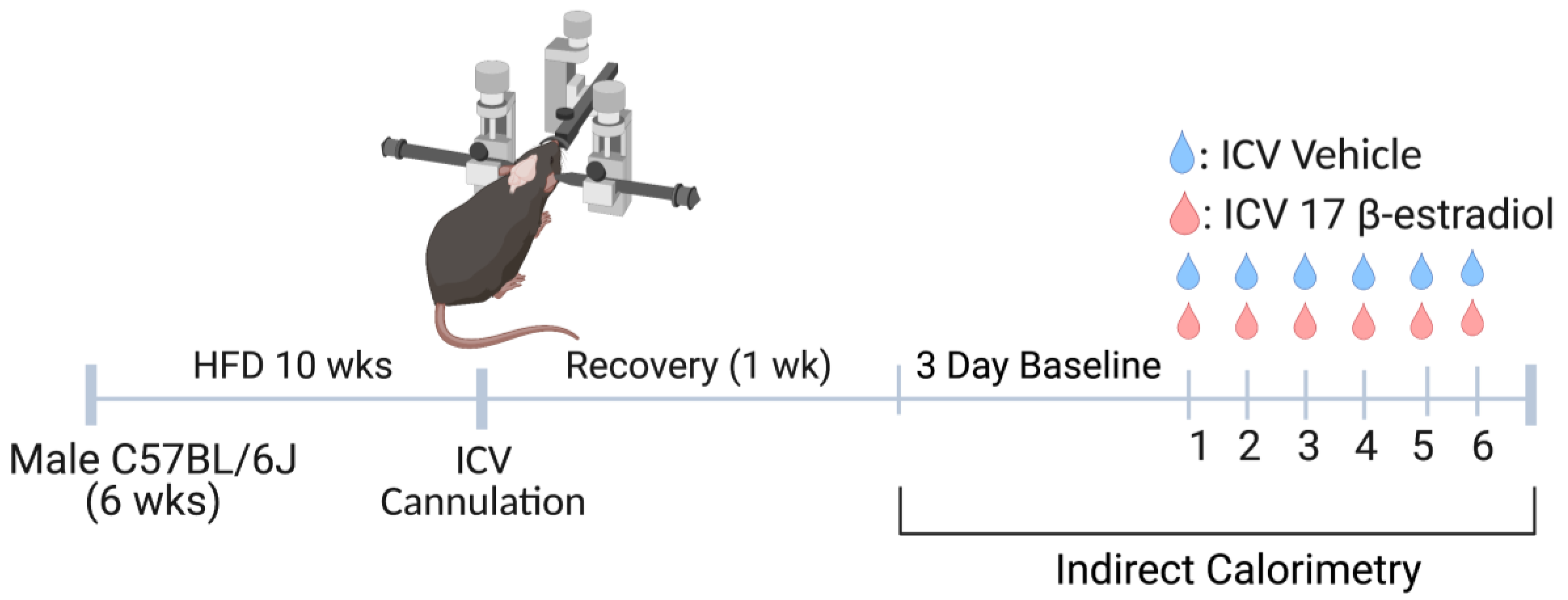
2.1. Experimental Model
2.2. Pharmacological Agents
2.3. Lateral Cerebroventricular Cannulation
2.4. Indirect Calorimetry
2.5. Liver Histology
2.6. Quantification and Statistical Analysis
3. Results
3.1. Central Feminization of Obese Male Mice Reduces Body Weight and Food Intake
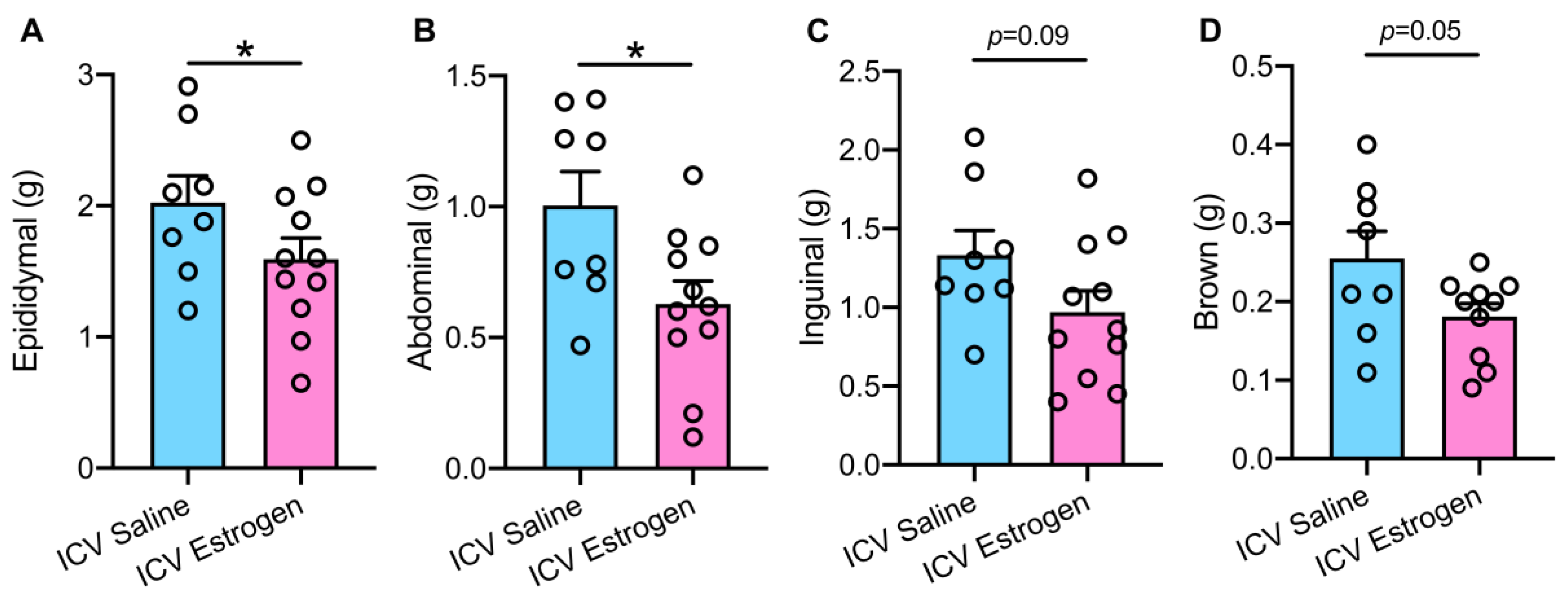
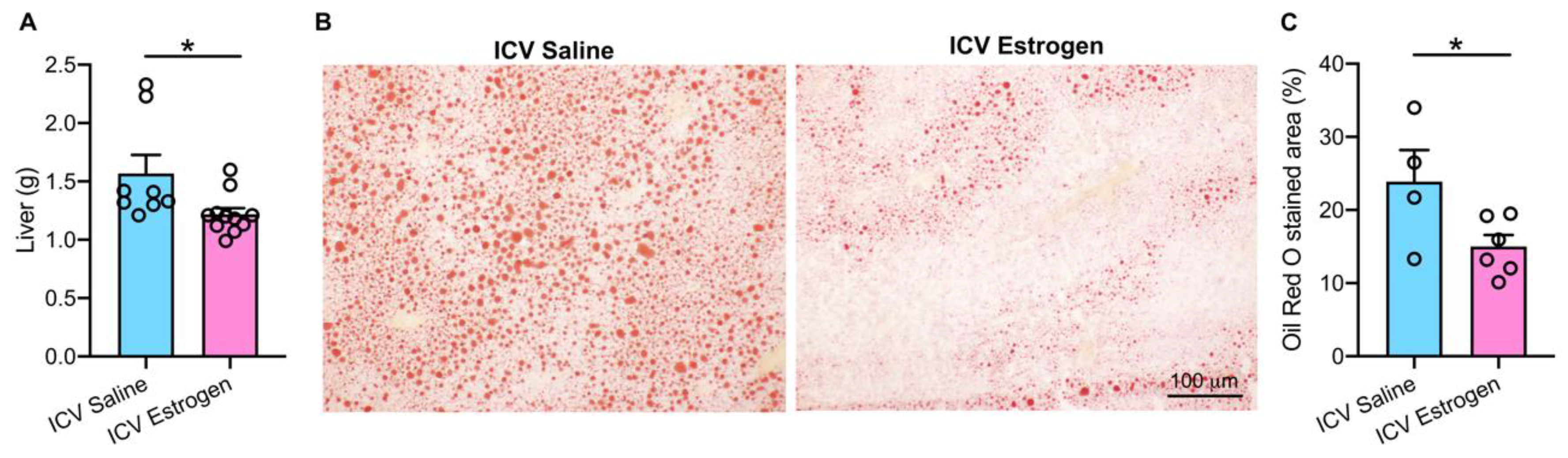
3.2. Central Estrogen Supplementation Reduces Visceral Adiposity and Rescues Hepatic Steatosis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eckel, R.H.; Alberti, K.G.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2010, 375, 181–183. [Google Scholar] [CrossRef]
- Alberti, K.G.M.; Zimmet, P.; Shaw, J. The metabolic syndrome—A new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Haffner, S.M. The metabolic syndrome: Inflammation, diabetes mellitus, and cardiovascular disease. Am. J. Cardiol. 2006, 97, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Velasco, M.; Ortiz-Huidobro, R.I.; Larqué, C.; Sánchez-Zamora, Y.I.; Romo-Yáñez, J.; Hiriart, M. Sexual dimorphism in insulin resistance in a metabolic syndrome rat model. Endocr. Connect. 2020, 9, 890–902. [Google Scholar] [CrossRef]
- Palmer, B.F.; Clegg, D.J. The sexual dimorphism of obesity. Mol. Cell. Endocrinol. 2015, 402, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Lobo, R.A. Metabolic syndrome after menopause and the role of hormones. Maturitas 2008, 60, 10–18. [Google Scholar] [CrossRef]
- Carr, M.C. The emergence of the metabolic syndrome with menopause. J. Clin. Endocrinol. Metab. 2003, 88, 2404–2411. [Google Scholar] [CrossRef]
- Ambikairajah, A.; Walsh, E.; Tabatabaei-Jafari, H.; Cherbuin, N. Fat mass changes during menopause: A metaanalysis. Obstet. Gynecol. 2019, 221, 393–409.e50. [Google Scholar] [CrossRef]
- Korljan, B.; Bagatin, J.; Kokić, S.; Matulić, N.B.; Ostojić, S.B.; Deković, A. The impact of hormone replacement therapy on metabolic syndrome components in perimenopausal women. Med. Hypotheses 2010, 74, 162–163. [Google Scholar] [CrossRef]
- Salpeter, S.R.; Walsh, J.; Ormiston, T.M.; Greyber, E.; Buckley, N.S.; Salpeter, E.E. Meta-analysis: Effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes. Metab. 2006, 8, 538–554. [Google Scholar] [CrossRef] [PubMed]
