High-Intensity Interval Training-Induced Hippocampal Molecular Changes Associated with Improvement in Anxiety-like Behavior but Not Cognitive Function in Rats with Type 2 Diabetes
Abstract
1. Introduction
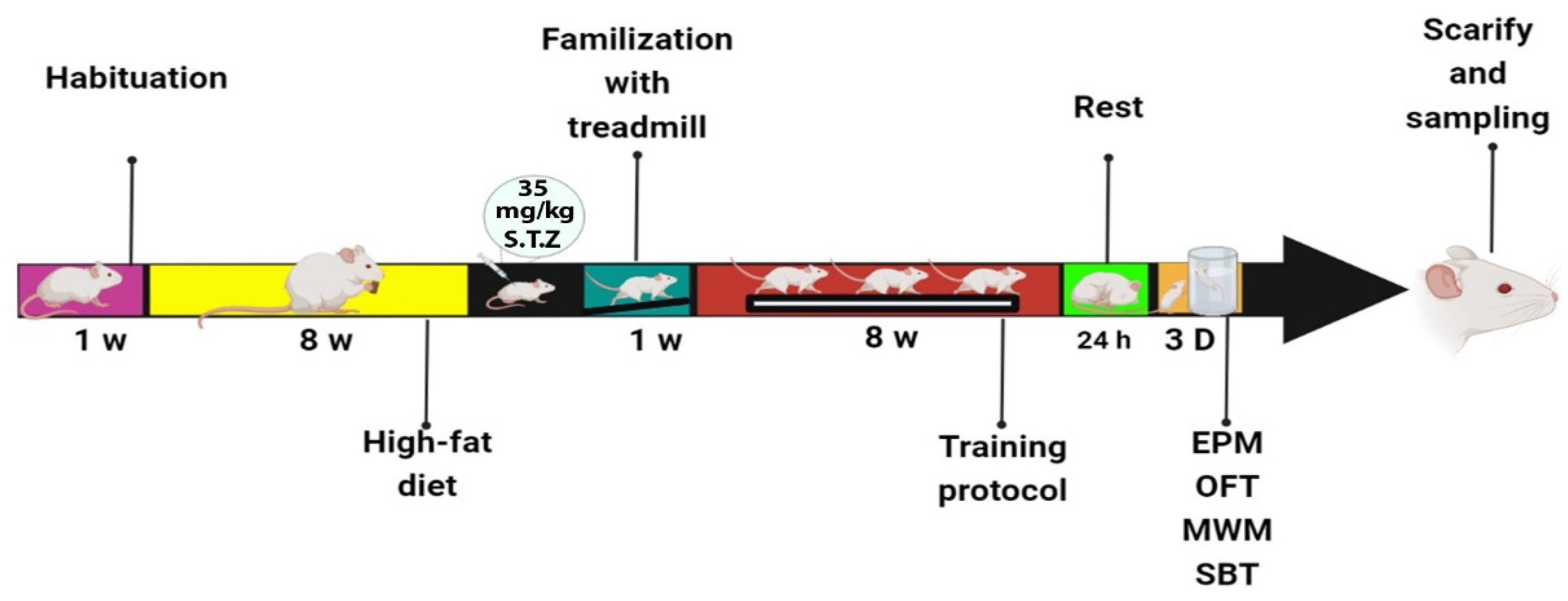
2. Materials and Methods
2.1. High-Fat Diet
2.2. High-Intensity Interval Training Protocol (HIIT)
2.3. Open Field Test (OFT)
2.4. Elevated Plus Maze (EPM)
2.5. Morris Water Maze (MWM)
2.6. Shuttle Box Test
2.7. Western Blot
2.8. Statistical Analysis
3. Results
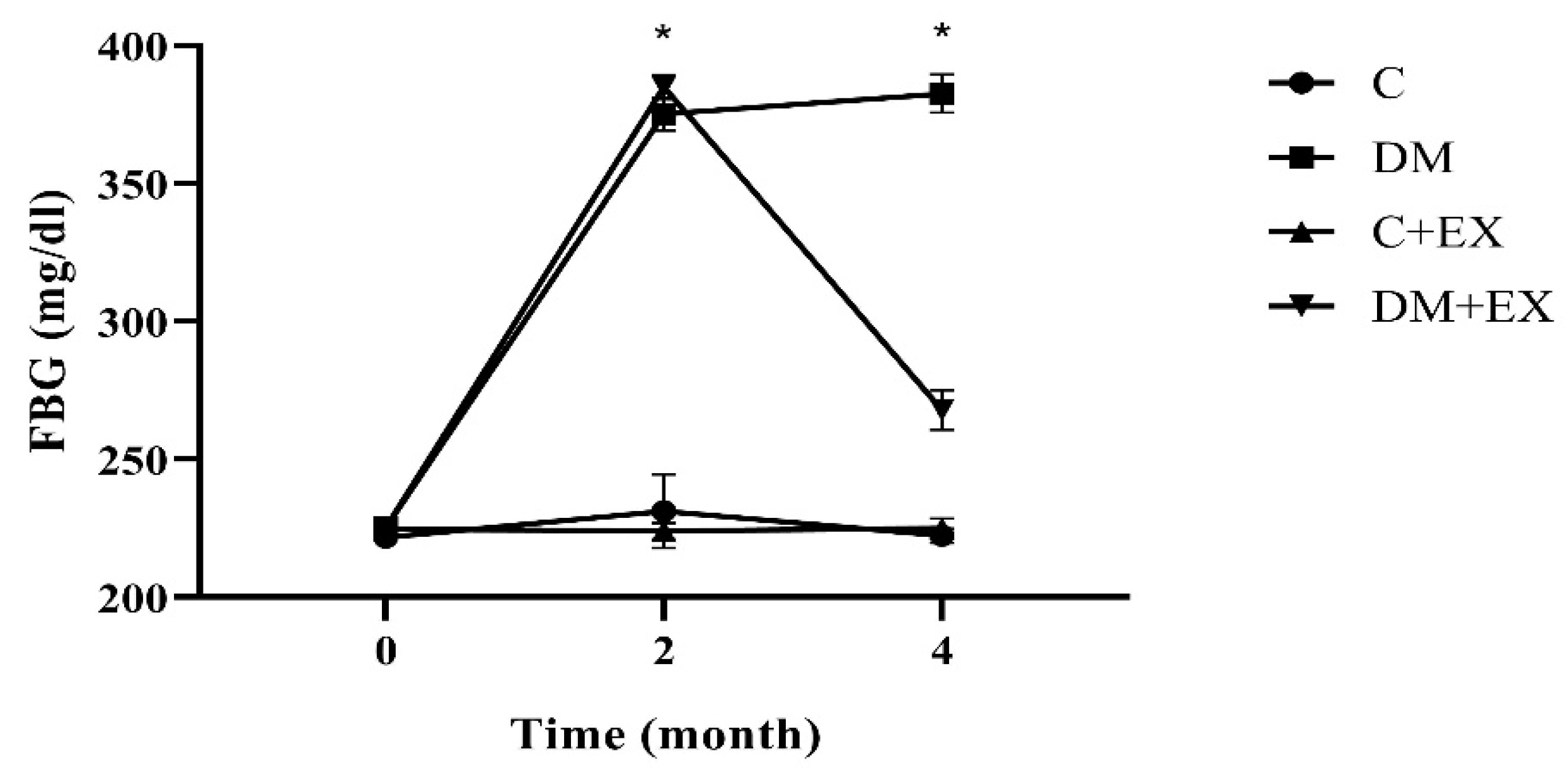
3.1. The Fasting Blood Glucose Results
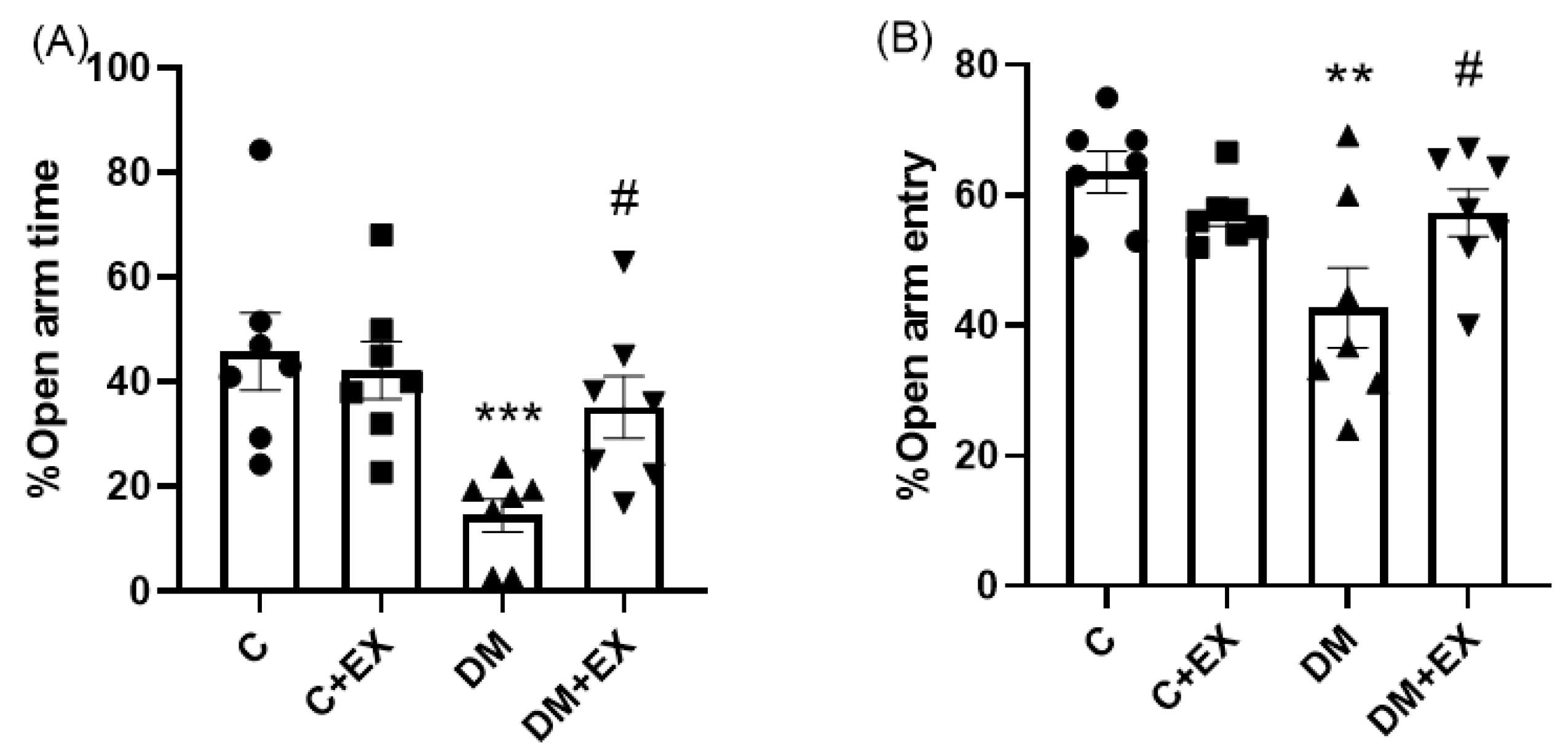
3.2. The Effects of HIIT on the %Open Arm Time (%OAT( and %Open Arm Entry (%OAE) of Rats with T2D in the Elevated Plus Maze (EPM)
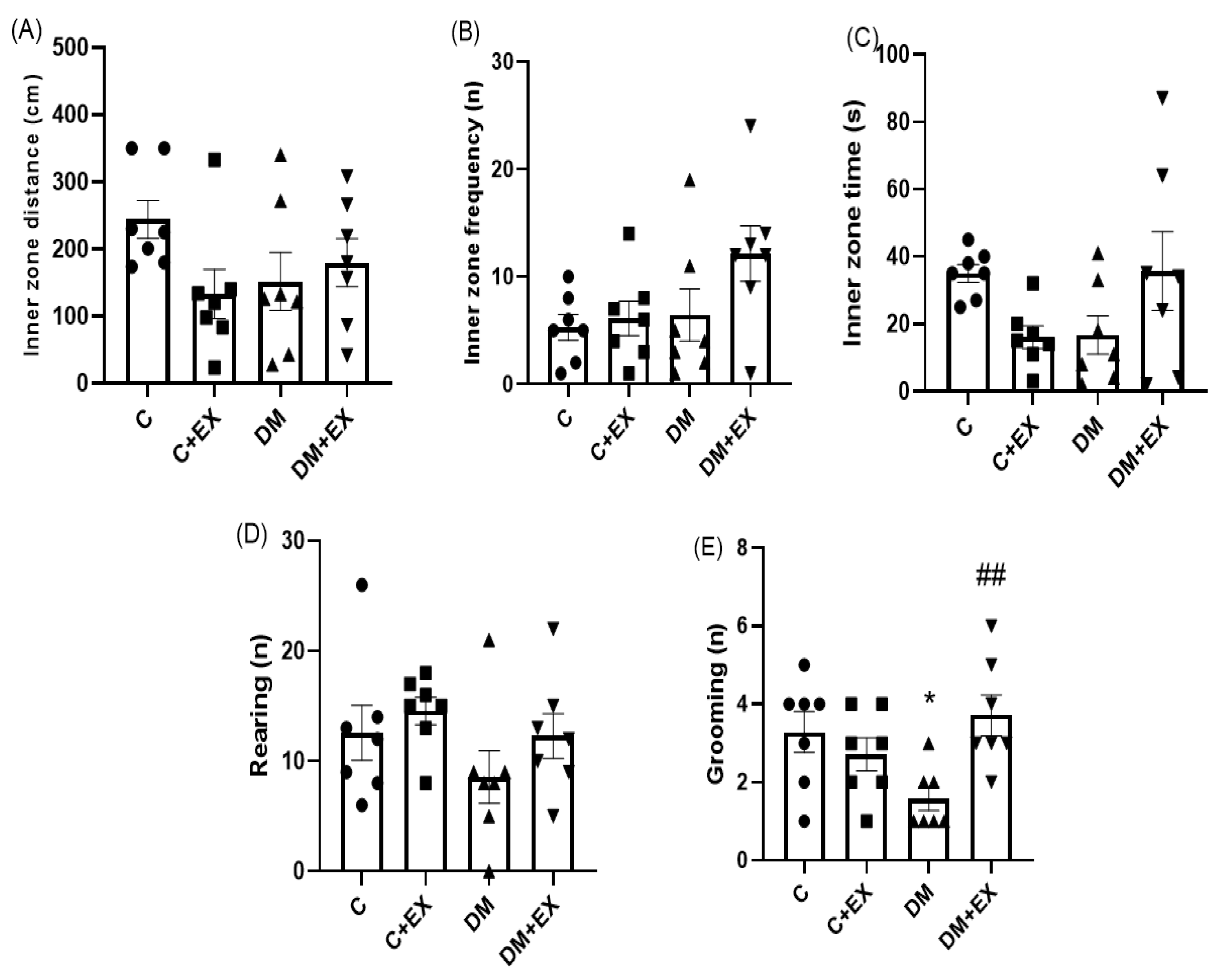
3.3. The Effects of HIIT on the Inner Zone Distance, Inner Zone Frequency, Inner Zone Time, and Rearing and Grooming of Rats with T2D in the Open Field Test (OFT)
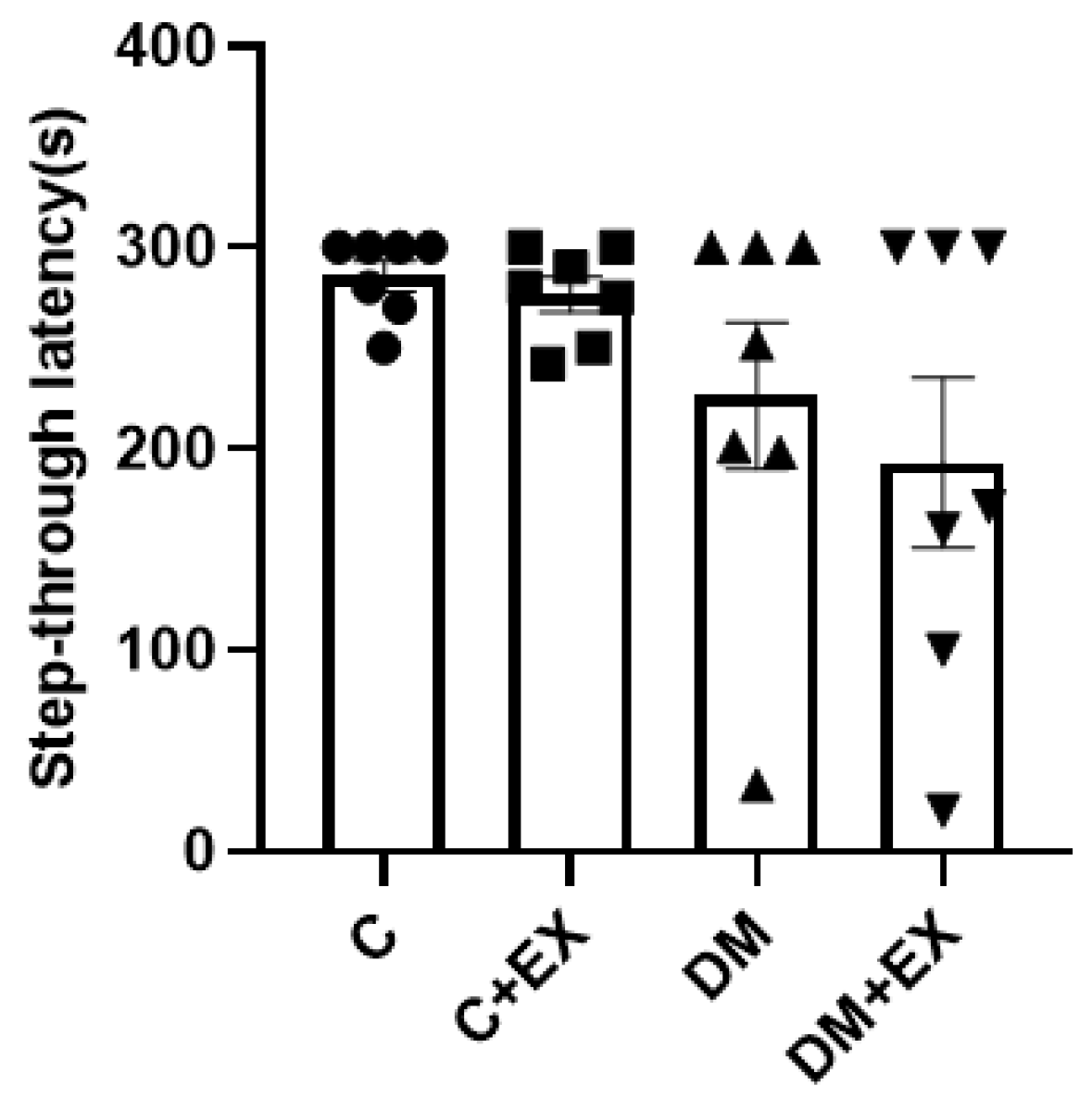
3.4. The Effects of HIIT on the Learning of Rats with T2D in the Shuttle Box
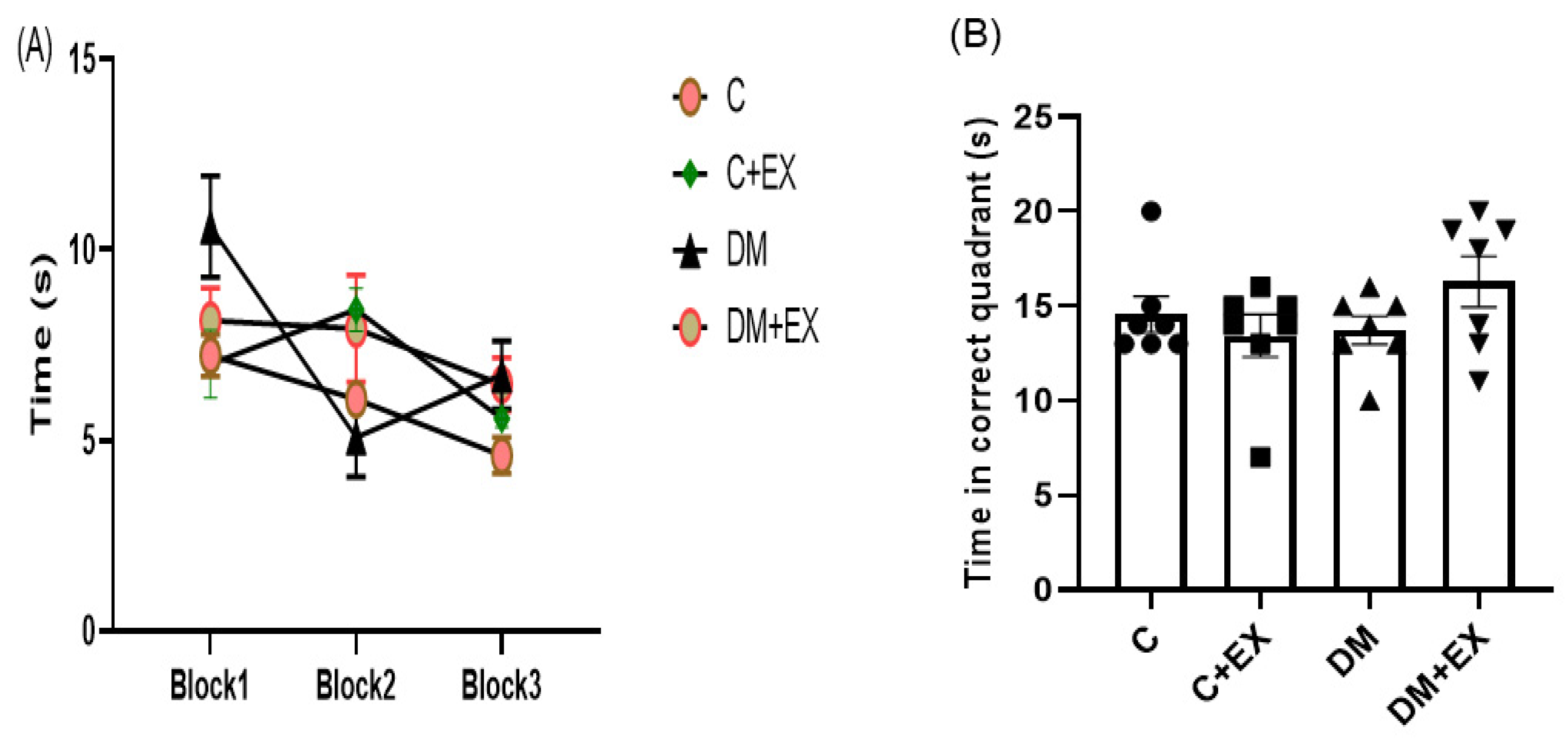
3.5. The Effects of HIIT on the Learning and Memory of Rats with T2D in the Morris Water Maze (MWM)
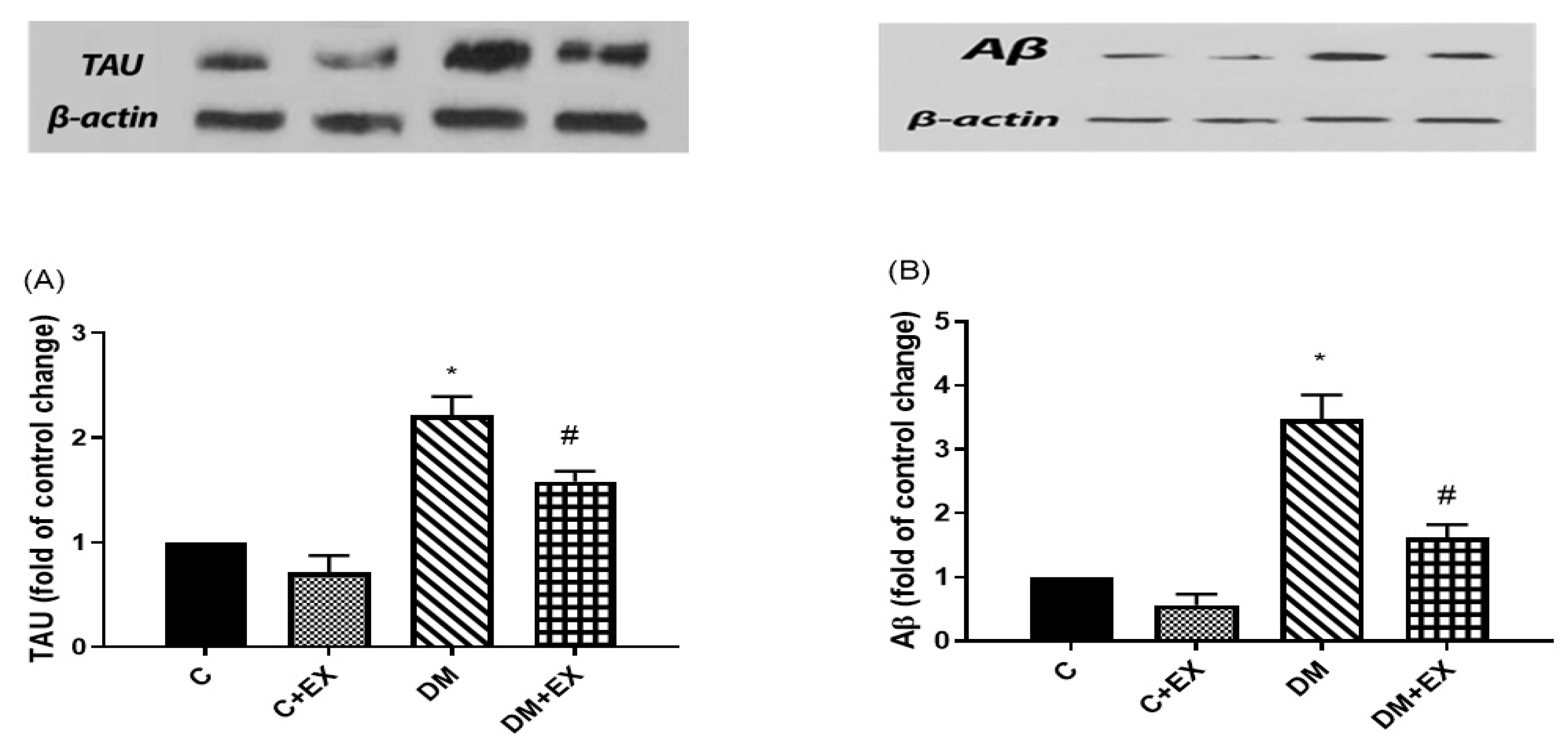
3.6. The Effects of HIIT on the Tau and Aβ Protein Level in the Hippocampus of Rats with T2D
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaul, K.; Tarr, J.M.; Ahmad, S.I.; Kohner, E.M.; Chibber, R. Introduction to diabetes mellitus. In Diabetes; Springer: New York, NY, USA, 2013; pp. 1–11. [Google Scholar]
- Alam, U.; Asghar, O.; Azmi, S.; Malik, R.A. General aspects of diabetes mellitus. Handb. Clin. Neurol. 2014, 126, 211–222. [Google Scholar]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2004, 27 (Suppl. S1), S5–S10. [Google Scholar] [CrossRef] [PubMed]
- Blair, M. Diabetes mellitus review. Urol. Nurs. 2016, 36, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Kharroubi, A.T.; Darwish, H.M. Diabetes mellitus: The epidemic of the century. World J. Diabetes 2015, 6, 850. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Seo, H.I.; Cha, H.Y.; Yang, Y.J.; Kwon, S.H.; Yang, S.J. Diabetes and Alzheimer’s Disease: Mechanisms and Nutritional Aspects. Clin. Nutr. Res. 2018, 7, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Muacevic, A.; Adler, J.; Nisar, O.; Pervez, H.; Mandalia, B.; Waqas, M.; Sra, H. Type 3 Diabetes Mellitus: A Link Between Alzheimer’s Disease and Type 2 Diabetes Mellitus. Cureus 2021, 12. [Google Scholar] [CrossRef]
