SUNCT/SUNA in Pediatric Age: A Review of Pathophysiology and Therapeutic Options
Abstract
1. Introduction
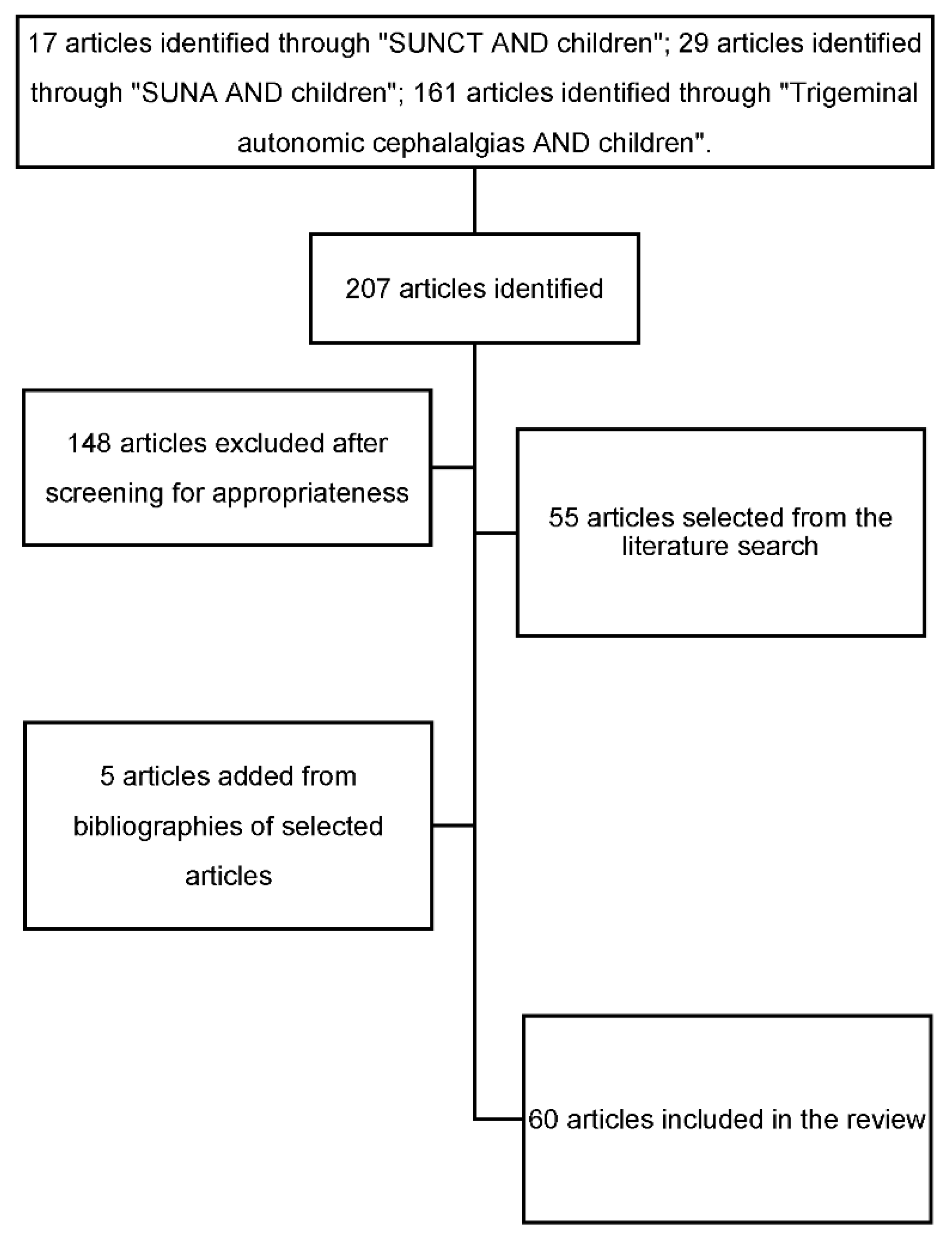
2. Materials and Methods
3. Results
3.1. Etiology
| Reference | Sex | Age at Onset | Diagnosis | Symptoms | Imaging | Therapy and Outcome |
|---|---|---|---|---|---|---|
| D’andrea, G. & Granella, F. 2001 [2] | F | 10 yr | SUNCT | Moderate/severe, right-sided (seldom left-sided), stabbing pain attacks, lasting 2–180 s, 10–180 s per hour over the first 2 months; ipsilateral conjunctival injection, lacrimation and occasional nasal obstruction | Normal MRI and CT | Indomethacin (100 mg daily), other NSAIDs (aspirin, nimesulide, ketoprofen) with no effect; spontaneous remission over 6 months |
| Blattler, T., Capone Mori, A., Boltshause, E. & Bassetti, C. (2003) [14] | F | 11 yr | SUNCT | Moderate/severe, strictly right-sided, sharp pain attacks, lasting 30–60 s, 20 per day; ipsilateral conjunctival injection, lacrimation and salivation | Pylocitic astrocytoma | Indomethacin (100 mg daily): frequency dropped from 20 to 10 per day, with no effect on pain intensity |
| Sékhara, T., Pelc, K., Mewasingh, L. D., Boucquey, D. & Dan, B. (2005) [16] | M | 5 yr | SUNCT | Mostly left-sided, burning or stabbing pain, lasting 2–50 s, 4–6 per hour every 2–3 days; conjunctival injection, lacrimation, nasal congestion | Normal MRI | No medication was administered; spontaneous remission over five months |
| Ünalp, A. & Öztürk, A. (2008) [18] | M | 6 yr | SUNCT | Shooting pain, lasting 5–10 min, 3–4 per day; swelling, rash, ptosis | Normal MRI | Lamotrigine, 25 up to 100 mg/day, with benefit |
| Sciruicchio, V. et al. (2010) [3] | F | 2 yr | SUNCT | Severe, right-sided pain attacks, lasting 5–30 s, 10 per hour, occurring at awakening; impressive ipsilateral conjunctival injection and tearing | Normal MRI | The spontaneous remission within a few hours made prophylactic therapy unnecessary |
| Zhang, Y et al. (2016) [9] | M | 12 yr | SUNCT | Severe, left-sided, lasting 60 s; ipsilateral conjunctival injection and tearing, facial flushing and running nose | Normal MRI | Oral carbamazepine (200 mg daily) discontinued due to an allergic reaction; gabapentin (100 mg) three times daily, pregabalin (75 mg) twice daily, indomethacin (25 mg) three times daily, flunarizine (5 mg) at night, ibuprofen (300 mg) four times daily, topiramate (25 mg) twice daily, methylprednisolone (80 mg) daily, 7–10 L/min of pure oxygen for 10–20 min per day, 2% lidocaine (2 mL) nasal drops, with no changes in the severity or frequency of pain attacks |
