A Meta-Analysis of fMRI Studies of Youth Cannabis Use: Alterations in Executive Control, Social Cognition/Emotion Processing, and Reward Processing in Cannabis Using Youth
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
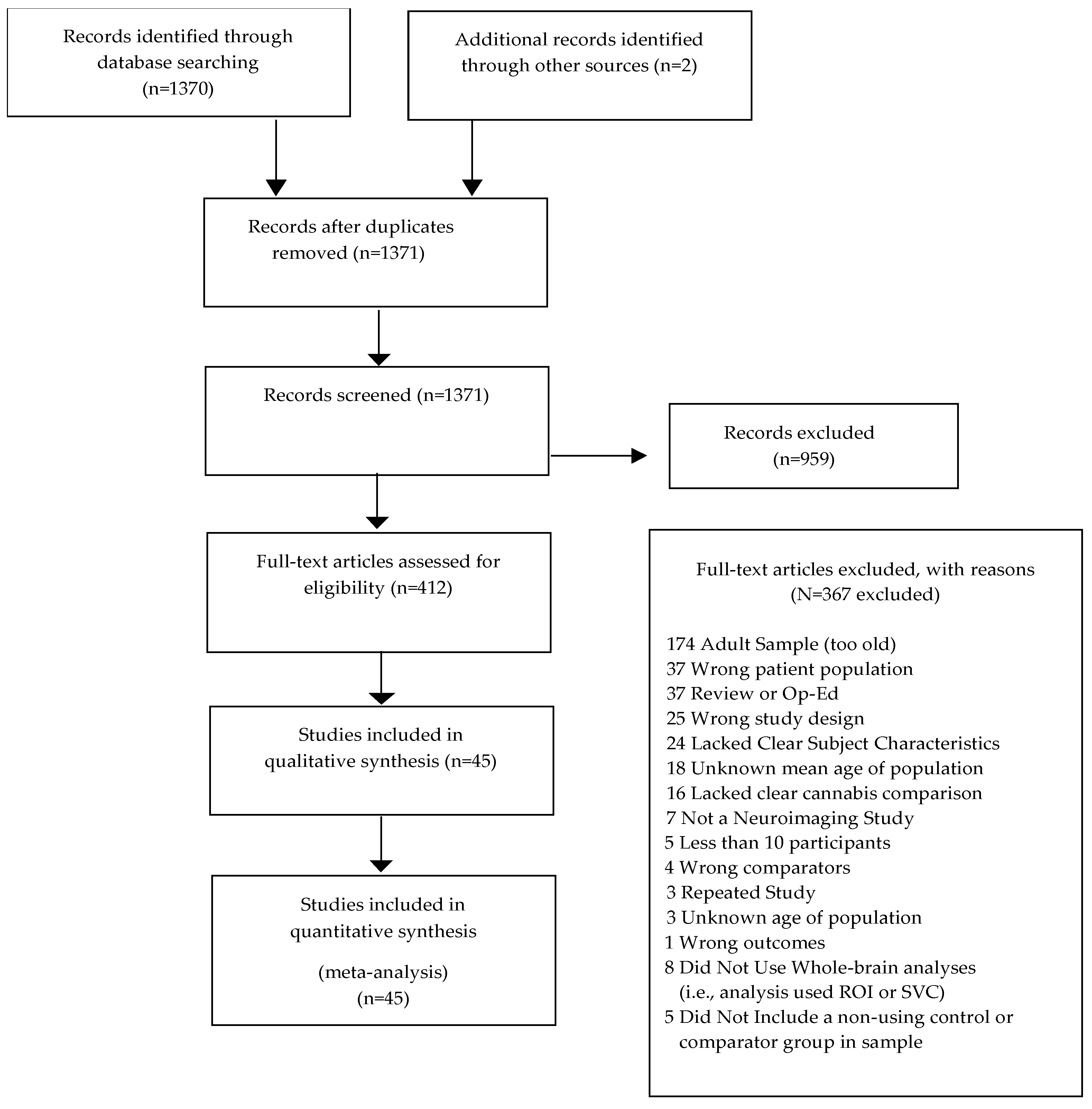
2.2. Study Selection
2.3. Data Extraction
2.4. Data Analysis
3. Results
3.1. Systematic Review and Qualitative Analysis
3.2. Study and Sample Characteristics
3.3. Meta-Analysis: BOLD Signal Differences in CU vs. TD Youth within and across Domains
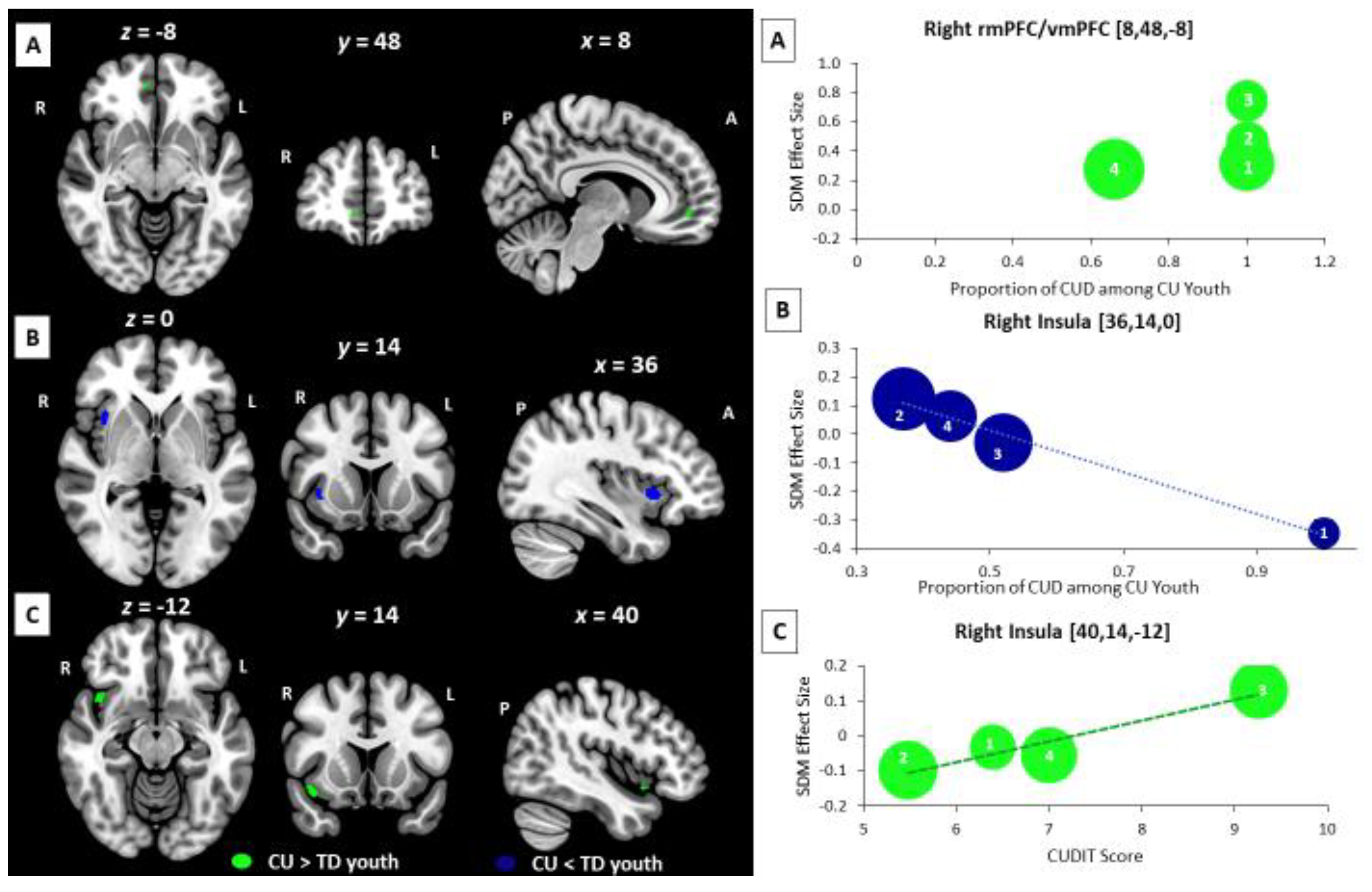
3.4. Meta-Regression Analysis: Age-Related, Sex-Related, and Cannabis-Related BOLD Effects
3.5. Supplemental Subgroup Meta-Analyses and Meta-Regression Analyses
3.6. Reliability Analysis and Publication Bias Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hammond, C.J.; Chaney, A.; Hendrickson, B.; Sharma, P. Cannabis use among U.S. adolescents in the era of marijuana legalization: A review of changing use patterns, comorbidity, and health correlates. Int. Rev. Psychiatry 2020, 32, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings; NSDUH Series H-48; HHS Publication No. (SMA) 14-4863; Substance Abuse and Mental Health Services Administration: Rockville, MD, USA, 2014.