- Suppakitjanusant, P.; Ji, Y.; Stevenson, M.O.; Chantrapanichkul, P.; Sineath, R.C.; Goodman, M.; Alvarez, J.A.; Tangpricha, V. Effects of gender affirming hormone therapy on body mass index in transgender individuals: A longitudinal cohort study. J. Clin. Transl. Endocrinol. 2020, 21, 100230. [Google Scholar] [CrossRef] [PubMed]
- Auer, M.K.; Ebert, T.; Pietzner, M.; Defreyne, J.; Fuss, J.; Stalla, G.K.; T’Sjoen, G. Effects of sex hormone treatment on the metabolic syndrome in transgender individuals: Focus on metabolic cytokines. J. Clin. Endocrinol. Metab. 2018, 103, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, N.; Nolan, N.; Chinnakotla, V. Estrogen-induced pancreatitis: Transgender females at risk. Am. J. Hosp. Med. 2018, 2, 2018.023. [Google Scholar] [CrossRef]
- Streed, C.G., Jr.; Harfouch, O.; Marvel, F.; Blumenthal, R.S.; Martin, S.S.; Mukherjee, M. Cardiovascular disease among transgender adults receiving hormone therapy: A narrative review. Ann. Intern. Med. 2017, 167, 256–267. [Google Scholar] [CrossRef]
- Khan, J.; Schmidt, R.L.; Spittal, M.J.; Goldstein, Z.; Smock, K.J.; Greene, D.N. Venous thrombotic risk in transgender women undergoing estrogen therapy: A systematic review and metaanalysis. Clin. Chem. 2019, 65, 57–66. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Clegg, D.J.; Hevener, A.L. The role of estrogens in control of energy balance and glucose homeostasis. Endocr. Rev. 2013, 34, 309–338. [Google Scholar] [CrossRef]
- Rivera, H.M.; Stincic, T.L. Estradiol and the control of feeding behavior. Steroids 2018, 133, 44–52. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, J.; Veldhuis-Vlug, A.G.; Su, Y.; Foppen, E.; van der Eerden, B.; Koedam, M.; Bravenboer, N.; Kalsbeek, A.; Boelen, A. Effects of chronic estrogen administration in the ventromedial nucleus of the hypothalamus (VMH) on fat and bone metabolism in ovariectomized rats. Endocrinology 2016, 157, 4930–4942. [Google Scholar] [CrossRef]
- Musatov, S.; Chen, W.; Pfaff, D.W.; Mobbs, C.V.; Yang, X.; Clegg, D.J.; Kaplitt, M.G.; Ogawa, S. Silencing of estrogen receptor α in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc. Natl. Acad. Sci. USA 2007, 104, 2501–2506. [Google Scholar] [CrossRef]
