Age-Related Changes in Hemispherical Specialization for Attentional Networks
Abstract
1. Introduction
Aims
2. Method
2.1. Participants
2.2. Cognitive Status
2.3. Apparatus
2.4. Stimuli
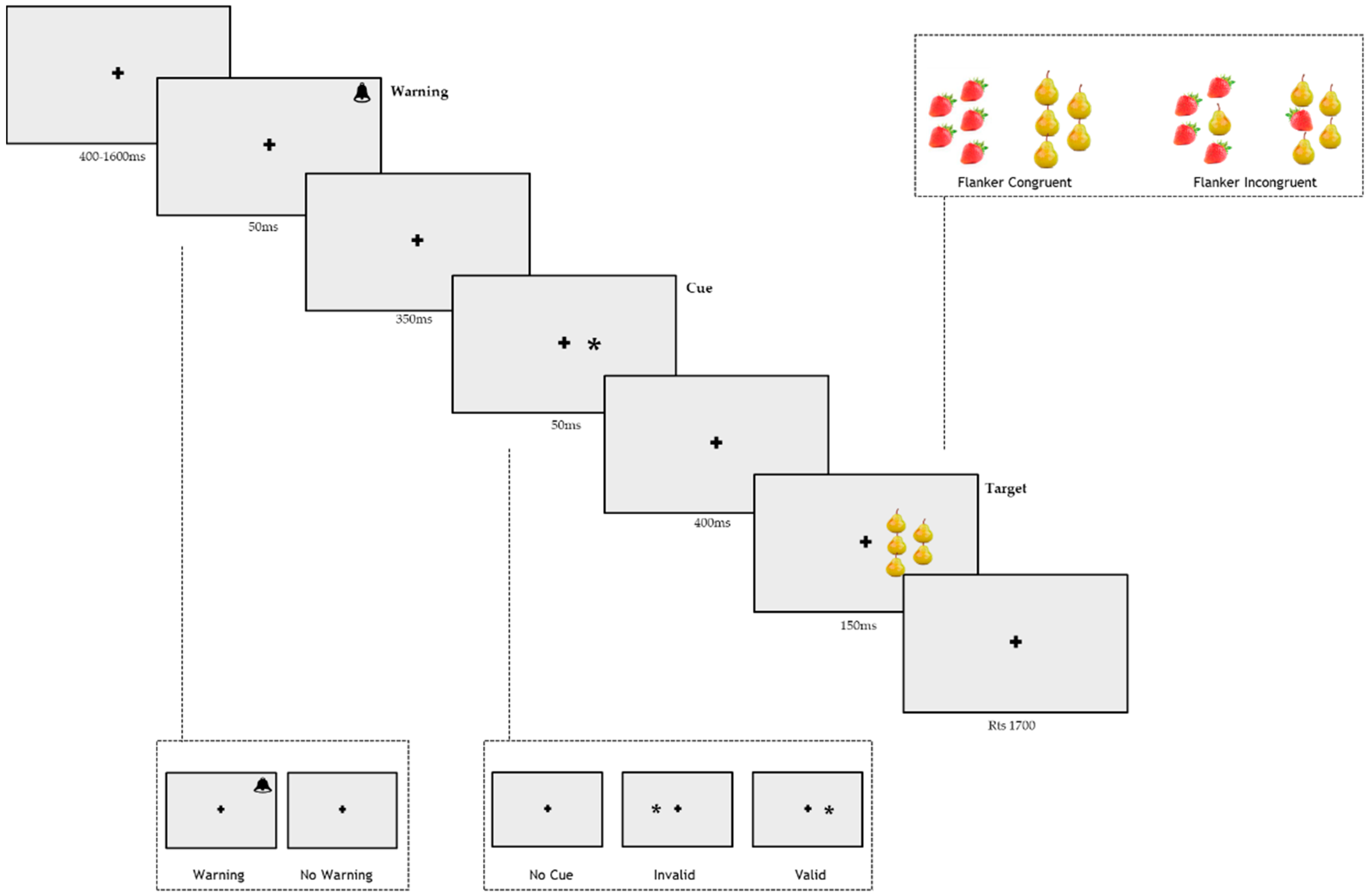
2.5. Procedure
2.5.1. Procedure
2.5.2. General Procedure
2.6. Data Analysis
3. Results
3.1. LANTI-F
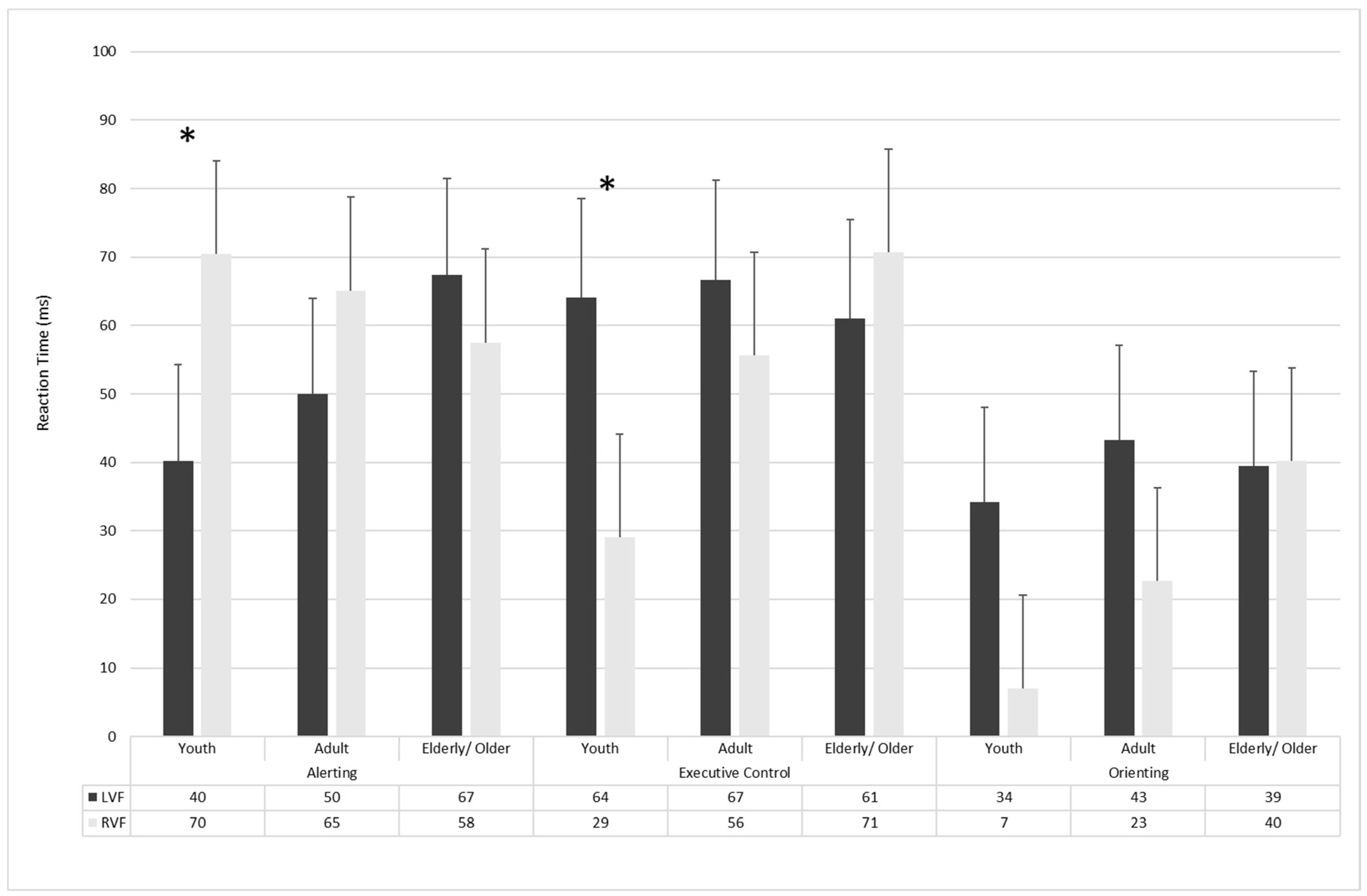
3.2. Attentional Effects
4. Discussion
4.1. Attentional Networks in Aging
4.2. Hemispheric Specialization of Attentional Networks in Aging
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Craik, F.I.; Bialystok, E. Cognition through the lifespan: Mechanisms of change. Trends Cogn. Sci. 2006, 10, 131–138. [Google Scholar] [CrossRef]
- Zelazo, P.D.; Craik, F.I.; Booth, L. Executive function across the life span. Acta Psychol. 2004, 115, 167–183. [Google Scholar] [CrossRef] [PubMed]
- Favieri, F.; Forte, G.; Casagrande, M. The executive functions in overweight and obesity: A systematic review of neuropsychological cross-sectional and longitudinal studies. Front. Psychol. 2019, 10, 2126. [Google Scholar] [CrossRef]
- Barbosa, M.B.; Pereira, C.V.; Cruz, D.T.D.; Leite, I.C.G. Prevalence and factors associated with alcohol and tobacco use among non-institutionalized elderly persons. Rev. Bras. Geriatr. Geront. 2018, 21, 123–133. [Google Scholar] [CrossRef]
- Farina, M.; Paloski, L.H.; de Oliveira, C.R.; de Lima Argimon, I.I.; Irigaray, T.Q. Cognitive reserve in elderly and its connection with cognitive performance: A systematic review. Age Internl. 2018, 43, 496–507. [Google Scholar] [CrossRef]
- Forte, G.; Casagrande, M. Effects of Blood Pressure on Cognitive Performance in Aging: A Systematic Review. Brain Sci. 2020, 10, 919. [Google Scholar] [CrossRef] [PubMed]