- Diniz Pereira, J.; Gomes Fraga, V.; Morais Santos, A.L.; Carvalho, M.D.G.; Caramelli, P.; Braga Gomes, K. Alzheimer’s disease and type 2 diabetes mellitus: A systematic review of proteomic studies. J. Neurochem. 2021, 156, 753–776. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Ta, Q.T.H.; Nguyen, T.K.O.; Nguyen, T.T.D.; Van Giau, V. Type 3 diabetes and its role implications in Alzheimer’s disease. Int. J. Mol. Sci. 2020, 21, 3165. [Google Scholar] [CrossRef]
- Castellani, R.J.; Rolston, R.K.; Smith, M.A. Alzheimer disease. Dis.-A-Mon. DM 2010, 56, 484. [Google Scholar] [CrossRef]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological Alterations in Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef]
- Lopes, S.; Vaz-Silva, J.; Pinto, V.; Dalla, C.; Kokras, N.; Bedenk, B.; Mack, N.; Czisch, M.; Almeida, O.F.X.; Sousa, N.; et al. Tau protein is essential for stress-induced brain pathology. Proc. Natl. Acad. Sci. USA 2016, 113, E3755–E3763. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, R.A.L.; Santos, L.G.; Lopes, P.M.; Cavalcante, B.R.R.; Improta-Caria, A.C.; Cassilhas, R.C. Physical exercise consequences on memory in obesity: A systematic review. Obes. Rev. 2021, 22, e13298. [Google Scholar] [CrossRef] [PubMed]
- Tonoli, C.; Heyman, E.; Buyse, L.; Roelands, B.; Piacentini, M.F.; Bailey, S.; Pattyn, N.; Berthoin, S.; Meeusen, R. Neurotrophins and cognitive functions in T1D compared with healthy controls: Effects of a high-intensity exercise. Appl. Physiol. Nutr. Metab. 2015, 40, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Forny-Germano, L.; De Felice, F.G.; Vieira, M.N.D.N. The role of leptin and adiponectin in obesity-associated cognitive decline and Alzheimer’s disease. Front. Neurosci. 2019, 12, 1027. [Google Scholar] [CrossRef] [PubMed]
- Duzel, E.; van Praag, H.; Sendtner, M. Can physical exercise in old age improve memory and hippocampal function? Brain 2016, 139, 662–673. [Google Scholar] [CrossRef]