- Heine, P.A.; Taylor, J.A.; Iwamoto, G.A.; Lubahn, D.B.; Cooke, P.S. Increased adipose tissue in male and female estrogen receptor-α knockout mice. Proc. Natl. Acad. Sci. USA 2000, 97, 12729–12734. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Nedungadi, T.P.; Zhu, L.; Sobhani, N.; Irani, B.G.; Davis, K.E.; Zhang, X.; Zou, F.; Gent, L.M.; Hahner, L.D. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011, 14, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Sano, K.; Tsuda, M.C.; Musatov, S.; Sakamoto, T.; Ogawa, S. Differential effects of site-specific knockdown of estrogen receptor α in the medial amygdala, medial pre-optic area, and ventromedial nucleus of the hypothalamus on sexual and aggressive behavior of male mice. Eur. J. Neurosci. 2013, 37, 1308–1319. [Google Scholar] [CrossRef] [PubMed]
- Asarian, L.; Geary, N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm. Behav. 2002, 42, 461–471. [Google Scholar] [CrossRef]
- Butcher, R.L.; Collins, W.E.; Fugo, N.W. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17β throughout the 4-day estrous cycle of the rat. Endocrinology 1974, 94, 1704–1708. [Google Scholar] [CrossRef]
- González-García, I.; Contreras, C.; Estévez-Salguero, Á.; Ruíz-Pino, F.; Colsh, B.; Pensado, I.; Liñares-Pose, L.; Rial-Pensado, E.; de Morentin, B.P.M.; Fernø, J. Estradiol Regulates Energy Balance by Ameliorating Hypothalamic Ceramide-Induced ER Stress. Cell Rep. 2018, 25, 413–423.e5. [Google Scholar] [CrossRef]
- Kalu, D.N.; Liu, C.C.; Salerno, E.; Hollis, B.; Echon, R.; Ray, M. Skeletal response of ovariectomized rats to low and high doses of 17β-estradiol. Bone Min. 1991, 14, 175–187. [Google Scholar] [CrossRef]
- Xue, B.; Zhao, Y.; Johnson, A.K.; Hay, M. Central estrogen inhibition of angiotensin II-induced hypertension in male mice and the role of reactive oxygen species. Am. J. Physiol.-Heart Circ. Physiol. 2008, 295, H1025–H1032. [Google Scholar] [CrossRef]
- Mehlem, A.; Hagberg, C.E.; Muhl, L.; Eriksson, U.; Falkevall, A. Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease. Nat. Protoc. 2013, 8, 1149–1154. [Google Scholar] [CrossRef]
- Curtis, K.S. Estrogen and the central control of body fluid balance. Physiol. Behav. 2009, 97, 180–192. [Google Scholar] [CrossRef]
- Koo, S. Nonalcoholic fatty liver disease: Molecular mechanisms for the hepatic steatosis. Clin. Mol. Hepatol. 2013, 19, 210. [Google Scholar] [CrossRef] [PubMed]