- Eckert, M.A.; Keren, N.I.; Roberts, D.R.; Calhoun, V.D.; Harris, K.C. Age-related changes in processing speed: Unique contributions of cerebellar and prefrontal cortex. Front. Hum. Neurosci. 2014, 4, 10. [Google Scholar] [CrossRef]
- Yuan, P.; Raz, N. Prefrontal cortex and executive functions in healthy adults: A meta-analysis of structural neuroimaging studies. Neurosci. Biobehav. Rev. 2014, 42, 180–192. [Google Scholar] [CrossRef]
- Gazzaley, A.; Cooney, J.W.; Rissman, J.; D’esposito, M. Top-down suppression deficit underlies working memory impairment in normal aging. Nat. Neurosci. 2005, 8, 1298. [Google Scholar] [CrossRef] [PubMed]
- Murman, D.L. The impact of age on cognition. In Seminars Hearing; Thieme Medical Publishers: New York, NY, USA, 2015; Volume 36, pp. 111–121. [Google Scholar]
- Turgeon, M.; Lustig, C.; Meck, W.H. Cognitive aging and time perception: Roles of Bayesian optimization and degeneracy. Front. Aging Neurosci. 2016, 8, 102. [Google Scholar] [CrossRef]
- Binetti, N.; Lecce, F.; Doricchi, F. Time-dilation and time-contraction in an anisochronous and anisometric visual scenery. J. Vis. 2012, 12, 8. [Google Scholar] [CrossRef]
- Maldonado, T.; Orr, J.M.; Goen, J.R.; Bernard, J.A. Age differences in the subcomponents of executive functioning. J. Geront. Ser. B 2020, 75, e31–e55. [Google Scholar] [CrossRef]
- Posner, M.I.; Petersen, S.E. The attention system of the human brain. Annu. Rev. Neurosci. 1990, 13, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.E.; Posner, M.I. The attention system of the human brain: 20 years after. Annu. Rev. Neurosci. 2012, 35, 73–89. [Google Scholar] [CrossRef]