| Qaiser, S., Hershey, A.D., Kacperski, J. (2020) [22] | 6 M 7 F | 3–18 yr | SUNCT SUNA | 13 pts: unilateral, stabbing pain attacks, lasting 1 s–10 m, for more than 3 months; 4 pts: conjunctival injection, tearing 2 pts: tearing, eyelid edema 2 pts: facial swelling, tearing 2 pts: eyelid edema, tearing, facial swelling 1 pt: facial swelling 1 pt: eyelid edema, injection, tearing 1 pt: facial flush, tearing | 8 pts: normal MRI 1 pt: left cerebellar hemangioma 1 pt: multifocal demyelinating lesions 1 pt: Chiari I post surgical decompresson 1 pt: cavum septum pellucidum post fenestration 1 pt: low lying cerebellar tonsils | 7 pts: indomethacin (1 mg/kg with max 150 mg/day), 5 cases had resolution of attacks 2 pts: oxygen, good response 1 pt: cyprohepatine, non resp 1 pt: amitriptyline, non resp 1 pt: topiramate, good response 1 pt: lost to follow-up |
| Posterior Fossa Pathologies | Pituitary Pathology | Cavernous Sinus/Orbits | Other |
|---|---|---|---|
| Pilocytic astrocytoma Cavernous hemangioma Arteriovenous malformation Basilar impression Dorsal–lateral brainstem ischemic lesions Skull malformation HIV-related lesions Congenital skull bone malformations (e.g., osteogenesis imperfecta) Ischemic infarction Cysts Vascular malformations or venous angioma of the cerebellopontine junction Brainstem angiocavernoma | Micro/macroadenomas (prolactinomas the most frequent) | Neurofibromatosis type 2 Intracranial intraorbital metastasis Extracranial intraorbital cystic tumors Invasion of the cavernous sinus by macroprolactinomas | Vascular loops and trigeminal neurovascular conflict Eye trauma Leiomyosarcoma of the venous sinus |
3.2. Clinical Features
3.2.1. Pain
3.2.2. Autonomic Signs
3.2.3. Differential Diagnoses
3.3. Pathophysiology
3.4. SUNCT/SUNA Therapy
3.4.1. Lamotrigine
3.4.2. Topiramate
3.4.3. Other Treatments
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd ed. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef]
- D’Andrea, G.; Granella, F. SUNCT Syndrome: The First Case in Childhood. Cephalalgia 2001, 21, 701–702. [Google Scholar] [CrossRef]
- Sciruicchio, V.; Sardaro, M.; Gagliardi, D.; Trabacca, A.; Galeone, D.; De Tommaso, M. A case of early-onset and monophasic trigeminal autonomic cephalalgia: Could it be a SUNCT? J. Headache Pain 2010, 11, 363–365. [Google Scholar] [CrossRef][Green Version]
- Lambru, G.; Matharu, M. Management of Trigeminal Autonomic Cephalalgias in Children and Adolescents. Curr. Pain Headache Rep. 2013, 17, 323. [Google Scholar] [CrossRef]
- Sebastian, S.; Schweitzer, D.; Tan, L.; Broadley, S.A. Role of Trigeminal Microvascular Decompression in the Treatment of SUNCT and SUNA. Curr. Pain Headache Rep. 2013, 17, 332. [Google Scholar] [CrossRef] [PubMed]
- Favoni, V.; Grimaldi, D.; Pierangeli, G.; Cortelli, P.; Cevoli, S. SUNCT/SUNA and neurovascular compression: New cases and critical literature review. Cephalalgia 2013, 33, 1337–1348. [Google Scholar] [CrossRef] [PubMed]
- Pomeroy, J.L.; Nahas, S.J. SUNCT/SUNA: A Review. Curr. Pain Headache Rep. 2015, 19, 38. [Google Scholar] [CrossRef] [PubMed]