- Postic, C.; Girard, J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: Lessons from genetically engineered mice. J. Clin. Investig. 2008, 118, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Hurr, C.; Simonyan, H.; Morgan, D.A.; Rahmouni, K.; Young, C.N. Liver sympathetic denervation reverses obesity-induced hepatic steatosis. J. Physiol. 2019, 597, 4565–4580. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.M.; Clegg, D.J. Central effects of estradiol in the regulation of food intake, body weight, and adiposity. J. Steroid Biochem. Mol. Biol. 2010, 122, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Gambacciani, M.; Ciaponi, M.; Cappagli, B.; Piaggesi, L.; De Simone, L.; Orlandi, R.; Genazzani, A.R. Body weight, body fat distribution, and hormonal replacement therapy in early postmenopausal women. J. Clin. Endocrinol. Metab. 1997, 82, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Bryzgalova, G.; Lundholm, L.; Portwood, N.; Gustafsson, J.; Khan, A.; Efendic, S.; Dahlman-Wright, K. Mechanisms of antidiabetogenic and body weight-lowering effects of estrogen in high-fat diet-fed mice. Am. J. Physiol.-Endocrinol. Metab. 2008, 295, E904–E912. [Google Scholar] [CrossRef]
- McElroy, J.F.; Wade, G.N. Short-and long-term effects of ovariectomy on food intake, body weight, carcass composition, and brown adipose tissue in rats. Physiol. Behav. 1987, 39, 361–365. [Google Scholar] [CrossRef]
- Xu, P.; Cao, X.; He, Y.; Zhu, L.; Yang, Y.; Saito, K.; Wang, C.; Yan, X.; Hinton, A.O.; Zou, F. Estrogen receptor–α in medial amygdala neurons regulates body weight. J. Clin. Investig. 2015, 125, 2861–2876. [Google Scholar] [CrossRef]
- Ter Haar, M.B. Circadian and estrual rhythms in food intake in the rat. Horm. Behav. 1972, 3, 213–219. [Google Scholar] [CrossRef]
- Blaustein, J.D.; Wade, G.N. Ovarian influences on the meal patterns of female rats. Physiol. Behav. 1976, 17, 201–208. [Google Scholar] [CrossRef]
- Buffenstein, R.; Poppitt, S.D.; McDevitt, R.M.; Prentice, A.M. Food intake and the menstrual cycle: A retrospective analysis, with implications for appetite research. Physiol. Behav. 1995, 58, 1067–1077. [Google Scholar] [CrossRef]
- Schulster, M.; Bernie, A.M.; Ramasamy, R. The role of estradiol in male reproductive function. Asian J. Androl. 2016, 18, 435. [Google Scholar] [PubMed]
- Hess, R.A.; Bunick, D.; Lee, K.; Bahr, J.; Taylor, J.A.; Korach, K.S.; Lubahn, D.B. A role for oestrogens in the male reproductive system. Nature 1997, 390, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Bjørndal, B.; Burri, L.; Staalesen, V.; Skorve, J.; Berge, R.K. Different adipose depots: Their role in the development of metabolic syndrome and mitochondrial response to hypolipidemic agents. J. Obes. 2011, 2011, 490650. [Google Scholar] [CrossRef] [PubMed]