- Schulenberg, J.; Johnston, L.; O’Malley, P.; Bachman, J.; Miech, R.; Patrick, M. Monitoring the Future National Survey Results on Drug Use, 1975–2019: Volume II, College Students and Adults Ages 19–60; Institute for Social Research. University of Michigan: Ann Arbor, MI, USA, 2020. [Google Scholar]
- Pacula, R.L.; Smart, R. Medical marijuana and marijuana legalization. Annu. Rev. Clin. Psychol. 2017, 13, 397–419. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, D.M.; Mathias, C.W.; Dawes, M.A.; Furr, R.M.; Charles, N.E.; Liguori, A.; Shannon, E.E.; Acheson, A. Impulsivity, attention, memory, and decision-making among adolescent marijuana users. Psychopharmacology 2013, 226, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Gruber, S.A.; Sagar, K.A.; Dahlgren, M.K.; Racine, M.; Lukas, S.E. Age of onset of marijuana use and executive function. Psychol. Addict. Behav. 2012, 26, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Jacobus, J.; Bava, S.; Cohen-Zion, M.; Mahmood, O.; Tapert, S.F. Functional consequences of marijuana use in adolescents. Pharmacol. Biochem. Behav. 2009, 92, 559–565. [Google Scholar] [CrossRef]
- Kelly, T.M.; Daley, D.C. Integrated treatment of substance use and psychiatric disorders. Soc. Work Public Health 2013, 28, 388–406. [Google Scholar] [CrossRef]
- Hasin, D.; Walsh, C. Cannabis use, cannabis use disorder, and comorbid psychiatric illness: A narrative review. J. Clin. Med. 2020, 10, 15. [Google Scholar] [CrossRef]
- Chadwick, B.; Miller, M.L.; Hurd, Y.L. Cannabis use during adolescent development: Susceptibility to psychiatric illness. Front. Psychiatry 2013, 4, 129. [Google Scholar] [CrossRef]
- Moore, T.H.; Zammit, S.; Lingford-Hughes, A.; Barnes, T.R.; Jones, P.B.; Burke, M.; Lewis, G. Cannabis use and risk of psychotic or affective mental health outcomes: A systematic review. Lancet 2007, 370, 319–328. [Google Scholar] [CrossRef]
- Hall, W.; Degenhardt, L. Cannabis use and the risk of developing a psychotic disorder. World Psychiatry 2008, 7, 68–71. [Google Scholar] [CrossRef]
- Fridberg, D.J.; Vollmer, J.M.; O’Donnell, B.F.; Skosnik, P.D. Cannabis users differ from non-users on measures of personality and schizotypy. Psychiatry Res. 2011, 186, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Di Forti, M.; Sallis, H.; Allegri, F.; Trotta, A.; Ferraro, L.; Stilo, S.A.; Marconi, A.; La Cascia, C.; Reis Marques, T.; Pariante, C.; et al. Daily use, especially of high-potency cannabis, drives the earlier onset of psychosis in cannabis users. Schizophr. Bull. 2014, 40, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Morse, M.C.; Benson, K.; Flory, K. Disruptive behavior disorders and marijuana use: The role of depressive symptoms. Subst. Abuse 2016, 9 (Suppl. 1), 69–76. [Google Scholar] [CrossRef] [PubMed]
- Schweinsburg, A.D.; Brown, S.A.; Tapert, S.F. The influence of marijuana use on neurocognitive functioning in adolescents. Curr. Drug Abuse Rev. 2008, 1, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Schweinsburg, A.D.; Nagel, B.J.; Schweinsburg, B.C.; Park, A.; Theilmann, R.J.; Tapert, S.F. Abstinent adolescent marijuana users show altered fMRI response during spatial working memory. Psychiatry Res. 2008, 163, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Schweinsburg, A.D.; Schweinsburg, B.C.; Cheung, E.H.; Brown, G.G.; Brown, S.A.; Tapert, S.F. fMRI response to spatial working memory in adolescents with comorbid marijuana and alcohol use disorders. Drug Alcohol Depend. 2005, 79, 201–210. [Google Scholar] [CrossRef]
- Tapert, S.F.; Schweinsburg, A.D.; Drummond, S.P.; Paulus, M.P.; Brown, S.A.; Yang, T.T.; Frank, L.R. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology 2007, 194, 173–183. [Google Scholar] [CrossRef]
- Hester, R.; Nestor, L.; Garavan, H. Impaired error awareness and anterior cingulate cortex hypoactivity in chronic cannabis users. Neuropsychopharmacology 2009, 34, 2450–2458. [Google Scholar] [CrossRef]
- Nestor, L.; Roberts, G.; Garavan, H.; Hester, R. Deficits in learning and memory: Parahippocampal hyperactivity and frontocortical hypoactivity in cannabis users. Neuroimage 2008, 40, 1328–1339. [Google Scholar] [CrossRef]
- Sagar, K.A.; Gruber, S.A. Marijuana matters: Reviewing the impact of marijuana on cognition, brain structure and function, & exploring policy implications and barriers to research. Null 2018, 30, 251–267. [Google Scholar] [CrossRef]
- Camchong, J.; Lim, K.O.; Kumra, S. Adverse effects of cannabis on adolescent brain development: A longitudinal study. Cereb. Cortex 2016, 27, 1922–1930. [Google Scholar] [CrossRef] [PubMed]
- Cousijn, J.; Goudriaan, A.E.; Ridderinkhof, K.R.; van den Brink, W.; Veltman, D.J.; Wiers, R.W. Neural responses associated with cue-reactivity in frequent cannabis users. Addict. Biol. 2013, 18, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Cousijn, J.; van Benthem, P.; van der Schee, E.; Spijkerman, R. Motivational and control mechanisms underlying adolescent cannabis use disorders: A prospective study. Dev. Cogn. Neurosci. 2015, 16, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.; Paulsen, D.J.; Geier, C.F.; Luna, B.; Clark, D.B. Regional brain activation supporting cognitive control in the context of reward is associated with treated adolescents’ marijuana problem severity at follow-up: A preliminary study. Dev. Cogn. Neurosci. 2015, 16, 93–100. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jager, G.; Block, R.I.; Luijten, M.; Ramsey, N.F. Tentative evidence for striatal hyperactivity in adolescent cannabis-using boys: A cross-sectional multicenter fMRI study. J. Psychoact. Drugs 2013, 45, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Coronado, C.; Wade, N.E.; Aguinaldo, L.D.; Mejia, M.H.; Jacobus, J. Neurocognitive correlates of adolescent cannabis use: An overview of neural activation patterns in task-based functional MRI studies. J. Pediatr. NeuroPsychol. 2020, 6, 1–13. [Google Scholar] [CrossRef]
- Padula, C.B.; Schweinsburg, A.D.; Tapert, S.F. Spatial working memory performance and fMRI activation interaction in abstinent adolescent marijuana users. Psychol. Addict. Behav. 2007, 21, 478–487. [Google Scholar] [CrossRef]
- Smith, A.; López Zunini, R.; Anderson, C.; Longo, C.; Cameron, I.; Hogan, M.; Fried, P. Impact of marijuana on response inhibition: An fMRI study in young adults. J. Behav. Brain Sci. 2011, 1, 124–133. [Google Scholar] [CrossRef]
- Claus, E.D.; Feldstein Ewing, S.W.; Magnan, R.E.; Montanaro, E.; Hutchison, K.E.; Bryan, A.D. Neural mechanisms of risky decision making in adolescents reporting frequent alcohol and/or marijuana use. Brain Imaging Behav. 2018, 12, 564–576. [Google Scholar] [CrossRef]
- Dalley, J.W.; Everitt, B.J.; Robbins, T.W. Impulsivity, compulsivity, and top-down cognitive control. Neuron 2011, 69, 680–694. [Google Scholar] [CrossRef]
- Radua, J.; Borgwardt, S.; Crescini, A.; Mataix-Cols, D.; Meyer-Lindenberg, A.; McGuire, P.K.; Fusar-Poli, P. Multimodal meta-analysis of structural and functional brain changes in first episode psychosis and the effects of antipsychotic medication. Neurosci. Biobehav. Rev. 2012, 36, 2325–2333. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Adamson, S.J.; Kay-Lambkin, F.J.; Baker, A.L.; Lewin, T.J.; Thornton, L.; Kelly, B.J.; Sellman, J.D. An improved brief measure of cannabis misuse: The Cannabis Use Disorders Identification Test-Revised (CUDIT-R). Drug Alcohol Depend. 2010, 110, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, L.K.; Pugh, K.R.; Constable, R.T.; Westerveld, M.; Mencl, W.E. Functional correlates of verbal memory deficits emerging during nicotine withdrawal in abstinent adolescent cannabis users. Biol. Psychiatry 2007, 61, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Ford, K.A.; Wammes, M.; Neufeld, R.W.; Mitchell, D.; Théberge, J.; Williamson, P.; Osuch, E.A. Unique functional abnormalities in youth with combined marijuana use and depression: An FMRI study. Front. Psychiatry 2014, 5, 130. [Google Scholar] [CrossRef] [PubMed]
- Schweinsburg, A.D.; Schweinsburg, B.C.; Nagel, B.J.; Eyler, L.T.; Tapert, S.F. Neural correlates of verbal learning in adolescent alcohol and marijuana users. Addiction 2011, 106, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Abdullaev, Y.; Posner, M.I.; Nunnally, R.; Dishion, T.J. Functional MRI evidence for inefficient attentional control in adolescent chronic cannabis abuse. Behav. Brain Res. 2010, 215, 45–57. [Google Scholar] [CrossRef]
- Acheson, A.; Ray, K.L.; Hines, C.S.; Li, K.; Dawes, M.A.; Mathias, C.W.; Dougherty, D.M.; Laird, A.R. Functional activation and effective connectivity differences in adolescent marijuana users performing a simulated gambling task. J. Addict. 2015, 2015, 783106. [Google Scholar] [CrossRef]
- Aloi, J.; Blair, K.S.; Crum, K.I.; Meffert, H.; White, S.F.; Tyler, P.M.; Thornton, L.C.; Mobley, A.M.; Killanin, A.D.; Adams, K.O.; et al. Adolescents show differential dysfunctions related to alcohol and cannabis use disorder severity in emotion and executive attention neuro-circuitries. Neuroimage Clin. 2018, 19, 782–792. [Google Scholar] [CrossRef]
- Aloi, J.; Meffert, H.; White, S.F.; Blair, K.S.; Hwang, S.; Tyler, P.M.; Thornton, L.C.; Crum, K.I.; Adams, K.O.; Killanin, A.D.; et al. Differential dysfunctions related to alcohol and cannabis use disorder symptoms in reward and error-processing neuro-circuitries in adolescents. Dev. Cogn. Neurosci. 2019, 36, 100618. [Google Scholar] [CrossRef]
- Aloi, J.; Blair, K.S.; Crum, K.I.; Bashford-Largo, J.; Zhang, R.; Lukoff, J.; Carollo, E.; White, S.F.; Hwang, S.; Filbey, F.M.; et al. Alcohol Use Disorder, But Not Cannabis Use Disorder, Symptomatology in Adolescents Is Associated With Reduced Differential Responsiveness to Reward Versus Punishment Feedback During Instrumental Learning. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2020, 5, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Aloi, J.; Blair, K.S.; Meffert, H.; White, S.F.; Hwang, S.; Tyler, P.M.; Crum, K.I.; Thornton, L.C.; Mobley, A.; Killanin, A.D.; et al. Alcohol use disorder and cannabis use disorder symptomatology in adolescents is associated with dysfunction in neural processing of future events. Addict. Biol. 2021, 26, e12885. [Google Scholar] [CrossRef] [PubMed]
- Aloi, J.; Crum, K.I.; Blair, K.S.; Zhang, R.; Bashford-Largo, J.; Bajaj, S.; Schwartz, A.; Carollo, E.; Hwang, S.; Leiker, E.; et al. Individual associations of adolescent alcohol use disorder versus cannabis use disorder symptoms in neural prediction error signaling and the response to novelty. Dev. Cogn. Neurosci. 2021, 48, 100944. [Google Scholar] [CrossRef] [PubMed]