- Leite, A.B.; Lima, H.N.; Flores, C.D.O.; Oliveira, C.A.; Cunha, L.E.C.; Neves, J.L.; Correia, T.M.L.; de Melo, F.F.; Oliveira, M.V.; de Magalhães, A.C.M.; et al. High-intensity interval training is more effective than continuous training to reduce inflammation markers in female rats with cisplatin nephrotoxicity. Life Sci. 2020, 266, 118880. [Google Scholar] [CrossRef] [PubMed]
- Chéilleachair, N.N.; Harrison, A.J.; Warrington, G.D. HIIT enhances endurance performance and aerobic characteristics more than high-volume training in trained rowers. J. Sports Sci. 2016, 37, 1052–1058. [Google Scholar] [CrossRef]
- Abbasi, S.; Khaledi, N.; Askari, H. High intensity interval training increases the expression of hippocampus BDNF gene and decreases the serum tnf-α in Diabetic Rat. Med. J. Tabriz Univ. Med. Sci. 2020, 42, 591–600. [Google Scholar] [CrossRef]
- Kim, T.-W.; Baek, K.-W.; Yu, H.S.; Ko, I.-G.; Hwang, L.; Park, J.-J. High-intensity exercise improves cognitive function and hippocampal brain-derived neurotrophic factor expression in obese mice maintained on high-fat diet. J. Exerc. Rehabil. 2020, 16, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.M.; Peiffer, J.; Rainey-Smith, S.R. Exploring the relationship between physical activity, beta-amyloid and tau: A narrative review. Ageing Res. Rev. 2019, 50, 9–18. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Y.; Wang, Y.; Song, L.; Zhang, R.; Du, Y. Long-term treadmill exercise attenuates Aβ burdens and astrocyte activation in APP/PS1 mouse model of Alzheimer’s disease. Neurosci. Lett. 2018, 666, 70–77. [Google Scholar] [CrossRef]
- Tan, Z.-X.; Dong, F.; Wu, L.-Y.; Feng, Y.-S.; Zhang, F. The Beneficial Role of Exercise on Treating Alzheimer’s Disease by Inhibiting β-Amyloid Peptide. Mol. Neurobiol. 2021, 58, 5890–5906. [Google Scholar] [CrossRef]
- Dolan, P.J.; Johnson, G.V. The role of tau kinases in Alzheimer’s disease. Curr. Opin. Drug Discov. Dev. 2010, 13, 595–603. [Google Scholar]