- Karastergiou, K.; Smith, S.R.; Greenberg, A.S.; Fried, S.K. Sex differences in human adipose tissues–the biology of pear shape. Biol. Sex Differ. 2012, 3, 13. [Google Scholar] [CrossRef]
- Zore, T.; Palafox, M.; Reue, K. Sex differences in obesity, lipid metabolism, and inflammation—A role for the sex chromosomes? Mol. Metab. 2018, 15, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Roesch, D.M. Effects of selective estrogen receptor agonists on food intake and body weight gain in rats. Physiol. Behav. 2006, 87, 39–44. [Google Scholar] [CrossRef]
- Patel, P.; Abate, N. Body fat distribution and insulin resistance. Nutrients 2013, 5, 2019–2027. [Google Scholar]
- Freemantle, N.; Holmes, J.; Hockey, A.; Kumar, S. How strong is the association between abdominal obesity and the incidence of type 2 diabetes? Int. J. Clin. Pract. 2008, 62, 1391–1396. [Google Scholar] [CrossRef]
- Katzmarzyk, P.T.; Mire, E.; Bouchard, C. Abdominal obesity and mortality: The Pennington Center longitudinal study. Nutr. Diabetes 2012, 2, e42. [Google Scholar] [CrossRef]
- Zhang, C.; Rexrode, K.M.; Van Dam, R.M.; Li, T.Y.; Hu, F.B. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality. Circulation 2008, 117, 1658–1667. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Corey, K.E.; Byrne, C.D.; Roden, M. The complex link between NAFLD and type 2 diabetes mellitus—Mechanisms and treatments. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 599–612. [Google Scholar] [PubMed]
- Liu, Y.; Zhong, G.; Tan, H.; Hao, F.; Hu, J. Nonalcoholic fatty liver disease and mortality from all causes, cardiovascular disease, and cancer: A meta-analysis. Sci. Rep. 2019, 9, 11124. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.D.; Jones, M.E.; Prelle, K.; Simpson, E.R.; Boon, W.C. A selective estrogen receptor agonist ameliorates hepatic steatosis in the male aromatase knockout mouse. J. Endocrinol. 2011, 210, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Pura, M.; Mittre, H.; Carreau, S.; Kottler, M.L. Clinical findings in an adult man with a novel mutation in the aromatase gene. In Program of the 85th Annual Meeting of the Endocrine Society; Abstracts Book: Philadelphia, PA, USA, 2003. [Google Scholar]