- Ames, S.L.; Grenard, J.L.; Stacy, A.W.; Xiao, L.; He, Q.; Wong, S.W.; Xue, G.; Wiers, R.W.; Bechara, A. Functional imaging of implicit marijuana associations during performance on an implicit association test (IAT). Behav. Brain Res. 2013, 256, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Behan, B.; Connolly, C.G.; Datwani, S.; Doucet, M.; Ivanovic, J.; Morioka, R.; Stone, A.; Watts, R.; Smyth, B.; Garavan, H. Response inhibition and elevated parietal-cerebellar correlations in chronic adolescent cannabis users. Neuropharmacology 2014, 84, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Berk, L.; Stewart, J.L.; May, A.C.; Wiers, R.W.; Davenport, P.W.; Paulus, M.P.; Tapert, S.F. Under pressure: Adolescent substance users show exaggerated neural processing of aversive interoceptive stimuli. Addiction 2015, 110, 2025–2036. [Google Scholar] [CrossRef] [PubMed]
- Blair, R.J.R.; White, S.F.; Tyler, P.M.; Johnson, K.; Lukoff, J.; Thornton, L.C.; Leiker, E.K.; Filbey, F.; Dobbertin, M.; Blair, K.S. Threat responsiveness as a function of cannabis and alcohol use disorder severity. J. Child. Adolesc. Psychopharmacol. 2019, 29, 526–534. [Google Scholar] [CrossRef]
- Blair, R.J.R.; Bajaj, S.; Sherer, N.; Bashford-Largo, J.; Zhang, R.; Aloi, J.; Hammond, C.; Lukoff, J.; Schwartz, A.; Elowsky, J.; et al. Alcohol use disorder and cannabis use disorder symptomatology in adolescents and aggression: Associations with recruitment of neural regions implicated in retaliation. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 536–544. [Google Scholar] [CrossRef]
- Cousijn, J.; Wiers, R.W.; Ridderinkhof, K.R.; van den Brink, W.; Veltman, D.J.; Porrino, L.J.; Goudriaan, A.E. Individual differences in decision making and reward processing predict changes in cannabis use: A prospective functional magnetic resonance imaging study. Addict. Biol. 2013, 18, 1013–1023. [Google Scholar] [CrossRef]
- Cyr, M.; Tau, G.Z.; Fontaine, M.; Levin, F.R.; Marsh, R. Deficient functioning of frontostriatal circuits during the resolution of cognitive conflict in cannabis-using youth. J. Am. Acad. Child. Adolesc. Psychiatry 2019, 58, 702–711. [Google Scholar] [CrossRef]
- De Bellis, M.D.; Wang, L.; Bergman, S.R.; Yaxley, R.H.; Hooper, S.R.; Huettel, S.A. Neural mechanisms of risky decision-making and reward response in adolescent onset cannabis use disorder. Drug Alcohol Depend. 2013, 133, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Gilman, J.M.; Lee, S.; Kuster, J.K.; Lee, M.J.; Kim, B.W.; van der Kouwe, A.; Blood, A.J.; Breiter, H.C. Variable activation in striatal subregions across components of a social influence task in young adult cannabis users. Brain Behav. 2016, 6, e00459. [Google Scholar] [CrossRef] [PubMed]
- Gilman, J.M.; Schuster, R.M.; Curran, M.T.; Calderon, V.; van der Kouwe, A.; Evins, A.E. Neural mechanisms of sensitivity to peer information in young adult cannabis users. Cogn. Affect. Behav. Neurosci. 2016, 16, 646–661. [Google Scholar] [CrossRef] [PubMed]
- Gilman, J.M.; Curran, M.T.; Calderon, V.; Schuster, R.M.; Evins, A.E. Altered neural processing to social exclusion in young adult marijuana users. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2016, 1, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Hatchard, T.; Fried, P.; Hogan, M.; Cameron, I.; Smith, A. Marijuana use impacts cognitive interference: An fMRI investigation in young adults performing the counting stroop task. J. Addict. Res. Ther. 2014, 5, 197. [Google Scholar] [CrossRef]
- Heitzeg, M.M.; Cope, L.M.; Martz, M.E.; Hardee, J.E.; Zucker, R.A. Brain activation to negative stimuli mediates a relationship between adolescent marijuana use and later emotional functioning. Dev. Cogn. Neurosci. 2015, 16, 71–83. [Google Scholar] [CrossRef]
- Jager, G.; Block, R.I.; Luijten, M.; Ramsey, N.F. Cannabis use and memory brain function in adolescent boys: A cross-sectional multicenter functional magnetic resonance imaging study. J. Am. Acad. Child. Adolesc. Psychiatry 2010, 49, 561–563. [Google Scholar] [CrossRef]
- Kroon, E.; Kuhns, L.; Cousijn, J. Context dependent differences in working memory related brain activity in heavy cannabis users. Psychopharmacology 2022, 239, 1373–1385. [Google Scholar] [CrossRef]
- Leiker, E.K.; Meffert, H.; Thornton, L.C.; Taylor, B.K.; Aloi, J.; Abdel-Rahim, H.; Shah, N.; Tyler, P.M.; White, S.F.; Blair, K.S.; et al. Alcohol use disorder and cannabis use disorder symptomatology in adolescents are differentially related to dysfunction in brain regions supporting face processing. Psychiatry Res. Neuroimaging 2019, 292, 62–71. [Google Scholar] [CrossRef]
- Lopez-Larson, M.P.; Rogowska, J.; Bogorodzki, P.; Bueler, C.E.; McGlade, E.C.; Yurgelun-Todd, D.A. Cortico-cerebellar abnormalities in adolescents with heavy marijuana use. Psychiatry Res. 2012, 202, 224–232. [Google Scholar] [CrossRef][Green Version]
- May, A.C.; Jacobus, J.; Stewart, J.L.; Simmons, A.N.; Paulus, M.P.; Tapert, S.F. Do adolescents use substances to relieve uncomfortable sensations? A preliminary examination of negative reinforcement among adolescent cannabis and alcohol users. Brain Sci. 2020, 10, 214. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, R.; Stewart, J.L.; May, A.C.; Tapert, S.F.; Paulus, M.P. What do you feel? adolescent drug and alcohol users show altered brain response to pleasant interoceptive stimuli. Drug Alcohol Depend. 2013, 133, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Raymond, D.R.; Paneto, A.; Yoder, K.K.; O’Donnell, B.F.; Brown, J.W.; Hetrick, W.P.; Newman, S.D. Does chronic cannabis use impact risky decision-making: An examination of fMRI activation and effective connectivity? Front. Psychiatry 2020, 11, 599256. [Google Scholar] [CrossRef] [PubMed]
- Schweinsburg, A.D.; Schweinsburg, B.C.; Medina, K.L.; McQueeny, T.; Brown, S.A.; Tapert, S.F. The influence of recency of use on fMRI response during spatial working memory in adolescent marijuana users. J. Psychoact. Drugs 2010, 42, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Tervo-Clemmens, B.; Simmonds, D.; Calabro, F.J.; Montez, D.F.; Lekht, J.A.; Day, N.L.; Richardson, G.A.; Luna, B. Early cannabis use and neurocognitive risk: A prospective functional neuroimaging study. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2018, 3, 713–725. [Google Scholar] [CrossRef]
- Thayer, R.E.; Feldstein Ewing, S.W.; Dodd, A.B.; Hansen, N.S.; Mayer, A.R.; Ling, J.M.; Bryan, A.D. Functional activation during the stroop is associated with recent alcohol but not marijuana use among high-risk youth. Psychiatry Res. 2015, 234, 130–136. [Google Scholar] [CrossRef][Green Version]
- Zhou, X.; Zimmermann, K.; Xin, F.; Zhao, W.; Derckx, R.T.; Sassmannshausen, A.; Scheele, D.; Hurlemann, R.; Weber, B.; Kendrick, K.M.; et al. Cue reactivity in the ventral striatum characterizes heavy cannabis use, whereas reactivity in the dorsal striatum mediates dependent use. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2019, 4, 751–762. [Google Scholar] [CrossRef]
- Zimmermann, K.; Walz, C.; Derckx, R.T.; Kendrick, K.M.; Weber, B.; Dore, B.; Ochsner, K.N.; Hurlemann, R.; Becker, B. Emotion regulation deficits in regular marijuana users. Hum. Brain Mapp. 2017, 38, 4270–4279. [Google Scholar] [CrossRef]
- Lévesque, J.; Joanette, Y.; Mensour, B.; Beaudoin, G.; Leroux, J.M.; Bourgouin, P.; Beauregard, M. Neural basis of emotional self-regulation in childhood. Neuroscience 2004, 129, 361–369. [Google Scholar] [CrossRef]
- Lévesque, J.; Eugène, F.; Joanette, Y.; Paquette, V.; Mensour, B.; Beaudoin, G.; Leroux, J.M.; Bourgouin, P.; Beauregard, M. Neural circuitry underlying voluntary suppression of sadness. Biol. Psychiatry 2003, 53, 502–510. [Google Scholar] [CrossRef]
- Booth, J.R.; Burman, D.D.; Meyer, J.R.; Lei, Z.; Trommer, B.L.; Davenport, N.D.; Li, W.; Parrish, T.B.; Gitelman, D.R.; Mesulam, M.M. Neural development of selective attention and response inhibition. Neuroimage 2003, 20, 737–751. [Google Scholar] [CrossRef]
- Durston, S.; Thomas, K.M.; Yang, Y.; Uluğ, A.M.; Zimmerman, R.D.; Casey, B.J. A neural basis for the development of inhibitory control. Dev. Sci. 2002, 5, F9–F16. [Google Scholar] [CrossRef]
- Tamm, L.; Menon, V.; Reiss, A.L. Maturation of brain function associated with response inhibition. J. Am. Acad. Child. Adolesc. Psychiatry 2002, 41, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.; Zaitchik, D.; Tager-Flusberg, H. Preschoolers can attribute second-order beliefs. Dev. Psychol. 1994, 30, 395–402. [Google Scholar] [CrossRef]
- Somerville, L.H.; Hare, T.; Casey, B.J. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. J. Cogn. Neurosci. 2011, 23, 2123–2134. [Google Scholar] [CrossRef]
- D’Souza, D.C.; Cortes-Briones, J.A.; Ranganathan, M.; Thurnauer, H.; Creatura, G.; Surti, T.; Planeta, B.; Neumeister, A.; Pittman, B.; Normandin, M.D.; et al. Rapid Changes in Cannabinoid 1 Receptor Availability in Cannabis-Dependent Male Subjects After Abstinence From Cannabis. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2016, 1, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Allick, A.; Kim, K.; Parg, G.; Lebo, R.; Nanavati, K.; Hammond, C.J. Abberant brain function in cannabis using compared to non-using youth is associated with cannabis type 1 receptor availability: A meta-analysis of youth fMRI studies. In Proceedings of the American Society of Addiction Medicine 2021 Virtual Annual Meeting, online, 22–24 April 2021. [Google Scholar]
- Orr, C.; Spechler, P.; Cao, Z.; Albaugh, M.; Chaarani, B.; Mackey, S.; D’Souza, D.; Allgaier, N.; Banaschewski, T.; Bokde, A.L.W.; et al. Grey Matter Volume Differences Associated with Extremely Low Levels of Cannabis Use in Adolescence. J. Neurosci. 2019, 39, 1817–1827. [Google Scholar] [CrossRef]
- Heller, A.S.; Cohen, A.O.; Dreyfuss, M.F.; Casey, B.J. Changes in cortico-subcortical and subcortico-subcortical connectivity impact cognitive control to emotional cues across development. Soc. Cogn. Affect. Neurosci. 2016, 11, 1910–1918. [Google Scholar] [CrossRef]
- Bailen, N.H.; Wu, H.; Thompson, R.J. Meta-emotions in daily life: Associations with emotional awareness and depression. Emotion 2019, 19, 776–787. [Google Scholar] [CrossRef]
- Klimes-Dougan, B.; Pearson, T.; Jappe, L.; Mathieson, L.; Simard, M.R.; Hastings, P.; Zahn-Waxler, C. Adolescent emotion socialization: A longitudinal study of friends’ responses to negative emotions. Soc. Dev. 2014, 23, 395–412. [Google Scholar] [CrossRef]