- Eller, M.; Goadsby, P.J. Trigeminal autonomic cephalalgias. Oral Dis. 2016, 22, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, H.; Lian, Y.-J.; Ma, Y.-Q.; Xie, N.-C.; Cheng, X.; Zhang, L. Botulinum Toxin A for the Treatment of a Child with SUNCT Syndrome. Pain Res. Manag. 2016, 2016, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Lambru, G.; Byrne, S. Trigeminal autonomic cephalalgias in children and adolescents. Neurol. Sci. 2018, 39, 105–106. [Google Scholar] [CrossRef]
- Groenke, B.R.; Daline, I.H.; Nixdorf, D.R. SUNCT/SUNA: Case series presenting in an orofacial pain clinic. Cephalalgia 2020, 41, 665–676. [Google Scholar] [CrossRef]
- Cohen, A. Short-Lasting Unilateral Neuralgiform Headache Attacks with Conjunctival Injection and Tearing. Cephalalgia 2007, 27, 824–832. [Google Scholar] [CrossRef]
- Lambru, G.; Matharu, M.S. SUNCT, SUNA and trigeminal neuralgia: Different disorders or variants of the same disorder? Curr. Opin. Neurol. 2014, 27, 325–331. [Google Scholar] [CrossRef]
- Blättler, T.; Mori, A.C.; Boltshauser, E.; Bassetti, C. Symptomatic SUNCT in an eleven-year-old girl. Neurology 2003, 60, 2012–2013. [Google Scholar] [CrossRef] [PubMed]
- Trucco, M.; Mainardi, F.; Maggioni, F.; Badino, R.; Zanchin, G. Chronic Paroxysmal Hemicrania, Hemicrania Continua and Sunct Syndrome in Association with Other Pathologies: A Review. Cephalalgia 2004, 24, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Sékhara, T.; Pelc, K.; Mewasingh, L.D.; Boucquey, D.; Dan, B. Pediatric SUNCT Syndrome. Pediatr. Neurol. 2005, 33, 206–207. [Google Scholar] [CrossRef] [PubMed]
- Pareja, J.A.; Álvarez, M.; Montojo, T. SUNCT and SUNA: Recognition and Treatment. Curr. Treat. Options Neurol. 2012, 15, 28–39. [Google Scholar] [CrossRef]
- Unalp, A.; Ozturk, A. SUNCT syndrome in a child: A rare cause of paroxysmal headache. Ann. Saudi Med. 2008, 28, 386. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Cittadini, E.; Burns, B.; Cohen, A.S. Trigeminal autonomic cephalalgias: Diagnostic and therapeutic developments. Curr. Opin. Neurol. 2008, 21, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Broadley, S. Microvascular decompression of the trigeminal nerve in the treatment of SUNCT and SUNA. J. Neurol. Neurosurg. Psychiatry 2010, 81, 992–996. [Google Scholar]
- Favier, I.; van Vliet, J.A.; Roon, K.I.; Witteveen, R.J.; Verschuuren, J.J.; Ferrari, M.D.; Haan, J. Trigeminal Autonomic Cephalgias Due to Structural Lesions: A Review of 31 Cases. Arch. Neurol. 2007, 64, 25. [Google Scholar] [CrossRef]
- Qaiser, S.; Hershey, A.D.; Kacperski, J. SUNCT/SUNA in children and adolescents: Application of ICHD-3 criteria and treatment response: Case series of 13 SUNCT/SUNA pediatric cases. Cephalalgia 2020, 41, 112–116. [Google Scholar] [CrossRef]
- Dao, J.M.; Qubty, W. Headache Diagnosis in Children and Adolescents. Curr. Pain Headache Rep. 2018, 22, 17. [Google Scholar] [CrossRef]