- Lecce, F.; Rotondaro, F.; Bonni, S.; Carlesimo, A.; De Schotten, M.T.; Tomaiuolo, F.; Doricchi, F. Cingulate neglect in humans: Disruption of contralesional reward learning in right brain damage. Cortex 2015, 62, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; McCandliss, B.D.; Sommer, T.; Raz, A.; Posner, M.I. Testing the efficiency and independence of attentional networks. J. Cogn. Neurosci. 2002, 14, 340–347. [Google Scholar] [CrossRef]
- Posner, M.I. Orienting of attention. Q. J. Exp. Psychol. 1980, 32, 3–25. [Google Scholar] [CrossRef]
- Eriksen, B.A.; Eriksen, C.W. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept. Psychoph. 1974, 16, 143–149. [Google Scholar] [CrossRef]
- Posner, M.I.; Rothbart, M.K. Research on attention networks as a model for the integration of psychological science. Annu. Rev. Psychol. 2007, 58, 1–23. [Google Scholar] [CrossRef]
- Callejas, A.; Lupianez, J.; Funes, M.J.; Tudela, P. Modulations among the alerting, orienting and executive control networks. Exp. Brain Res. 2005, 167, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Spagna, A.; Martella, D.; Sebastiani, M.; Maccari, L.; Marotta, A.; Casagrande, M. Efficiency and interactions of alerting, orienting and executive networks: The impact of imperative stimulus type. Acta Psychol. 2014, 148, 209–215. [Google Scholar] [CrossRef]
- Spagna, A.; Martella, D.; Fuentes, L.J.; Marotta, A.; Casagrande, M. Hemispheric modulations of the attentional networks. Brain Cogn. 2016, 108, 73–80. [Google Scholar] [CrossRef]