- Ernst, M.; Fudge, J.L. A developmental neurobiological model of motivated behavior: Anatomy, connectivity and ontogeny of the triadic nodes. Neurosci. Biobehav. Rev. 2009, 33, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Dorard, G.; Berthoz, S.; Phan, O.; Corcos, M.; Bungener, C. Affect dysregulation in cannabis abusers: A study in adolescents and young adults. Eur. Child. Adolesc. Psychiatry 2008, 17, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Micalizzi, L.; Brick, L.A.; Thomas, S.A.; Wolff, J.; Esposito-Smythers, C.; Spirito, A. Cannabis Use and Emotional Awareness Difficulties in Adolescents with Co-Occurring Substance Use and Psychiatric Disorders. Subst. Use Misuse 2020, 55, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Limonero, J.T.; Tomás-Sábado, J.; Fernández-Castro, J. Perceived emotional intelligence and its relation to tobacco and cannabis use among university students. Psicothema 2006, 18, 95–100. [Google Scholar] [PubMed]
- Etkin, A.; Egner, T.; Kalisch, R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci. 2011, 15, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Martz, M.E.; Trucco, E.M.; Cope, L.M.; Hardee, J.E.; Jester, J.M.; Zucker, R.A.; Heitzeg, M.M. Association of Marijuana Use With Blunted Nucleus Accumbens Response to Reward Anticipation. JAMA Psychiatry 2016, 73, 838–844. [Google Scholar] [CrossRef]
- Savulich, G.; Rychik, N.; Lamberth, E.; Hareli, M.; Evins, A.; Shakian, B.; Schuster, R. Sex Differences in Neuropsychological Functioning are Domain-Specific in Adolescent and Young Adult Regular Cannabis Users. J. Int. NeuroPsychol. Soc. 2021, 27, 592–606. [Google Scholar] [CrossRef]
- Francis, A.M.; Bissonnette, J.N.; MacNeil, S.E.; Crocker, C.E.; Tibbo, P.G.; Fisher, D.J. Interaction of sex and cannabis in adult in vivo brain imaging studies: A systematic review. Brain Neurosci. Adv. 2022, 6, 23982128211073431. [Google Scholar] [CrossRef]
- Allick, A.; Park, G.; Kim, K.; Vintimilla, M.; Rathod, K.; Lebo, R.; Nanavati, J.; Hammond, C.J. Age- and Sex-Related Cortical Gray Matter Volume Differences in Adolescent Cannabis Users: A Systematic Review and Meta-Analysis of Voxel-Based Morphometry Studies. Front. Psychiatry 2021, 12, 745193. [Google Scholar] [CrossRef]
- Blest-Hopley, G.; O’Neill, A.; Wilson, R.; Giampietro, V.; Lythgoe, D.; Egerton, A.; Bhattacharyya, S. Adolescent-onset heavy cannabis use associated with significantly reduced glial but not neuronal markers and glutamate levels in the hippocampus. Addict. Biol. 2019, 25, e12827. [Google Scholar] [CrossRef]
- Wiers, C.E.; Shokri-Kojori, E.; Wong, C.T.; Abi-Dargham, A.; Demiral, Ş.B.; Tomasi, D.; Wang, G.J.; Volkow, N.D. Cannabis abusers show hypofrontality and blunted brain responses to a stimulant challenge in females but not in males. Neuropsychopharmacology 2016, 41, 2596–2605. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.M.; Wallace, A.L.; Wade, N.E.; Swartz, A.M.; Lisdahl, K.M. Assessing the role of cannabis use on cortical surface structure in adolescents and young adults: Exploring gender and aerobic fitness as potential moderators. Brain Sci. 2020, 10, 117. [Google Scholar] [CrossRef] [PubMed]
- Medina, K.L.; McQueeny, T.; Nagel, B.J.; Hanson, K.L.; Yang, T.T.; Tapert, S.F. Prefrontal cortex morphometry in abstinent adolescent marijuana users: Subtle gender effects. Addict. Biol. 2009, 14, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Ritchay, M.M.; Huggins, A.A.; Wallace, A.L.; Larson, C.L.; Lisdahl, K.M. Resting state functional connectivity in the default mode network: Relationships between cannabis use, gender, and cognition in adolescents and young adults. Neuroimage Clin. 2021, 30, 102664. [Google Scholar] [CrossRef]
- Filbey, F.M.; Aslan, S.; Lu, H.; Peng, S.L. Residual Effects of THC via Novel Measures of Brain Perfusion and Metabolism in a Large Group of Chronic Cannabis Users. Neuropsychopharmacology 2018, 43, 700–707. [Google Scholar] [CrossRef]
- Calakos, K.C.; Bhatt, S.; Foster, D.W.; Cosgrove, K.P. Mechanisms Underlying Sex Differences in Cannabis Use. Curr. Addict. Rep. 2017, 4, 439–453. [Google Scholar] [CrossRef]
- Ruiz, C.M.; Torrens, A.; Castillo, E.; Perrone, C.R.; Cevallos, J.; Inshishian, V.C.; Harder, E.V.; Justeson, D.N.; Huestis, M.A.; Swarup, V.; et al. Pharmacokinetic, behavioral, and brain activity effects of Δ9-tetrahydrocannabinol in adolescent male and female rats. Neuropsychopharmacology 2021, 46, 959–969. [Google Scholar] [CrossRef]
- Hernandez-Avila, C.A.; Rounsaville, B.J.; Kranzler, H.R. Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend. 2004, 74, 265–272. [Google Scholar] [CrossRef]
- Cooper, Z.D.; Craft, R.M. Sex-Dependent Effects of Cannabis and Cannabinoids: A Translational Perspective. Neuropsychopharmacology 2018, 43, 34–51. [Google Scholar] [CrossRef]
- Lenroot, R.K.; Gogtay, N.; Greenstein, D.K.; Wells, E.M.; Wallace, G.L.; Clasen, L.S.; Blumenthal, J.D.; Lerch, J.; Zijdenbos, A.P.; Evans, A.C.; et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage 2007, 36, 1065–1073. [Google Scholar] [CrossRef]
- Burgess, P.W.; Gilbert, S.J.; Dumontheil, I. Function and localization within rostral prefrontal cortex (area 10). Philos Trans. R. Soc. Lond B Biol. Sci. 2007, 362, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F. Addiction is a Reward Deficit and Stress Surfeit Disorder. Front. Psychiatry 2013, 4, 72. [Google Scholar] [CrossRef]
- Filbey, F.M.; Dunlop, J. Differential reward network functional connectivity in cannabis dependent and non-dependent users. Drug Alcohol Depend. 2014, 140, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Hammond, C.J.; Wu, J.; Krishnan-Sarin, S.; Mayes, L.C.; Potenza, M.N.; Crowley, M.J. Co-occurring tobacco and cannabis use in adolescents: Dissociable relationships with mediofrontal electrocortical activity during reward feedback processing. Neuroimage Clin. 2021, 30, 102592. [Google Scholar] [CrossRef] [PubMed]
- Hammond, C.J.; Allick, A.; Rahman, N.; Nanavati, J. Structural and Functional Neural Targets of Addiction Treatment in Adolescents and Young Adults: A Systematic Review and Meta-Analysis. J. Child. Adolesc. Psychopharmacol. 2019, 29, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Waldron, H.B.; Turner, C.W. Evidence-Based Psychosocial Treatments for Adolescent Substance Abuse. J. Clin. Child. Adolesc. Psychol. 2008, 37, 238–261. [Google Scholar] [CrossRef]
- Horigian, V.E.; Anderson, A.R.; Szapocznik, J. Family-Based Treatments for Adolescent Substance Use. Child. Adolesc. Psychiatr. Clin. N. Am. 2016, 25, 603–628. [Google Scholar] [CrossRef]
- Müller, V.I.; Cieslik, E.C.; Laird, A.R.; Fox, P.T.; Radua, J.; Mataix-Cols, D.; Tench, C.R.; Yarkoni, T.; Nichols, T.E.; Turkeltaub, P.E.; et al. Ten simple rules for neuroimaging meta-analysis. Neurosci. Biobehav. Rev. 2018, 84, 151–161. [Google Scholar] [CrossRef]
- Loflin, M.J.E.; Kiluk, B.D.; Huestis, M.A.; Aklin, W.M.; Budney, A.J.; Carroll, K.M.; D’Souza, D.C.; Dworkin, R.H.; Gray, K.M.; Hasin, D.S.; et al. The state of clinical outcome assessments for cannabis use disorder clinical trials: A review and research agenda. Drug Alcohol Depend. 2020, 212, 107993. [Google Scholar] [CrossRef]
- Wall, M.B.; Pope, R.; Freeman, T.P.; Kowalczyk, O.S.; Demetriou, L.; Mokrysz, C.; Hindocha, C.; Lawn, W.; Bloomfield, M.A.; Freeman, A.M.; et al. Dissociable effects of cannabis with and without cannabidiol on the human brain’s resting-state functional connectivity. J. Psychopharmacol. 2019, 33, 822–830. [Google Scholar] [CrossRef]
- Gunasekera, B.; Davies, C.; Martin-Santos, R.; Bhattacharyya, S. The Yin and Yang of cannabis: A systematic review of human neuroimaging evidence of the differential effects of d(9)-tetrahydrocannabinol and cannabidiol. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 636–645. [Google Scholar] [CrossRef] [PubMed]
| Study | Sample Characteristics | Male Sex (%) by Group | Mean Age (Years) by Group | Quantity of CU among Participants | Sample Type | Abstinence at MRI Scan Session | MRI Scanner | Task Type | Task Contrast(s) | Analytic Method, MC, and Sampling Approach | Results of Whole-Brain Voxel-Wise Analysis: |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abdullaev et al., 2010 [39] | N = 14 Chronic CU youth and N = 14 matched healthy CON | CU: 71.4% CON: 71.4% | CU: 19.5 yrs. CON: 19.6 yrs. | CU group used on 132 days per year and for an average of 5 yrs. | Community | ≥48-h | 3.0 T | Attention Network task | Alerting effect: Center cue vs. No cue contrast; Orienting effect: Spatial cue vs. center cue contrast; Conflict effect: Incongruent vs. congruent contrast | Analysis: WB GLM FWHM: 6 mm MC: WB cluster corrected p < 0.05, Z > 2.3 Sampling: CU vs. TD group comparison | Alerting and Oriented effects: MJ > CON: None MJ < CON: None Conflict effec: MJ > CON: R lateral prefrontal cortex (BA 47) R supplemental motor cortex (BA 6) b/l lateral parietal corext (BA 40) MJ < CON: none |
| Acheson et al., 2015 [40] | N = 14 CU youth and N = 14 CON, ages 15–19 yrs | CU: 78.6% CON: 78.6% | CU: 17.3 yrs. CON: 17.6 yrs. | CU group used ≥5-days per week | Community | >12-h | 3.0 T | Win/Loss Feedback task | Win vs. Neutral contrast; Loss vs. Neutral contrast | Analysis: WB and ROI, SEM FWHM: 5 mm MC: WB: k > 15 voxels, Cluster corrected p < 0.01, z ≥ 2.3 Sampling: CU vs. TD group comparison | Win vs. Neutral: MJ > CON: R middle frontal gyrus R caudate L middle frontal gyrus L caudate L claustrum R claustrum L middle frontal gyrus MJ < CON: None Loss vs. Neutral: MJ > CON: R middle frontal gyrus R posterior cingulate R anterior cingulate R claustrum L insula L claustrum L declive R declive MJ < CON: None |
| Aloi et al., 2018 [41] | N = 150 youth ages 14–18-years with variable levels of CUD and AUD severity recruited from residential program and community | Total Sample: 62% CU: 76% CON: 64% | Total Sample: 16.1 yrs. CU: 16.2 yrs. CON: 15.6 yrs. | Mean CUDIT score of Total sample = 7.0 | Clinical & Community (combined sample) | ≥30-days | 3.0 T | Affective Stroop task | 9 emotion-by-task contrasts were included based upon 3 emotional stimuli (positive vs. neutral vs. negative images) and 2 conditions (congruent vs. incongruent trials) | Analysis: WB + amygdala ROI, GLM + ANCOVA FWHM: 6 mm MC: WB: k > 19 voxels, p < 0.001 (via AFNI3dClustSIM) Sampling: Combined Sample | CUDIT-by-Task Condition effect was observed within the PCC, precuneus, IPL with Incongruent vs. Congruent contrast showing: Participants w/High-CUD symptoms > Participants w/Low/No-CUD symptoms: R PCC b/l precuneus R IPL R middle temporal gyrus L Culmen L cerebellum AUDIT-by-CUDIT-by-emotion-by-task condition interaction: Significant 4-way interaction was observed in the L IFG whereby AUDIT scores were negatively associated with IFG BOLD response to negative stimuli at low CUD levels (CUDIT < 4) but positively associated at high CUD levels (CUDIT > 27). |