- Copp, S.R.; Leblanc, C. A Case of Ophthalmic Branch Trigeminal Neuralgia in the Emergency Department. Cureus 2019, 11, e3831. [Google Scholar] [CrossRef]
- Stafstrom, C.E.; Goldenholz, S.R.; Dulli, D.A. Serial headache drawings by children with migraine: Correlation with clinical head-ache status. J. Child Neurol. 2005, 20, 809–813. [Google Scholar] [CrossRef]
- Goadsby, P.; Cittadini, E.; Cohen, A. Trigeminal Autonomic Cephalalgias: Paroxysmal Hemicrania, SUNCT/SUNA, and Hemicrania Continua. Semin. Neurol. 2010, 30, 186–191. [Google Scholar] [CrossRef][Green Version]
- Mack, K.J.; Goadsby, P. Trigeminal Autonomic Cephalalgias in Children and Adolescents: Cluster Headache and Related Conditions. Semin. Pediatr. Neurol. 2016, 23, 23–26. [Google Scholar] [CrossRef]
- Wei, D.Y.; Jensen, R.H. Therapeutic Approaches for the Management of Trigeminal Autonomic Cephalalgias. Neurotherapeutics 2018, 15, 346–360. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.H.; Broadley, S.A. SUNCT and SUNA: Clinical features and medical treatment. J. Clin. Neurosci. 2008, 15, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Matharu, M.; Cohen, A.S.; Goadsby, P.J. SUNCT Syndrome Responsive to Intravenous Lidocaine. Cephalalgia 2004, 24, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, D.; Duggal, A.K. SUNCT and SUNA: An Update. Neurol. India 2021, 69, S144–S159. [Google Scholar] [CrossRef] [PubMed]
- Barloese, M.C.J. The pathophysiology of the trigeminal autonomic cephalalgias, with clinical implications. Clin. Auton. Res. 2017, 28, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Leone, M.; Franzini, A.; D’Andrea, G.; Broggi, G.; Casucci, G.; Bussone, G. Deep brain stimulation to relieve drug-resistant SUNCT. Ann. Neurol. 2005, 57, 924–927. [Google Scholar] [CrossRef] [PubMed]
- May, A.; Bahra, A.; Turner, R.; Goadsby, P.J. Functional magnetic resonance imaging in spontaneous attacks of SUNCT: Short-lasting neuralgiform headache with conjunctival injection and tearing. Ann. Neurol. 1999, 46, 791–794. [Google Scholar] [CrossRef]
- Sprenger, T.; Valet, M.; Platzer, S.; Pfaffenrath, V.; Steude, U.; Tolle, T.R. SUNCT: Bilateral hypothalamic activation during headache attacks and resolving of symptoms after tri-geminal decompression. Pain 2005, 113, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, T.; Levy, M.J.; Knight, Y.E.; Goadsby, P.J. Differential modulation of nociceptive dural input to [hypocretin] orexin A and B receptor activation in the posterior hypothalamic area. Pain 2004, 109, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.S.; Kaube, H. Rare nocturnal headaches. Curr. Opin. Neurol. 2004, 17, 295–299. [Google Scholar] [CrossRef]