- Elahi, M.; Motoi, Y.; Matsumoto, S.-E.; Hasan, Z.; Ishiguro, K.; Hattori, N. Short-term treadmill exercise increased tau insolubility and neuroinflammation in tauopathy model mice. Neurosci. Lett. 2016, 610, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Eliza, J.; Daisy, P.; Ignacimuthu, S.; Duraipandiyan, V. Antidiabetic and antilipidemic effect of eremanthin from Costus speciosus (Koen.)Sm., in STZ-induced diabetic rats. Chem. Interact. 2009, 182, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Racil, G.; Ben Ounis, O.; Hammouda, O.; Kallel, A.; Zouhal, H.; Chamari, K.; Amri, M. Effects of high vs. moderate exercise intensity during interval training on lipids and adiponectin levels in obese young females. Eur. J. Appl. Physiol. 2013, 113, 2531–2540. [Google Scholar] [CrossRef]
- Lee, M.R.; Kim, J.E.; Choi, J.Y.; Park, J.J.; Kim, H.R.; Song, B.R.; Choi, Y.W.; Kim, K.M.; Song, H.; Hwang, D.Y. Anti-obesity effect in high-fat-diet-induced obese C57BL/6 mice: Study of a novel extract from mulberry (Morus alba) leaves fermented with Cordyceps militaris. Exp. Ther. Med. 2019, 17, 2185–2193. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Son, C.; Aotani, D.; Nomura, H.; Hikida, T.; Hosoda, K.; Nakao, K. Role of leptin in conditioned place preference to high-fat diet in leptin-deficient ob/ob mice. Neurosci. Lett. 2017, 640, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Freitas, D.A.; Rocha-Vieira, E.; Soares, B.A.; Nonato, L.F.; Fonseca, S.R.; Martins, J.B.; Mendonça, V.A.; Lacerda, A.C.; Massensini, A.; Poortamns, J.R.; et al. High intensity interval training modulates hippocampal oxidative stress, BDNF and inflammatory mediators in rats. Physiol. Behav. 2018, 184, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Hamidkhaniha, S.; Bashiri, H.; Omidi, A.; Hosseini-Chegeni, A.; Tavangar, S.M.; Sabouri, S.; Montazeri, H.; Sahebgharani, M. Effect of pretreatment with intracerebroventricular injection of minocycline on morphine-induced memory impairment in passive avoidance test: Role of P-CREB and c-Fos expression in the dorsal hippocampus and basolateral amygdala regions. Clin. Exp. Pharmacol. Physiol. 2019, 46, 711–722. [Google Scholar] [CrossRef]