| Aloi et al., 2019 [42] | N = 150 youth ages 14–18-years with variable levels of CUD and AUD severity recruited from residential program and community | Total Sample: 61% | Total Sample: 16.1 yrs. | Mean CUDIT score of Total sample = 7.31 | Clinical & Community (combined sample) | ≥30-days | 3.0 T | Monetary Incentive Delay (MID) task | Reinforcement and Accuracy Effects: 4 reinforcement-by-accuracy contrasts were included based upon 2 reinforcement cues (reward vs. punishment cues) and 2 response outcomes (accurate vs. inaccurate response) on unmodulated BOLD response data. | Analysis: WB + ROI (striatum, ACC/dmPFC), GLM, ANCOVA FWHM: 6 mm MC: WB k > 26 voxels, voxelwise: p < 0.002, Cluster corrected p < 0.05 (via AFNI3dClustSIM) Sampling: Combined Sample | CUDIT-by-Accuracy Effect: Significant CUDIT-by-Accuracy effects were observed showing a strong negative correlation between CUDIT score and BOLD response in lingual gyrus and putamen during inaccurate compared to accurate trials. CUDIT-by-Reinforcement-by-Accuracy Interaction Effect: A significant CUDIT-by-Reinforcement-by-Accuracy interaction was observed within the R putamen and L ACC/dmPFC showing a negative correlation between CUDIT score and BOLD response during feedback on inaccurate punishment trials relative to all other outcomes. REW: In whole-brain voxel-wise analyses, no main effect of CUDIT was observed on BOLD response during reward feedback of accurate trials in the sample |
| Aloi et al., 2020 [43] | N = 104 youth ages 14–18-years with variable levels of CUD and AUD severity recruited from residential program and community | Total Sample: 64% | Total Sample: 16.1 yrs. | Clinical & Community (combined sample) | ≥30-days | 3.0 T | Passive avoidance task | 4-stimulus-by-feedback contrasts were included based upon 2 stimulus types (high punishment probability stimulus vs. high reward probability stimulus) and 2 feedback outcomes (reward vs. punishment) | Analysis: WB, GLM, ANCOVA FWHM: 6 mm MC: WB k > 16 voxels, voxelwise: p < 0.001, cluster corrected p < 0.05 (via AFNI3dClustSIM) Sampling: Combined Sample | In whole-brain voxel-wise analyses no regions showed either a CUDIT-by-Feedback effect or an AUDIT-by-CUDIT-by-Feedback interaction effect that survived correction for multiple comparisons. | |
| Aloi et al., 2021a [44] | N = 141 youth ages 14–18-years with variable levels of CUD and AUD severity recruited from residential program and community | Total Sample: 57% | Total Sample: 16.3 yrs. | Clinical & Community (combined sample) | ≥30-days | 3.0 T | Comparative optimism (CO) task | BOLD response was measured while participants were presented with future events that varied in valence and intensity and were asked to rate the probability of those events occurring. Contrasts of Interest: 4 valence-by-intensity contrasts based upon 2 valences of future events (positively valenced vs. negatively valenced) and 2 levels of intensity (high-intensity vs. low-intensity) future events. | Analysis: WB, GLM, ANCOVA FWHM: 6 mm MC: k > 23 voxels, voxelwise: p < 0.001, cluster corrected p < 0.05 (via AFNI3dClustSIM) Sampling: Combined Sample | CUDIT-by-Intensity effect: R/L subgenual ACC R/L PCC R superior temporal gyrus L fusiform L culmen R putamen In total sample, greater CUDIT scores were associated with greater differential BOLD responsiveness within the R/L sg ACC, R/L PCC, R superior temporal gyrus, L fusiform, L culmen, and R putamen to high-relative to low-intensity future events. CUDIT-by-Valence-by-Intensity effect: L precentral gyrus R/L cuneus L Occipital cortex R culmen In the total sample, there was a significant negative relationship between CUDIT scores and differential BOLD responsiveness within the precentral gyrus, cuneus, and occipital cortex to high-intensity relative to low-intensity negative future events. AUDIT-by-CUDIT-by-Intensity interaction effect: R/L rmFC | |
| Aloi et al., 2021b [45] | N = 128 youth ages 14–18-years with variable levels of CUD and AUD severity recruited from residential program and community | Total Sample: 61% | Total Sample: 16.7 yrs. | Clinical & Community (combined sample) | ≥30-days | 3.0 T | Novelty task (three-armed bandit paradigm) | RPE during Explore vs. Non-Explore trials contrast Behavioral outcome: Novelty Propensity (NP) score Main analysis examined RPE-modulated BOLD responsiveness during explore vs. non-explore trials using a one-way ANCOVA with the following between-subject variables: AUDIT score, CUDIT score, NP score, Sex, AUDIT-by-NP interaction, and CUDIT-by-NP interaction. | Analysis: WB, GLM, ANCOVA FWHM: 6 mm MC: WB k > 17 voxels, voxelwise: p < 0.001, cluster corrected p < 0.05 (via AFNI3dClustSIM) Sampling: Combined Sample of CU and TD youth | Main effect of CUDIT: No main effect of CUDIT on RPE-modulated BOLD response was observed. CUDIT-by- NP score: There was a significant CUDIT-by-NP interaction within IPL and Cerebellum—whereby NP score was positively associated w/ RPE modulated BOLD response in youth with low CUDIT scores (<6) within IPL and cerebellum and was negatively associated with RPE modulated BOLD response in youth with high CUDIT scores (>12) [i.e., CUD < CON] in the cerebellum. CUDIT-by-NPS-by-Explore condition: Significant CUDIT-by-NP-by-explore interaction within dmPFC, IPL, and STG whereby NP score was positively associated w/RPE modulated BOLD response in youth with low CUDIT scores (<6) within dmPFC, STG, and IPL, and was negatively associated with RPE modulated BOLD response in youth with high CUDIT scores (>12) [i.e., CUD < CON] in the dmPFC, STG, and IPL. | |
| Ames et al., 2013 [46] | N = 13 heavy MJ users and N = 15 healthy CON young adults (ages 19–25-years) | MJ: 85% CON: 33% | MJ: 21.2 yrs. CON: 20.3 yrs. | MJ group had >300 use episode over prior 3-years | Community | ≥24-h | 3.0 T | Marijuana Implicit Association task | Compatible association vs. fixation contrast; Incompatible association vs. fixation contrast | Analysis: WB + ROI, GLM FWHM: 4 mm MC: WB k > 30 voxels, voxelwise: p < 0.005, cluster corrected p < 0.05 w/MCS Sampling: CU vs. TD group comparison | Compatible associations: MJ > CON: L caudate R caudate R putamen L putamen R inferior frontal cortex MJ < CON: none Incompatible associations: MJ > CON: none MJ < CON: R inferior frontal cortex |
| Behan et al., 2014 [47] | N = 17 heavy CU youth and N = 18 non-using CON between ages 15–18-years | CU: 94.1% CON: 94.4% | CU: 16.5 yrs. CON: 16.1 yrs. | Cannabis user smoked 178.4 joints on average and smoked 4168 joints in their lifetime. | Clinical (drug treatment center in Dublin, IR) | ≥12-h | 3.0 T | Go/No-Go task | Successful inhibition of prepotent response (STOP) trials and Unsuccessful inhibition of prepotent response (ERROR) trials | Analysis: WB: FWHM: 4.2 mm MC: WB: Voxel-wise: p < 0.005, t = 3.01; Cluster-level: k > 277 μL, Cluster corrected p < 0.05 based on MCS Sampling: CU vs. TD group comparison | MJ vs. CON: No group differences |
| Berk et al., 2015 [48] | N = 15 adolescents (ages 15–17-yrs. With SUD related to cannabis and/or alcohol and N = 18 matched CON | SUD: 67% CON: 61% | SUD: 16.6 yrs. CON: 16.5 yrs. | 73% of SUD group met criteria for CUD | Community (California high schools) | ≥72-h | Aversive inspiratory breathing load task | Anticipation vs. baseline contrast; breathing load vs. baseline contrast | Analysis: WB + insula and ACC ROI FWHM: 4 mm MC: WB k > 768 µL (12 voxesl), cluster corrected p < 0.05 (via AFNI3dClustSIM) Sampling: CU vs. TD group comparison | Main group effects across phases: SUD > CON: None SUD < CON: L precentral gyrus L superior temporal gyrus Group effects-by-phase: Anticipation Phase: SUD > CON: None SUD < CON: Posterior insula Parahippocampal gyrus Superior temporal gyrus Breathing load Phase: SUD > CON: Posterior insula Middle frontal gyrus Uncus Middle temporal gyrus Anterior insula Inferior frontal gyrus SUD < CON: None | |
| Blair et al., 2019 [49] | N = 87 youth ages 14–18-years with variable levels of CUD and AUD severity recruited from residential program and community | Total Sample: 49% | Total Sample: 16.48 yrs. | Mean CUDIT score of Total Sample = 6.4 | Community/Clinical | ≥30-days | 3.0 T | Looming threat task | Direction (Looming vs. receding) by Type (animal vs. human) by Emotion (threatening vs. neutral) contrasts; main contrast: Looming vs. receding threat contrast; secondary contrast: threatening vs. neutral stimuli contrast | Analysis: WB, GLM, ANCOVA FWHM: 6 mm MC: WB k > 23 voxels, voxelwise: p < 0.001, cluster corrected p < 0.05 (via AFNI3dClustSIM) Sampling: Combined Sample of CU and TD youth | CUDIT-by-Direction contrast: Increasing CUD symptoms were associated with reducing BOLD response differentiation of looming vs. receding stimuli in rostromedial frontal cortex (rmPFC), L fusiform gyrus, cerebellum Traditional group-based analysis: Looming vs. receding threat contrast: MJ < CON: Rostromedial PFC Threatening vs. Neutral contrast: No main or interaction effect related to CUD symptoms. |
| Blair et al., 2021 [50] | N = 102 youth ages 14–18-years with variable levels of CUD and AUD severity recruited from residential program and community | Total Sample: 66% | Total Sample: 16.5 yrs. | Mean CUDIT score of Total Sample = 9.3 | ≥30-days | Retaliation task (variation on ultimatum game) | Primary contrast of interest: BOLD response when retaliating to unfair offers Task included 3 phases (offer vs. decision vs. outcome phases), 4 offers with variable levels of fairness or unfairness (fair vs. 3-levels of unfair offers), and 4 decision response options (accept offer vs. reject offer and punish partner by spending $1, $2, or $3 as punishment dollars). | Analysis: WB, GLM, ANCOVA FWHM: 6 mm MC: WB k > 19 voxels, voxelwise: p < 0.001, cluster corrected p < 0.05 (via AFNI3dClustSIM) Sampling: Combined Sample of CU and TD youth | In whole-brain voxel-wise analyses no regions showed either a CUDIT-by-phase effect or an AUDIT-by-CUDIT-by-Phase interaction effect that survived correction for multiple comparisons. | ||
| Claus et al., 2018 [31] | N= 39 MJ users, N = 90 MJ + ALC users, N = 23 ALC users, and N = 37 healthy CON adolescents ages 14–18-yrs. | MJ: 72% MJ + ALC: 88% ALC: 61% CON: 54% 129 (82.3%) | MJ: 16.0 yrs. MJ + Alc: 16.3 yrs. ALC: 16.4 yrs. CON: 16.1 yrs. | MJ group used approximately 14.6 days in the past 30-days | Justice System (alternative to incarceration program ©n SW United States) | ≥24-h | 3.0 T | BART risky decision-making task | Mean risk vs. Mean non-risk contrast (mean level of response for risky vs. riskless decisions across balloons); Linear risk vs. Linear Non-risk contrast (difference in partial correlation coefficient between BOLD signal and # pumps during risky vs. riskless choices) | Analysis: WB, ANOVA FWHM: 5 mm MC: WB: voxel threshold Z > 2.33, corrected cluster p < 0.025 Sampling: CU vs. TD group comparison | Mean risk vs. non-risk contrast: MJ + AUD > CON: none MJ + AUD < CON: b/l ventral striatum, thalamus, brain stem L putamen, insula, IFG Linear risk vs. non-risk contrast: MJ + AUD > CON: none MJ + AUD < CON: L Pre/Post-central gyrus, SPL, R putamen, caudate, insula, dACC/SMA |