- Bosco, D.; Labate, A.; Mungari, P.; Vero, S.; Fava, A. SUNCT and high nocturnal prolactin levels: Some new unusual char-acteristics. J. Headache Pain 2007, 8, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Ben-Jonathan, N.; Mershon, J.L.; Allen, D.L.; Steinmetz, R.W. Extrapituitary Prolactin: Distribution, Regulation, Functions, and Clinical Aspects*. Endocr. Rev. 1996, 17, 639–669. [Google Scholar] [CrossRef]
- Egunsola, O.; Choonara, I.; Sammons, H.M. Safety of lamotrigine in paediatrics: A systematic review. BMJ Open 2015, 5, e007711. [Google Scholar] [CrossRef]
- Rogawski, M. Chapter 1: Principles of antiepileptic drug action. In Antiepileptic Drugs, 5th ed.; Levy, R.H., Mattson, R.H., Meldrum, B.S., Perucca, E., Eds.; Lippincott Williams & Wilkins: Philadelphia, PY, USA, 2002; pp. 3–22. [Google Scholar]
- Goadsby, P.J.; Lipton, R.B. A review of paroxysmal hemicranias, SUNCT syndrome and other short-lasting headaches with au-tonomic feature, including new cases. Brain 1997, 120, 193–209. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.S.; Lancaster, B.; Peakman, T.; Garthwaite, J. Interaction of the antiepileptic drug lamotrigine with recombinant rat brain type IIA Na+ channels and with native Na+ channels in rat hippocampal neurones. Pflugers Arch. 1995, 430, 437–446. [Google Scholar] [CrossRef]
- Nakamura-Craig, M.; Follenfant, R.L. Effect of lamotrigine in the acute and chronic hyperalgesia induced by PGE2 and in the chronic hyperalgesia in rats with streptozotocin-induced diabetes. Pain 1995, 63, 33–37. [Google Scholar] [CrossRef]
- Tremont-Lukats, I.W.; Megeff, C.; Backonja, M.-M. Anticonvulsants for Neuropathic Pain Syndromes. Drugs 2000, 60, 1029–1052. [Google Scholar] [CrossRef] [PubMed]
- Rosselli, J.L.; Karpinski, J.P. Ann, El Papel de Lamotrigina en el Tratamiento de Ataques de Dolor de Cabeza Neuralgiforme Unilateral de Corta Duración con Inyección y Rasgado Conjuntivales. Pharmacother 2011, 45, 108–113. [Google Scholar] [CrossRef] [PubMed]
- French, J.; Kanner, A.M.; Bautista, J.; Abou-Khalil, B.; Browne, T.; Harden, C.L.; Theodore, W.H.; Bazil, C.; Stern, J.; Schachter, S.C.; et al. Efficacy and tolerability of the new antiepileptic drugs II: Treatment of refractory epilepsy: Report of the Therapeutics and Technology Assessment Subcommittee and Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology 2004, 62, 1261–1273. [Google Scholar] [CrossRef] [PubMed]
- Brandes, J.L.; Saper, J.R.; Diamond, M.; Couch, J.R.; Lewis, D.W.; Schmitt, J.; Neto, W.; Schwabe, S.; Jacobs, D.; for the MIGR-002 Study Group. Topiramate for Migraine Prevention: A Randomized Controlled Trial. JAMA 2004, 291, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Le, K.; Yu, D.; Wang, J.; Ali, A.I.; Guo, Y. Is topiramate effective for migraine prevention in patients less than 18 years of age? A meta-analysis of randomized controlled trials. J. Headache Pain 2017, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- FDA approves Topamax for migraine prevention in adolescents. J. Pain Palliat. Care Pharmacother 2014, 28, 191.