- Bashiri, H.; Rezayof, A.; Sahebgharani, M.; Tavangar, S.M.; Zarrindast, M.-R. Modulatory effects of the basolateral amygdala α2-adrenoceptors on nicotine-induced anxiogenic-like behaviours of rats in the elevated plus maze. Neuropharmacology 2016, 105, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Vakili Shahrbabaki, S.S.; Jonaidi, H.; Sheibani, V.; Bashiri, H. Early postnatal handling alters social behavior, learning, and memory of pre- and postnatal VPA-induced rat models of autism in a context-based manner. Physiol. Behav. 2022, 249, 113739. [Google Scholar] [CrossRef]
- Inoue, D.S.; Monteiro, P.A.; Gerosa-Neto, J.; Santana, P.R.; Peres, F.P.; Edwards, K.M.; Lira, F.S. Acute increases in brain-derived neurotrophic factor following high or moderate-intensity exercise is accompanied with better cognition performance in obese adults. Sci. Rep. 2020, 10, 13493. [Google Scholar] [CrossRef] [PubMed]
- Narwal, S.; Saini, D.; Kumari, K. Behavior and Pharmacological Animal Models for the Evaluation of Learning & Memory Condition. Indo-Glob. Res. J. Pharm. Sci. 2001, 1, 121–129. [Google Scholar]
- Frankenberg ADv Reis, A.F.; Gerchman, F. Relationships between adiponectin levels, the metabolic syndrome, and type 2 diabetes: A literature review. Arch. Endocrinol. Metab. 2017, 61, 614–622. [Google Scholar] [CrossRef]
- Anderson, R.J.; Freedland, K.E.; Clouse, R.E.; Lustman, P.J. The prevalence of comorbid depression in adults with diabetes: A meta-analysis. Diabetes Care 2001, 24, 1069–1078. [Google Scholar] [CrossRef]
- Egede, L.E.; Zheng, D.; Simpson, K. Comorbid depression is associated with increased health care use and expenditures in individuals with diabetes. Diabetes Care 2002, 25, 464–470. [Google Scholar] [CrossRef]
- Šurkienė, G.; Mikaliūkštienė, A.; Zagminas, K.; Juozulynas, A.; Narkauskaitė, L.; Sąlyga, J.; Jankauskiene, K.; Stukas, R. Prevalence and determinants of anxiety and depression symptoms in patients with type 2 diabetes in Lithuania. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2014, 20, 182. [Google Scholar] [CrossRef]
- Xia, G.; Han, Y.; Meng, F.; He, Y.; Srisai, D.; Farias, M.; Dang, M.; Palmiter, R.D.; Xu, Y.; Wu, Q. Reciprocal control of obesity and anxiety–depressive disorder via a GABA and serotonin neural circuit. Mol. Psychiatry 2021, 26, 2837–2853. [Google Scholar] [CrossRef]