| Cousijn et al., 2012a [24] | N = 31 frequent CU, N = 20 sporadic CU, and N = 20 non-using CON youth | Frequent CU: 65% Sporadic CU: 65% CON: 64% | Frequent CU: 21.3 yrs. Sporadic CU: 22.1 yrs. CON: 22.1 yrs. | Frequent CU reported using cannabis > 10 days per month for past-2-years and not having CUD treatment. Sporadic CU had between 1 and 50 lifetime CU episode. | Community (Amsterdam) | ≥24-h | 3.0 T | Visual Cannabis Cue reactivity task | Cannabis vs. neutral cue contrast | Analysis: WB, GLM, Regressions FWHM: 5 mm MC: WB: Corrected cluster pFWE < 0.05, z > 2.3 Sampling: CU vs. TD group comparison | Cannabis vs. neutral cue contrast: WB analysis: No Frequent MJ or Sporadic MJ vs. CON group differences. ROI: analysis: Frequent MJ > Sporadic MJ and CON: VTA Sporadic MJ vs. CON: No group differences Dependent MJ users > Non-dependent MJ users: ROI analysis: b/l ACC b/l OFC L putamen b/l caudate b/l Nucleus accumbens WB analysis: L middle frontal gyrus L temporal pole |
| Cousijn et al., 2012b [25] | N = 33 heavy CU young adults and N = 36 matched healthy CON young adults ages 18–25 yrs. who completed an MRI session at baseline and then a follow-up assessment at 6-months. | CU: 64% CON: 64% | CU: 21.3 yrs. CON: 22.2 yrs. | Heavy CU used > 10 days per month over the past-2-yrs. | Community (Amsterdam) | ≥24-h | 3.0 T | Approach bias Stimulus Response compatibility (SRC) task | Task includes approach, avoid, and baseline blocks/trials and uses cannabis and neutral images. Primary contrast: cannabis approach-bias obtained by subtracting avoid block (avoid-cannabis & approach-control) from approach block (approach-cannabis & avoid-control). Additionally four secondary condition vs. baseline contrast(s) were also investigated. | Analysis: WB, GLM FWHM: 5 mm MC: WB: Cluster corrected p < 0.05, Z > 2.3 Sampling: CU vs. TD group comparison | Approach block > Avoid block: Group Comparisons: No significant MJ vs. CON group differences were observed in approach-bias BOLD response. Within MJ group association analyses: Lifetime cannabis use positive correlation: L parahippocampal gyrus R amygdala b/l Occipital cortex b/l Cerebellum R insula R inferior frontal gyrus b/l medial frontal gyrus R precuneus L supramarginal gyrus Change in CUDIT negative correlation: R dlPFC b/l ACC |
| Cousijn et al., 2013 [51] | N = 32 heavy CU youth and N = 41 matched non-using CON youth completed MRI scan at baseline and had a follow-up assessment at 6-months | CU: 66% CON: 63% | CU: 21.4 yrs. CON: 22.2 yrs. | CU group used cannabis an avg. of 4.0 days per week and had a mean CUDIT score of 12.2 at baseline. | Community (Amsterdam) | ≥24-h | 3.0 T | Iowa Gambling task | Decision making phase: disadvantageous vs. advantageous choices contrast Feedback phase: win vs. loss feedback | Analysis: WB, GLM FWHM: 5 mm MC: WB: Corrected cluster pFWE < 0.05, z > 2.3 Sampling: CU vs. TD group comparison | Decision making: Disadvantageous vs. advantageous contrast: No MJ vs. CON group differences in BOLD response at baseline visit Reward Feedback phase: Win > Loss feedback: MJ > CON: R orbitofrontal cortex R insula L posterior superior temporal gyrus MJ < CON: None |
| Cyr et al., 2019 [52] | N = 28 CU youth and N = 32 healthy CON youth ages 14–23-yrs. | CU: 61% CON: 53% | CU: 19.3 yrs. CON: 18.9 yrs. | CU group used > 2 times per week | Community/Clinical | ≥12-h | 3.0 T | Simon Spatial Incompatibility Task | Incongruent (I) vs. Congruent©) contrast | Analysis: WB, Multilevel Regressions FWHM: 8 mm MC: WB voxelwise: p < 0.001, cluster corrected pFWE < 0.05 in SPM Sampling: CU vs. TD group comparison | I vs. C contrast: MJ > CON: none MJ < CON: R orbitofrontal cortex (lateral) R inferior frontal gyrus (orbitalis) L thalamus B/l orbitofrontal cortex (medial) L anterior cingulate cortex R supramarginal gyrus R postcentral gyrus R Rolandic operculum |
| Debellis et al., 2013 [53] | N = 15 adolescents with CUD in post-treatment remission with >30-days abstinence compared to N = 18 healthy TD controls and N = 23 CON with psychiatric comorbidities, all groups ages 13–17-yrs. | CUD: 100% TD CON: 100% Psychiatric CON: 100% | CUD: 16.4 yrs. TD CON: 16.0 yrs. Psychiatric CON: 15.4 yrs. | All CUD youth received treatment, were in full remission, and had been >30 days abstinent at scan session | Community/Clinical (CUD and Psychiatric CON from clinic and TD CON from community) | ≥30-days | 3.0 T | Decision Reward Uncertainty task | DM: Decision-making phase: Uncertain reward risk vs. known reward probability risk and no-risk contrast; REW: Outcome phase: Reward vs. No-reward outcomes during risky decision trials (behavioral and reward risk trials) | Analysis: WB + ROI, GLM FWHM: 5 mm MC: Cluster corrected pFWE = 0.05 Sampling: CU vs. TD group comparison | Uncertain risk vs. known risk DM contrast: CUD > CON with psychopathology: L superior parietal lobule and left lateral occipital cortex, precuneus L superior parietal lobule L lateral occipital cortex L precuneus R precuneus; Reward Outcome Contrast: CUD > CON with psychopathology: None CUD < CON with psychopathology: L frontal lobe/middle frontal gyrus/OFC L frontal lobe/MFG L middle frontal gyrus L frontal pole/OFC L superior frontal gyrus L middle frontal gyrus |
| Ford et al., 2014 [37] | N = 15 MJ using youth, N = 14 MDD + MJ use youth, N = 15 MDD youth, and N = 17 healthy CON youth ages 16–25-yrs. | MJ: 67% MDD + MJ: 71% MDD: 13% CON: 35% | MJ: 20.2 yrs. MDD+ MJ: 19.9 yrs. MDD: 19.7 yrs. CON: 20.0 yrs. | MJ group and MDD + MJ groups used on 22 and 21 days in the past month respectively | Community/Clinical | 3.0 T | Passive music listening task | Preferred music selection vs. neutral music contrast | Analysis: WB, GLM, ANCOVA, regressions FWHM: 8 mm MC: pFDR < 0.05 Sampling: CU vs. TD group comparison | Preferred vs. neutral contrast: MJ > Other Groups: None MJ < Other Groups: None Preferred vs. neutral contrast: Preferred > neutral: MDD + MJ > Other Groups: R middle and inferior frontal gyrus R postcentral gyrus L precentral and postcentral gyrus L cingulate gyrus R inferior frontal and precentral gyrus extending to claustrum and putamen MDD + MJ < Other Groups: None | |
| Gilman et al., 2016a [54] | N = 20 social CU young adults and N = 20 non-using CON young adults ages 18–25-yrs. | CU: 50% CON: 50% | CU: 20.6 yrs. CON: 21.5 yrs. | All members of CU group reported weekly CU | Community | ≥12-h | 3.0 T | Social-influence Decision-Making task (using graph to represent peer choices) | Primary contrast: Social influence vs. No-influence contrast (during choice phase); Secondary contrast(s): Congruent vs. incongruent choice contrast (during choices w/social influence stimuli) and Win vs. Loss feedback contrast | Analysis: Wb, NAc ROI, two-way ANOVAs FWHM: 5 mm MC: k > 20 voxels, voxelwise p < 0.005, Z > 2.6, cluster corrected p < 0.05 Sampling: CU vs. TD group comparison | Primary contrast (social influence): MJ > CON: L frontal pole L superior temporal gyrus L superior parietal gyrus MJ < CON: none Secondary contrasts: Incongruent vs. congruent choice: No group differences Win vs. Loss feedback: No group differences |
| Gilman et al., 2016b [55] | N = 20 social CU young adults and N = 23 non-using CON young adults ages 18–25-yrs. | CU: 45% CON: 48% | CU: 20.6 yrs. CON: 21.6 yrs. | All members of CU group reported weekly CU | Community | ≥12-h | 3.0 T | Social-influence decision-making task (using peer images as social stimuli) | Social influence vs. No-influence contrast (during Choice phase); Congruent vs. incongruent choices (during choices w/social influence stimuli) | Analysis: Wb, NAc ROI FWHM: 5 mm MC:, Z > 2.3, cluster corrected p < 0.05 Sampling: CU vs. TD group comparison | Social influence vs. No-influence contrast: MJ > CON: R Caudate MJ < CON: None Incongruent vs. congruent choices: MJ > CON: None MJ < CON: None |
| Gilman et al., 2016c [56] | N = 20 heavy CU and N = 22 non-using CON young adults ages 18–25-yrs. | CU: 45% CON: 50% | CU: 21.4 yrs. CON: 20.4 yrs. | All members of CU group reported weekly use; 50% of CU group met criteria for current CUD | Community | ≥12-h | 3.0 T | Cyberball task (social exclusion paradigm) | Primary contrast: exclusion vs. inclusion contrast Secondary contrast(s): Inclusion vs. exclusion (social inclusion) and reinclusion vs. inclusion (response to reinclusion following exclusion) | Analysis: WB, right insula and ACC ROIs, Mixed effect analysis FWHM: 5 mm MC: Z > 2.3, cluster corrected p < 0.05 Sampling: CU vs. TD group comparison | Primary contrast: Exclusion vs. fair play: MJ > CON: none MJ < CON: R insula R orbitofrontal cortext/insula Secondary contrasts: No MJ vs. CON group differences for secondary contrasts (social inclusion or win vs. loss feedback) |
| Hatchard et al., 2014 [57] | N = 10 regular CU and N = 14 healthy CON young adults ages 19–21 yrs. | CU: 60% CON: 64% | CU: 20.0 yrs. CON: 20.0 yrs. | All CU participants were regular users defined as smoking > 1 joint per week for at least 3-yrs. | Community (Mixed risk community sample from Ottowa Prenatal Prospective Study) | Ad-lib use | 1.5 T | Counting Stroop Interference task | Incongruent (‘Numbers’)—Congruent (‘Animals’) contrast across all trials | Analysis: WB, independent sample t-tests FWHM: 8 mm MC: WB voxelwise p < 0.001, cluster corrected pFWE < 0.05 via SPM Sampling: CU vs. TD group comparison | Incongruent—Congruent: contrast: MJ > CON: R rolandic operculum R cerebellar tonsil R postcentral gyrus Cingulate gyrus L postcentral gyrus R SMA MJ < CON: None |
| Heitzeg et al., 2015 [58] | N = 20 heavy CU young adults and N = 20 healthy CON young adults ages 17–22 yrs. | CU: 60% CON: 70% | CU: 19.8 yrs. CON: 20.5 yrs. | All CU participants had > 100 lifetime use episodes | Community (Mixed risk community sample from Michigan Longitudinal Study) | ≥48-h | 3.0 T | Emotion arousal word task | Negative vs. neutral and positive vs. neutral word contrasts | Analysis: WB + amygdala ROI, GLM FWHM: 6 mm MC: WB: p < 0.005; k > 77 voxels (est. using AlphaSim) Sampling: CU vs. TD group comparison | Negative vs. neutral contrast: MJ > CON: none MJ < CON: R caudal dlPFC R MTG/STG R cuneus/lingual gyrus R STG/insula R amygdala L amygdala Positive vs. neutral contrast: MJ > CON: R dlPFC MJ < CON: R IPL R Amygdala L Amygdala |