- Maffei, L.; Murata, Y.; Rochira, V.; Tubert, G.; Aranda, C.; Vazquez, M.; Clyne, C.D.; Davis, S.; Simpson, E.R.; Carani, C. Dysmetabolic syndrome in a man with a novel mutation of the aromatase gene: Effects of testosterone, alendronate, and estradiol treatment. J. Clin. Endocrinol. Metab. 2004, 89, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, B.L.; Saller, B.; Janssen, O.E.; Gocke, P.; Bockisch, A.; Sperling, H.; Mann, K.; Broecker, M. Impact of estrogen replacement therapy in a male with congenital aromatase deficiency caused by a novel mutation in the CYP19 gene. J. Clin. Endocrinol. Metab. 2002, 87, 5476–5484. [Google Scholar] [CrossRef]
- La Fleur, S.E.; Kalsbeek, A.; Wortel, J.; Buijs, R.M. Polysynaptic neural pathways between the hypothalamus, including the suprachiasmatic nucleus, and the liver. Brain Res. 2000, 871, 50–56. [Google Scholar] [CrossRef]
- O’Hare, J.D.; Zsombok, A. Brain-liver connections: Role of the preautonomic PVN neurons. Am. J. Physiol. -Endocrinol. Metab. 2016, 310, E183–E189. [Google Scholar] [CrossRef]
- Liang, J.J.; Jolly, D.; Chan, K.J.; Safer, J.D. Testosterone levels achieved by medically treated transgender women in a United States endocrinology clinic cohort. Endocr. Pract. 2018, 24, 135–142. [Google Scholar] [CrossRef]
- de Blok, C.J.M.; Klaver, M.; Wiepjes, C.M.; Nota, N.M.; Heijboer, A.C.; Fisher, A.D.; Schreiner, T.; T’Sjoen, G.; den Heijer, M. Breast development in transwomen after 1 year of cross-sex hormone therapy: Results of a prospective multicenter study. J. Clin. Endocrinol. Metab. 2018, 103, 532–538. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blackmore, K.; Young, C.N. Central Feminization of Obese Male Mice Reduces Metabolic Syndrome. Brain Sci. 2022, 12, 1324. https://doi.org/10.3390/brainsci12101324
Blackmore K, Young CN. Central Feminization of Obese Male Mice Reduces Metabolic Syndrome. Brain Sciences. 2022; 12(10):1324. https://doi.org/10.3390/brainsci12101324
Chicago/Turabian StyleBlackmore, Katherine, and Colin N. Young. 2022. "Central Feminization of Obese Male Mice Reduces Metabolic Syndrome" Brain Sciences 12, no. 10: 1324. https://doi.org/10.3390/brainsci12101324
APA StyleBlackmore, K., & Young, C. N. (2022). Central Feminization of Obese Male Mice Reduces Metabolic Syndrome. Brain Sciences, 12(10), 1324. https://doi.org/10.3390/brainsci12101324