- Caliskan, H.; Akat, F.; Omercioglu, G.; Bastug, G.; Ficicilar, H.; Bastug, M. Aerobic exercise has an anxiolytic effect on streptozotocin-induced diabetic rats. Acta Neurobiol. Exp. 2020, 80, 245–255. [Google Scholar] [CrossRef]
- Aksu, I.; Ates, M.; Baykara, B.; Kiray, M.; Sisman, A.R.; Buyuk, E.; Baykara, B.; Cetinkaya, C.; Gümüş, H.; Uysal, N. Anxiety correlates to decreased blood and prefrontal cortex IGF-1 levels in streptozotocin induced diabetes. Neurosci. Lett. 2012, 531, 176–181. [Google Scholar] [CrossRef]
- Hilakivi-Clarke, L.; Wozniak, K.; Durcan, M.; Linnoila, M. Behavior of streptozotocin-diabetic mice in tests of exploration, locomotion, anxiety, depression and aggression. Physiol. Behav. 1990, 48, 429–433. [Google Scholar] [CrossRef]
- Bangasser, D.A.; Cuarenta, A. Sex differences in anxiety and depression: Circuits and mechanisms. Nat. Rev. Neurosci. 2021, 22, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Fulk, L.J.; Stock, H.S.; Lynn, A.; Marshall, J.; Wilson, M.A.; Hand, G.A. Chronic physical exercise reduces anxiety-like behavior in rats. Endoscopy 2004, 25, 78–82. [Google Scholar]
- Lin, T. Self-Assembly of Short Peptide Derivatives; The Ohio State University: Columbus, OH, USA, 2021. [Google Scholar]
- Costa, M.S.; Ardais, A.P.; Fioreze, G.T.; Mioranzza, S.; Botton, P.H.S.; Portela, L.V.; Souza, D.O.; Porciúncula, L.O. Treadmill running frequency on anxiety and hippocampal adenosine receptors density in adult and middle-aged rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2012, 36, 198–204. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Braszko, J.J.; Kamiñski, K.A.; Hryszko, T.; Jedynak, W.; Brzósko, S. Diverse effects of prolonged physical training on learning of the delayed non-matching to sample by rats. Neurosci. Res. 2001, 39, 79–84. [Google Scholar] [CrossRef]
- Sabaghi, A.; Heirani, A.; Yousofvand, N.; Sabaghi, S.; Sadeghi, F. Comparison of high-intensity interval training and moderate-intensity continuous training in their effects on behavioral functions and CORT levels in streptozotocin-induced diabetic mice. Sport Sci. Health 2020, 17, 119–126. [Google Scholar] [CrossRef]