- McElroy, S.L.; Arnold, L.M.; Shapira, N.A.; Keck, P.E.; Rosenthal, N.R.; Karim, M.R.; Kamin, M.; Hudson, J.I. Topiramate in the Treatment of Binge Eating Disorder Associated With Obesity: A Randomized, Placebo-Controlled Trial. Am. J. Psychiatry 2003, 160, 255–261. [Google Scholar] [CrossRef]
- Storer, R.; Goadsby, P. Trigeminovascular nociceptive transmission involves N-methyl-d-aspartate and non-N-methyl-d-aspartate glutamate receptors. Neuroscience 1999, 90, 1371–1376. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Edvinsson, L.; Ekman, R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann. Neurol. 1990, 28, 183–187. [Google Scholar] [CrossRef] [PubMed]
- White, H.; Brown, S.; Woodhead, J.H.; A Skeen, G.; Wolf, H.H. Topiramate enhances GABA-mediated chloride flux and GABA-evoked chloride currents in murine brain neurons and increases seizure threshold. Epilepsy Res. 1997, 28, 167–179. [Google Scholar] [CrossRef]
- Leniger, T.; Thöne, J.; Wiemann, M. Topiramate modulates pH of hippocampal CA3 neurons by combined effects on carbonic anhydrase and Cl−/HCO3−exchange. Br. J. Pharmacol. 2004, 142, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Minton, G.C.; Miller, A.D.; Bookstaver, P.B.; Love, B. Topiramate: Safety and Efficacy of its Use in the Prevention and Treatment of Migraine. J. Central Nerv. Syst. Dis. 2011, 3, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Hershey, A.; Powers, S.W.; Vockell, A.-L.B.; LeCates, S.; Kabbouche, M. Effectiveness of Topiramate in the Prevention of Childhood Headaches. Headache: J. Head Face Pain 2002, 42, 810–818. [Google Scholar] [CrossRef]
- Moorjani, B.I.; Rothner, A.D. Indomethacin-responsive headaches in children and adolescents. Semin. Pediatr. Neurol. 2001, 8, 40–45. [Google Scholar] [CrossRef]

| SUNHA Diagnostic criteria:
| SUNCT Diagnostic criteria:
| Episodic SUNCT/SUNA Diagnostic criteria:
|
| SUNA Diagnostic criteria:
| Chronic SUNCT/SUNA Diagnostic criteria:
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cesaroni, C.A.; Pruccoli, J.; Bergonzini, L.; Quatrosi, G.; Vetri, L.; Roccella, M.; Parmeggiani, A. SUNCT/SUNA in Pediatric Age: A Review of Pathophysiology and Therapeutic Options. Brain Sci. 2021, 11, 1252. https://doi.org/10.3390/brainsci11091252
Cesaroni CA, Pruccoli J, Bergonzini L, Quatrosi G, Vetri L, Roccella M, Parmeggiani A. SUNCT/SUNA in Pediatric Age: A Review of Pathophysiology and Therapeutic Options. Brain Sciences. 2021; 11(9):1252. https://doi.org/10.3390/brainsci11091252
Chicago/Turabian StyleCesaroni, Carlo Alberto, Jacopo Pruccoli, Luca Bergonzini, Giuseppe Quatrosi, Luigi Vetri, Michele Roccella, and Antonia Parmeggiani. 2021. "SUNCT/SUNA in Pediatric Age: A Review of Pathophysiology and Therapeutic Options" Brain Sciences 11, no. 9: 1252. https://doi.org/10.3390/brainsci11091252
APA StyleCesaroni, C. A., Pruccoli, J., Bergonzini, L., Quatrosi, G., Vetri, L., Roccella, M., & Parmeggiani, A. (2021). SUNCT/SUNA in Pediatric Age: A Review of Pathophysiology and Therapeutic Options. Brain Sciences, 11(9), 1252. https://doi.org/10.3390/brainsci11091252