| Jacobsen et al., 2007 [36] | N = 20 MJ + TOB users and N = 25 TOB users with limited MJ history scanned at satiety and 24-hr abstinence from nicotine. | MJ + TOB: 25% TOB: 28% | MJ + TOB: 17.3 yrs. TOB: 17.0 yrs. | MJ + TOB group had ≥60 MJ use episode; Both MJ + TOB and TOB groups were daily cigarette smokers | Community | ≥30-day | 1.5 T | Auditory N-Back task | Task Contrast: WM-load (2-back vs. 1-back); Within-Subject Tobacco smoking status contrast (Ad-lib tobacco smoking vs. 24-hr tobacco abstinence) | Analysis: WB, Linear Mixed Regression Models FWHM: 3.125 MC: WB voxelwise p < 0.001, k > 8 voxels Sampling: CU vs. TD group comparison | Group-by-WM-load (2-back vs. 1-back): No MJ + TOB vs. TOB-only group differences Group-by-WM load-by-smoking condition interaction effect in L IPL/STG, R STG, R posterior insula, L posterior cingulate. Group findings based upon the 3 contrasts showed: 2-Back vs. 1-Back: MJ + TOB 24-hr-Abst > TOB 24-hr-Abst L IPL/STG R posterior insula R STG L posterior cingulate Other MJ + TOB vs. TOB state-by-trait comparisons showed no differences |
| Jager et al., 2010 [59] | N = 21 regular CU male youth and N = 24 healthy CON male youth | CU: 100% CON: 100% | CU: 17.2 yrs. CON: 16.8 yrs. | CU participants had at least 200 lifetime CU episodes | Community (two sites: Netherlands and United States) | ≥24-h | 3.0 T and 3.0 T | Sternberg Verbal WM task and Pictorial Associative Memory Task | WM vs. Control; Practiced WM vs. Control; Novel WM vs. Control; Associative learning (collapsed across AL and AR conditions) vs. Classification phase; | Analysis: WB + ROI FWHM: 8 mm MC: WB, pFWE < 0.05 Sampling: CU vs. TD group comparison | WM (collapsed across Practiced and Novel WM trials) vs. Control condition contrast: No group differences PAMT: No group differences |
| Jager et al., 2013 [27] | N = 23 regular CU male youth and N = 24 healthy CON male youth | CU: 100% CON: 100% | CU: 17.2 yrs. CON: 16.8 yrs. | CU participants had at least 200 lifetime CU episodes | Community (two sites: Netherlands and United States) | ≥24-h | 3.0 T and 3.0 T | Monetary Incentive Delay (MID) task | Anticipation phase contrast: Reward vs. neutral anticipation Feedback phase: win vs. loss feedback during reward trials | Analysis: Analysis: WB + caudate, putamen, VS ROIs; GLM repeated measure analyses FWHM: 8 mm MC: WB: pFWE < 0.05 Sampling: CU vs. TD group comparison | Whole-brain voxel-wise analyses showed no MJ vs. CON group differences in reward vs. neutral anticipation contrast or win vs. loss feedback contrast. |
| Kroon et al., 2021 [60] | N = 36 daily CU youth and N = 33 healthy CON youth | CU: 53% CON: 49% | CU: 21.0 yrs. CON: 21.0 yrs. | All CU youth reported daily or near daily use. Mean CUDIT score of CU participants was 13.0. | ≥24-h | 3.0 T | N-back flanker WM task with neutral and cannabis flankers | 3 contrasts of interest: cannabis (c) > neutral (n) flanker contrast (main effect of flanker); 2-back (2) > 1-back (1) contrast (i.e., main effect of WM); and flanker-by-WM-interaction contrast ((2c > 1c) > (2n > 1n)) | Analysis: WB, Mixed effect group analysis and independent sample t-tests FWHM: 5 mm MC: WB k >10 voxels, z > 2.3, cluster corrected p < 0.05 Sampling: CU vs. TD group comparison | Flanker effect (c > n): MJ > CON: none MJ < CON: none WM performance: No MJ vs. CON group differences in accuracy or reaction time on N-back task. WM effect (2 vs. 1): MJ > CON: none MJ < CON: L STG L MTG L angular gyrus Flanker-by-WM effect: MJ > CON: none MJ < CON: L thalamus L operculum L insula R SPL R SMG R PCG | |
| Leiker et al., 2019 [61] | N = 104 youth ages 14–18-years with variable levels of CUD and AUD severity recruited from residential program and community | Total Sample: 64% | Total Sample: 16.0 yrs. | Mean CUDIT score of Total Sample = 5.5 | Community/Clinical | ≥30-days | 3.0 T | Emotional faces task | Fearful vs. happy vs. neutral faces contrasts | Analysis: WB, ANCOVAs FWHM: 6 mm MC: WB k > 24 voxels, voxelwise: p < 0.001, cluster corrected p < 0.05 (via AFNI3dClustSIM) Sampling: Combined Sample of CU and TD youth | Whole-brain meta-regression: Emotional vs. neutral faces contrast: Main effect of CUD symptoms: Negative association between CUDIT scores and BOLD response to emotional face stimuli in L rostromedial PFC including left caudal, ACC regions. Traditional group-based analyses: CUD < CON: R rostrommedial PFC/ACC |
| Lopez-Larson et al., 2012 [62] | N = 24 regular CU and N = 24 healthy CON youth ages 16–22 yrs. | CU: 92% CON: 71% | CU: 18.2 yrs. CON: 18.0 yrs. | All CU participants had at least 100 lifetime CU episodes | Community | ≥12-h | 3.0 T | Standard bilateral finger tapping task | No contrast, BOLD response during finger tapping compared between groups | Analysis: WB + ROI FWHM: 8 mm MC: WB: k > 20 voxels, Cluster corrected p < 0.005 Sampling: CU vs. TD group comparison | Finger tapping related BOLD activation: MJ > CON: R middle occipital lobe MJ < CON: R cingulate gyrus |
| May et al., 2020 [63] | N = 13 CAN + ALC-SUD, N = 16 CAN + ALC-EXP, and N = 18 CON adolescents ages 15–17 years | CAN + ALC-SUD: 69% CAN + ALC-EXP: 75% CON: 72% | CAN + ALC-SUD: 16.6 yrs. CAN + ALC-EXP: 16.7 yrs. CON: 16.3 yrs. | CAN + ALC-SUD: 92.3% CUD diagnoses and 61.5% AUD diagnoses with Mean lifetime CU episodes: 467.9 CAN + ALC-EXP: Mean lifetime CU episodes: 39.4 CON: Mean lifetime CU episodes: 0.1 | Community (California high schools) | ≥72-h | 3.0 T | Drug Cue Breathing fMRI paradigm that paired a cannabis/alcohol drug cue reactivity task with anticipation and experience of aversive interoceptive stimulus (inspiratory breathing load) | 9 task conditions: anticipation neutral images, anticipation substance images, anticipation scrambled images, breathing load neutral images, breathing load substance images, breathing load scrambled images, neutral images only, substance images only, and scrambled images only Contrasts of interest: BOLD signal differences between 3 groups (CAN + ALC-SUD vs. CAN + ALC-EXP vs. CON participants) across breathing load and cue image type conditions; group-by-image type (substance vs. neutral contrast); group-by-interoceptive condition (no breathing load [anticipation] vs. breathing load); and Group-by-Image type-by Interoceptive condition | Analysis: WB, linear mixed effect models FWHM: 6 mm MC: k > 1280 µL (20 voxels), voxelwise p < 0.002, cluster corrected p < 0.05 (via AFNI3dClustSIM) Sampling: CU vs. TD group comparison | Main Group and Image type interaction effects: No group differences were observed in main group comparison or in group-by-image type or group-by-image type-by interoceptive condition interactions. Group-by-interoceptive condition effect (anticipation vs. breathing load): R amgydala L IFG R posterior cingulate L parahippocampal gyrus Post Hoc Pairwise Group Comparisons from Group-by-Interoceptive Condition effect: Anticipation condition: CAN + ALC-SUD > CAN +ALC-EXP: R amgydala CAN + ALC-EXP < CON: L parahippocampal gyrus CAN + ALC-SUD < CAN +ALC-EXP: None Breathing load condition: CAN + ALC-SUD > CAN +ALC-EXP None CAN + ALC-SUD < CAN +ALC-EXP R amgydala CAN + ALC-SUD < CAN +ALC-EXP and CON: L inferior frontal gyrus L parahippocampal gyrus |
| Migliorini et al., 2013 [64] | N = 15 adolescents with SUD and N = 17 healthy CON adolescents ages 15–17 yrs. | SUD: 67% CON: 65% | SUD: 16.5 yrs. CON: 16.8 yrs. | All SUD participants met criteria for current CUD and/or AUD; 73% of SUD participants met criteria for CUD | Community (California high schools) | ≥72-h | 3.0 T | Interoceptive Stimulation task (Soft Touch task) | Soft touch vs. anticipation contrast | Analysis: WB + striatal and ant/post. Insula ROIs, LME FWHM: 4 mm MC: WB: k > 512μL (8 voxels), cluster corrected p < 0.05 based on MCS Sampling: CU vs. TD group comparison | Group main effect: SUD > CON: none SUD < CON: L posterior insula L cuneus R inferior temporal gyrus Group by condition interaction: SUD > CON: None SUD < CON: L postcentral gyrus R precentral gyrus L posterior insula L precentral gyrus R middle frontal gyrus L postcentral gyrus R medial frontal gyrus L cerebellar lingual gyrus R cingulate gyrus R cuneus R medial frontal gyrus L precuneus |
| Padula et al., 2007 [29] | N = 17 CU adolescents and N = 17 healthy CON adolescents ages 16–18 yrs. | CU: 82% CON: 71% | CU: 18.1 yrs. CON: 17.9 yrs. | CU participants had an average of 477 lifetime CU episodes | Community | >28-days | 1.5 T | Spatial WM N-Back task | SWM vs. vigilance condition (1-Back vs. 0-Back) | Analysis: WB, Regressions FWHM: 5 mm MC: WB; k > 50 voxels; Cluster-wise p < 0.05 corrected using MCS Sampling: CU vs. TD group comparison | 1-Back vs. 0-Back: MJ > CON: R claustrum, putamen, caudate, thalamus, globus pallidus, insula, globus pallidus R precuneus, superior parietal lobule, postcentral gyrus L superior parietal lobule, precuneus MJ < CON: none |
| Raymond et al., 2020 [65] | N = 17 regular CU young adults and N = 14 non-using CON young adults | CU: 47% CON: 43% | CU: 21.2 yrs. CON: 22.5 yrs. | The CU group used an avg. of 5.2 days per week and had mean CUDIT score of 13.4 | Community | ≥12-h | 3.0 T | Balloon Analogue Risk Task (BART) | Primary contrast: Risk taking condition which reflects the choice to inflate the balloon x the probability of explosion ChooseInflate*P(explode) | Analysis: WB, GLM FWHM: 8 mm MC: WB k > 150 voxels, p < 0.001 Sampling: CU vs. TD group comparison | Risk condition: MJ > CON: R lateral posterior PFC extending into frontal eye fields |
| Schweinsburg et al., 2005 [18] | N = 15 AUD, N = 15 comorbid CUD + AUD, and N = 19 healthy CON adolescents ages 15–17 yrs. | AUD: 67% CUD + AUD: 67% CON: 58% 15(66.7%) | AUD: 16.8 yrs. CUD+ AUD: 16.9 yrs. CON: 16.5 yrs. | CUD+ AUD participants met criteria for current CUD and AUD and had >100 lifetime CU episodes | Community | ≥48-h | 1.5 T | Spatial WM task | WM vs. Simple Attention/Vigilance Trial contrast | Analysis: WB, one-way ANOVAs FWHM: 3.5 mm MC: WB: k > 1072 μL (25 voxels), cluster corrected p < 0.016 Sampling: CU vs. TD group comparison | SWM > Attention: MJ + AUD > CON: R superior frontal and middle frontal gyri MJ + AUD < CON: R inferior frontal gyrus R superior temporal and supramarginal gyrus SWM < Attention: MJ + AUD > CON: L inferior frontal gyrus B/L inferior frontal and anterior cingulate gyri MJ + AUD < CON: None |
| Schweinsburg et al., 2008 [17] | N = 15 heavy CU youth and N = 17 healthy CON youth ages 16–18 yrs. | CU: 73% CON: 71% | CU: 18.1 yrs. CON: 17.9 yrs. | CU participants had an average of 480.7 lifetime use episode | Community | ≥28-days | 1.5 T | Spatial WM task | SWM vs. Attention/Vigilance trial contrast | Analysis: WB, independent sample t-tests FWHM: 5 mm MC: WB: k > 1328 μL, cluster corrected p < 0.05 Sampling: CU vs. TD group comparison | SWM > Vigilance: MJ > CON: R superior parietal gyrus MJ < CON: R middle frontal gyrus SWM < Vigilance: MJ > CON: R cuneus L lingual gyrus and cuneus MJ < CON: none |