- De Sousa, R.A.L.; Improta-Caria, A.C.; de Jesus-Silva, F.M.; Magalhães, C.O.D.e.; Freitas, D.A.; Lacerda, A.C.R.; Mendonça, V.A.; Cassilhas, R.C.; Leite, H.R. High-intensity resistance training induces changes in cognitive function, but not in locomotor activity or anxious behavior in rats induced to type 2 diabetes. Physiol. Behav. 2020, 223, 112998. [Google Scholar] [CrossRef]
- Xie, C.; Alderman, B.L.; Meng, F.; Ai, J.; Chang, Y.-K.; Li, A. Acute high-intensity interval exercise improves inhibitory control among young adult males with obesity. Front. Psychol. 2020, 11, 1291. [Google Scholar] [CrossRef]
- Rooijackers, H.M.; Wiegers, E.C.; van der Graaf, M.; Thijssen, D.H.; Kessels, R.P.; Tack, C.J.; de Galan, B.E. A single bout of high-intensity interval training reduces awareness of subsequent hypoglycemia in patients with type 1 diabetes. Diabetes 2017, 66, 1990–1998. [Google Scholar] [CrossRef]
- Raimundo, A.F.; Ferreira, S.; Martins, I.C.; Menezes, R. Islet Amyloid Polypeptide: A Partner in Crime with Aβ in the Pathology of Alzheimer’s Disease. Front. Mol. Neurosci. 2020, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Fu, X.; Han, R.; Liu, X.; Zhao, X.; Wei, J. Neuroprotective Effect of HIIT against GFAP Hypertrophy through Mitochondrial Dynamics in APP/PS1 Mice. Oxidative Med. Cell. Longev. 2022, 2022, 1764589. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Yang, L.; Li, Y.; Dong, Y.; Yang, B.; Tucker, L.D.; Zong, X.; Zhang, Q. Effects of Exercise Training on Anxious–Depressive-like Behavior in Alzheimer Rat. Med. Sci. Sports Exerc. 2020, 52, 1456–1469. [Google Scholar] [CrossRef]
- Pentkowski, N.S.; Berkowitz, L.E.; Thompson, S.M.; Drake, E.N.; Olguin, C.R.; Clark, B.J. Anxiety-like behavior as an early endophenotype in the TgF344-AD rat model of Alzheimer’s disease. Neurobiol. Aging 2018, 61, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.-J.; Tian, Y.; Ma, Y.-H.; Dong, Q.; Tan, L.; Yu, J.-T. Associations of Anxiety with Amyloid, Tau, and Neurodegeneration in Older Adults without Dementia: A Longitudinal Study. J. Alzheimer’s Dis. 2021, 82, 273–283. [Google Scholar] [CrossRef] [PubMed]
| Diet Ingredients | Fat | Carbohydrate | Protein | Fiber | Mineral | Vitamin |
|---|---|---|---|---|---|---|
| Regular diet | 10% | 70% | 20% | 50 g | 50 g | 3 g |
| High-fat diet | 60% | 20% | 20% | 50 g | 50 g | 3 g |
| Week | Intervals | High-Intensity Interval Duration (min) | Low-Intensity Interval Duration (min) | High-Intensity Interval Velocity (%VMax) | Low-Intensity Interval Velocity (%VMax) | Total Exercise Time in a Session (min) |
|---|---|---|---|---|---|---|
| 1 | 4 | 2 | 1 | 80 | 50 | 12 |
| 2 | 4 | 2 | 1 | 85 | 50 | 12 |
| 3 | 6 | 2 | 1 | 85 | 50 | 18 |
| 4 | 6 | 2 | 1 | 90 | 50 | 21 |
| 5 | 8 | 2 | 1 | 90 | 50 | 24 |
| 6 | 8 | 2 | 1 | 95 | 50 | 24 |
| 7 | 10 | 2 | 1 | 95 | 50 | 30 |
| 8 | 10 | 2 | 1 | 100 | 50 | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orumiyehei, A.; Khoramipour, K.; Rezaei, M.H.; Madadizadeh, E.; Meymandi, M.S.; Mohammadi, F.; Chamanara, M.; Bashiri, H.; Suzuki, K. High-Intensity Interval Training-Induced Hippocampal Molecular Changes Associated with Improvement in Anxiety-like Behavior but Not Cognitive Function in Rats with Type 2 Diabetes. Brain Sci. 2022, 12, 1280. https://doi.org/10.3390/brainsci12101280
Orumiyehei A, Khoramipour K, Rezaei MH, Madadizadeh E, Meymandi MS, Mohammadi F, Chamanara M, Bashiri H, Suzuki K. High-Intensity Interval Training-Induced Hippocampal Molecular Changes Associated with Improvement in Anxiety-like Behavior but Not Cognitive Function in Rats with Type 2 Diabetes. Brain Sciences. 2022; 12(10):1280. https://doi.org/10.3390/brainsci12101280
Chicago/Turabian StyleOrumiyehei, Amin, Kayvan Khoramipour, Maryam Hossein Rezaei, Elham Madadizadeh, Manzumeh Shamsi Meymandi, Fatemeh Mohammadi, Mohsen Chamanara, Hamideh Bashiri, and Katsuhiko Suzuki. 2022. "High-Intensity Interval Training-Induced Hippocampal Molecular Changes Associated with Improvement in Anxiety-like Behavior but Not Cognitive Function in Rats with Type 2 Diabetes" Brain Sciences 12, no. 10: 1280. https://doi.org/10.3390/brainsci12101280
APA StyleOrumiyehei, A., Khoramipour, K., Rezaei, M. H., Madadizadeh, E., Meymandi, M. S., Mohammadi, F., Chamanara, M., Bashiri, H., & Suzuki, K. (2022). High-Intensity Interval Training-Induced Hippocampal Molecular Changes Associated with Improvement in Anxiety-like Behavior but Not Cognitive Function in Rats with Type 2 Diabetes. Brain Sciences, 12(10), 1280. https://doi.org/10.3390/brainsci12101280