| Schweinsburg et al., 2010 [66] | N = 13 recent CU youth, N = 13 abstinent CU youth, and N = 18 healthy CON youth ages 15–18 yrs. | Recent CU: 69% Abstinent CU: 69% CON: 61% | Recent CU: 17.1 yrs. Abstinent CU: 17.6 yrs. CON: 17.3 yrs. | Recent CU: 342 lifetime CU episodes Abstinent CU: 515 lifetime CU episodes | Community | Recent Users: ≥24-h; Abstinent Users: ≥27-days | 1.5 T | Spatial WM task | SWM vs. vigilance contrasts | Analysis: WB, independent sample t-tests FWHM: 5 mm MC: k > 1328 µL (49 voxels), t > 2.06, cluster corrected p < 0.05 Sampling: CU vs. TD group comparison | Recent CU > Abstinent CU: Medial cingulate MFG L SFG and MFG Bilateral mPFC Bilateral insula L precentral gyrus R IFG Abstinent CU > Recent CU: Right precentral gyrus Post Hoc Pairwise Comparisons: Recent CU showed increased bilateral mPFC and insula activation to SWM compared to vigilance condition, while Abstinent CU showed decreased activation and CON showed no activation differences in these regions to SWM vs. vigilance condition. Recent CU showed decreased R precentral gyrus activation to SWM compared to vigilance condition, while abstinent CU and CON showed no activation difference during SWM vs. vigilance in this region. |
| Schweinsburg et al., 2011 [38] | N = 8 MJ users, N = 16 BD, N = 28 MJ + BD, and N = 22 healthy CON adolescents | MJ: 50% BD: 81% MJ+ BD: 82% CON: 73% | MJ: 18.1 yrs. BD: 18.1 yrs. MJ + BD: 18.0 yrs. CON: 17.6 yrs. | MJ and MJ + BD groups both had >180 lifetime MJ use episodes | Community | ≥21-days | 3.0 T | Verbal Paired Associations Test | Primary contrast: BOLD response to novel word pairs | Analysis: WB + hippocampal ROI, ANOVAs FWHM: 5 mm MC: WB k > 1512 µL, cluster corrected p < 0.05 Sampling: CU vs. TD group comparison | Main effect of Marijuana Use: (Examined by collapsing across Subgroups): MJ and BD + MJ > CON and BD: None MJ and BD + MJ < CON and BD: None Drinking x Marijuana Interaction: (Whole-brain): L superior and middle frontal gyri R inferior and middle frontal gyri R superior and middle frontal gyri Medial cuneus/lingual gyrus Post Hoc Pairwise Group Comparisons from Drinking X MJ interaction: MJ > CON and BD + MJ: L superior and middle frontal gyri MJ and BD > CON: R superior and middle frontal gyri MJ > CON: R middle and inferior frontal gyri MJ and BD < CON: b/l cuneus and lingual gyri |
| Smith et al., 2010 [30] | N = 10 current CU young adults and N = 14 non-using CON young adults ages 19–21 yrs. | CU: 60% CON: 64% | CU: 20.0 yrs. CON: 20.0 yrs. | All CU participants used cannabis weekly | Community | Ad-lib use | 1.5 T | N-Back WM task (Visuospatial 2-back task) | 2-back vs. 0-back contrast | Analysis: WB, two-sample t-tests FWHM: 8 mm MC: WB: Cluster corrected p < 0.05 Sampling: CU vs. TD group comparison | Behavioral: No group differences in WM performance on 2-back and 0-back 2-back vs. 0-back fMRI contrast: MJ > CON: R inferior frontal gyrus R superior temporal gyrus and temporal pole R cingulate gyrus MJ < CON: None |
| Tapert et al., 2007 [19] | N = 16 CU adolescdents and N = 17 healthy CON adolescents ages 16–18 yrs. | CU: 75% CON: 71% | CU: 18.1 yrs. CON: 17.9 yrs. | CU group endoresed and avg. of 500 lifetime CU episodes | Community (California high schools) | >28-days | 1.5 T | Go/No-Go task | Inhibition (No-Go) Trials vs. Baseline contrast (primary outcome); Go Trials vs. Baseline contrast | Analysis: WB, independent sample t-tests FWHM: 3.5 mm MC: WB: k > 22 voxels, Cluster corrected p < 0.05 Sampling: CU vs. TD group comparison | Inhibition (No-Go) trial contrast: MJ > CON: R Superior and middle frontal gyrus R middle frontal gyrus and insula L middle and superior frontal gyri b/l medial frontal cortex R inferior and superior parietal lobes L inferior and superior parietal lobes R lingual and middle occipital gyrus MJ < CON:None Go trial contrast; MJ > CON: R inferior frontal gyrus and insula R superior and middle frontal gyurs R superior parietal lobe R inferior parietal lobe R medial precuneus MJ < CON: None |
| Tervo-Clemmens et al., 2018 [67] | N = 85 participants completed a baseline MRI session at age 12-yrs and then followed up at age 15-yrs. At follow-up: N = 22 participants were CU and N = 63 were non-CU | CU group: 55% Non-CU group: 46% | CU group: 15.6 yrs. Non-CU group: 15.6 yrs. | Community (longitudinal sample of US youth enriched for SUD risk) | ≥24-h | 3.0 T | Spatial WM task | BOLD response during successful/correct WM trials at age 12 baseline visit and age 15 follow-up visit for MJ preusers/users and non-using CON. | Analysis: WB, Multivariate Models FWHM: 5 mm MC: WB: k > 11, voxelwise p < 0.005, cluster corrected pFDR < 0.05 (done w/AFNI 3dClustSIM) Sampling: CU vs. TD group comparison | Baseline group comparison: MJ Pre-Users > CON Non-Pre-Users: b/l MFG L inferior parietal lobule Paracentral lobule/cingulate gyrus MJ Pre-Users < CON Non-Pre-Users: b/l lingual gyrus L precuneus Pre-SMA L lateral occipital gyrus Follow-up group comparison: MJ users > CON: None MJ users < CON: R Cuneus Post hoc analysis of follow-up data showed a significant negative correlation between BOLD response in the R cuneus cluster and cannabis dose Group-by-Time effect: Posterior cingulate cortex | |
| Thayer et al., 2015 [68] | N = 80 high-risk adolescents with variable cannabis and alcohol use behaviors | Total sample: 74% | Total sample: 15.9 yrs. | Total sample reported a past-3-month avg. of 7–9 hits of MJ on 4–5 occasions per month and 2–3 drinks per month | Justice system (juvenile justice program in SW United States) | NP | 3.0 T | Stroop Color-Word Interference task | Contrasts of interest: Incongruent—Neutral and Incongruent—Congruent contrasts during correct trials | Analysis: WB, GLM FWHM: 8 mm MC: WB: k > 2496 µL, voxelwise: p < 0.005, Cluster corrected p < 0.05 (done w/AFNI 3dClustSIM) Sampling: Combined Sample | Incongruent—Neutral contrast: No main or interaction effects of MJ frequency on BOLD response Incongruent—Congruent contrast: No main or interaction effects of MJ frequency on BOLD response |
| Zhou et al., 2019 [69] | N = 26 Dependent MJ users, N = 25 Non-Dependent MJ users, and N = 52 healthy CON youth | Dep MJ: 100% Non-Dep MJ: 100% CON: 100% | Dep MJ: 22.9 yrs. Non-Dep MJ: 21.5 yrs. CON: 23.2 yrs. | Dep. MJ: 1538 g lifetime use Non-Dep MJ: 985 g lifetime use | Community | ≥24-h | 3.0 T | Drug Cue-reactivity task | Cannabis cue vs. neutral cue contrast | Analysis: WB + dorsal and ventral striatal ROI, mixed ANOVAs, t-tests FWHM: 6 mm MC: voxelwise p < 0.001, Cluster corrected pFWE < 0.05 Sampling: CU vs. TD group comparison | Cannabis vs. Neutral cue: Non-dependent MJ > CON: Ventral caudate Nucleus accumbens Superior parietal lobe and precuneus Non-dependent MJ < CON: None Dependent MJ > CON: Limbic lobe extending to temporal, occipital, and parietal lobes R inferior frontal gyrus extending to middle frontal gyrus L superior frontal gyrus extending to middle frontal gyrus L IPL extending to posterior cingulate cortex and precuneus L fusiform R inferior frontal gyrus Medial PFC extending to anterior cingulate cortex L inferior frontal gyrus extending to middle frontal gyrus L inferior frontal gyrus Dependent MJ < CON: None |
| Zimmerman et al., 2017 [70] | N = 23 regular recreational CU young adults and N = 22 non-using matched CON young adults | CU: 100% CON: 100% | CU: 21.2 yrs. CON: 21.1 yrs. | All CU participants used cannabis >3 times per week over the past-yr. and had >200 lifetime use episodes | Community | ≥48-h | 3.0 T | Cognitive reappraisal task | Primary contrast: distance vs. baseline contrast (emotion regulation using cognitive reappraisal). Secondary contrast: spontaneous negative vs. baseline contrast (emotional reactivity to negative stimuli) | Analysis: WB + amygdala ROI, GLM, also seed-based amygdala-ROI FC analysis FWHM: 8 mm MC: WB: pFWE < 0.05 Sampling: CU vs. TD group comparison | Distance vs. bsl contrast: MJ > CON: b/l precentral gyrus R superior frontal gyrus L mid-cingulate/SMA L precentral gyrus R amygdala MJ < CON: None Emotion reactivity: Spontaneous negative vs. bsl: No group differences |
| MNI Coordinates | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cluster #, Label | BA | Voxels | x | y | z | SDM-Z | p-Value | ||
| All studies (45 studies) | |||||||||
| CU > TD youth | |||||||||
| None | |||||||||
| CU < TD youth | |||||||||
| None | |||||||||
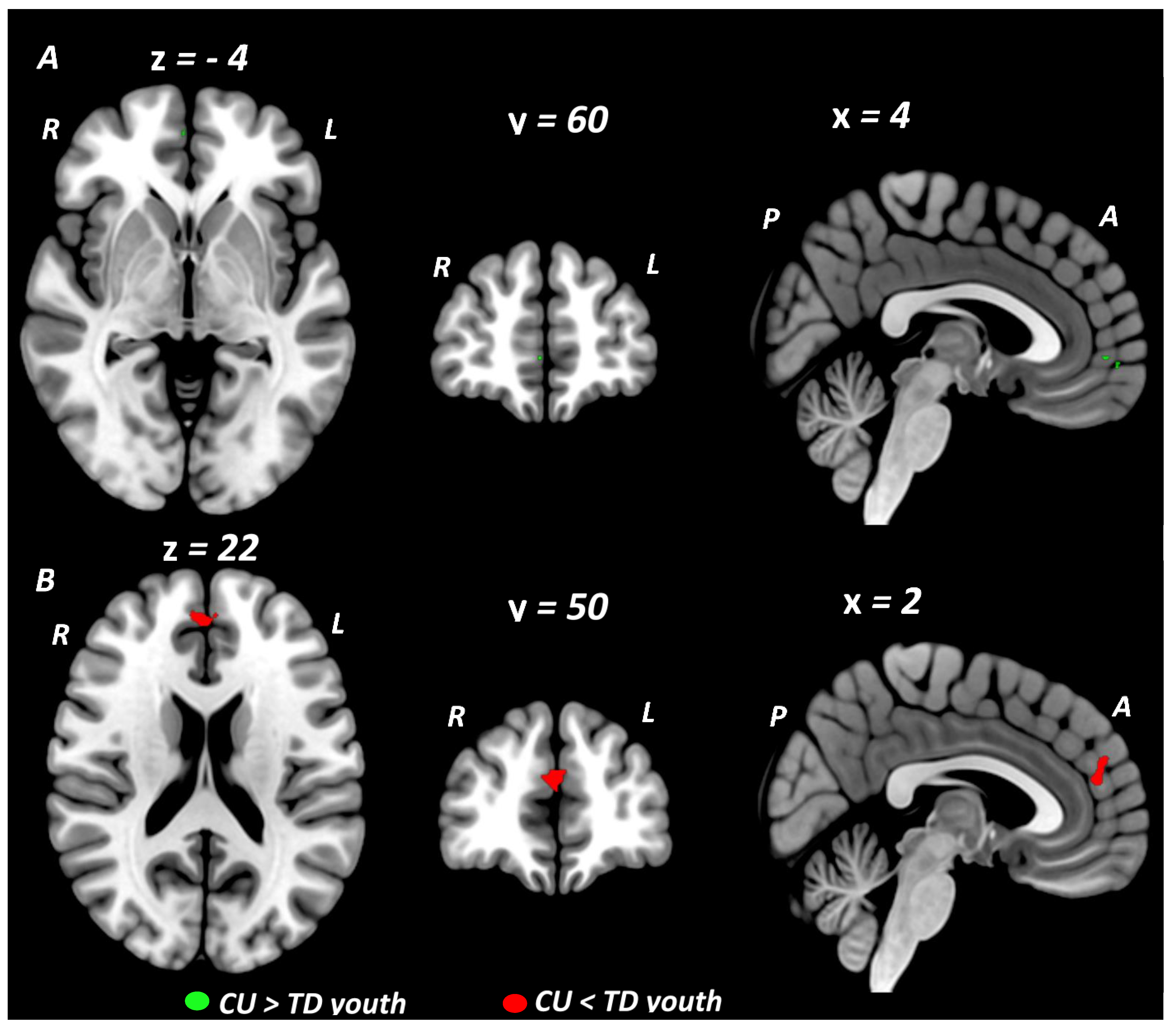
| Executive Function/Cognitive Control studies (16 studies) | |||||||||
| CU > TD youth | |||||||||
| Cluster #1 Right rostral mPFC | 10, 11 | 5 | 4 | 60 | −4 | 2.615 | 0.0044618 | ||
| CU < TD youth | |||||||||
| None | |||||||||
| Social Cognition/Emotion Processing studies (9 studies) | |||||||||
| CU > TD youth | |||||||||
| None | |||||||||
| CU < TD youth | |||||||||
| Cluster #2 Left dorsal mPFC Right dorsal mPFC Left dorsal ACC Right dorsal ACC | 10, 32 | 64 | 2 | 50 | 22 | −3.100 | 0.0009676 | ||
| Reward Processing studies (8 studies) | |||||||||
| CU > TD youth | |||||||||
| None | |||||||||
| CU < TD youth | |||||||||
| None | |||||||||
| Drug Cue Reactivity studies (5 studies) | |||||||||
| CU > TD youth | |||||||||
| None | |||||||||
| CU < TD youth | |||||||||
| None | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammond, C.J.; Allick, A.; Park, G.; Rizwan, B.; Kim, K.; Lebo, R.; Nanavati, J.; Parvaz, M.A.; Ivanov, I. A Meta-Analysis of fMRI Studies of Youth Cannabis Use: Alterations in Executive Control, Social Cognition/Emotion Processing, and Reward Processing in Cannabis Using Youth. Brain Sci. 2022, 12, 1281. https://doi.org/10.3390/brainsci12101281
Hammond CJ, Allick A, Park G, Rizwan B, Kim K, Lebo R, Nanavati J, Parvaz MA, Ivanov I. A Meta-Analysis of fMRI Studies of Youth Cannabis Use: Alterations in Executive Control, Social Cognition/Emotion Processing, and Reward Processing in Cannabis Using Youth. Brain Sciences. 2022; 12(10):1281. https://doi.org/10.3390/brainsci12101281
Chicago/Turabian StyleHammond, Christopher J., Aliyah Allick, Grace Park, Bushra Rizwan, Kwon Kim, Rachael Lebo, Julie Nanavati, Muhammad A. Parvaz, and Iliyan Ivanov. 2022. "A Meta-Analysis of fMRI Studies of Youth Cannabis Use: Alterations in Executive Control, Social Cognition/Emotion Processing, and Reward Processing in Cannabis Using Youth" Brain Sciences 12, no. 10: 1281. https://doi.org/10.3390/brainsci12101281
APA StyleHammond, C. J., Allick, A., Park, G., Rizwan, B., Kim, K., Lebo, R., Nanavati, J., Parvaz, M. A., & Ivanov, I. (2022). A Meta-Analysis of fMRI Studies of Youth Cannabis Use: Alterations in Executive Control, Social Cognition/Emotion Processing, and Reward Processing in Cannabis Using Youth. Brain Sciences, 12(10), 1281. https://doi.org/10.3390/brainsci12101281