- Casagrande, M.; Martella, D.; Ruggiero, M.C.; Maccari, L.; Paloscia, C.; Rosa, C.; Pasini, A. Assessing Attentional Systems in Children with Attention Deficit Hyperactivity Disorder. Arch. Clin. Neuropsychol. 2012, 27, 30–44. [Google Scholar] [CrossRef]
- Casagrande, M.; Marotta, A.; Canepone, V.; Spagna, A.; Rosa, C.; Bonocore, L.; Berloco, B.; Dimaggio, G.; Pasini, A. Dysfunctional personality traits in adolescence: Effects on alerting, orienting and executive control of attention. Cogn. Process. 2017, 18, 183–193. [Google Scholar] [CrossRef]
- Federico, F.; Marotta, A.; Adriani, T.; Maccari, L.; Casagrande, M. Attention Network Test—The impact of social information on executive control, alerting and orienting. Acta Psychol. 2013, 143, 65–70. [Google Scholar] [CrossRef]
- Federico, F.; Marotta, A.; Martella, D.; Casagrande, M. Development in attention functions and social processing: Evidence from the Attention Network Test. Brit. J. Dev. Psychol. 2017, 35, 169–185. [Google Scholar] [CrossRef] [PubMed]
- Marotta, A.; Delle Chiaie, R.; Spagna, A.; Bernabei, L.; Sciarretta, M.; Roca, J.; Biondi, M.; Casagrande, M. Impaired conflict resolution and vigilance in euthymic bipolar disorder. Psychiat. Res. 2015, 229, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Martella, D.; Casagrande, M.; Lupiáñez, J. Alerting, orienting and executive control: The effects of sleep deprivation on attentional networks. Exper. Brain Res. 2011, 210, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Giovannoli, J.; Martella, D.; Casagrande, M. Assessing the three attentional networks and vigilance in the adolescence stages. Brain Sci. 2021, 11, 503. [Google Scholar] [CrossRef]
- Casagrande, M.; Marotta, A.; Martella, D.; Volpari, E.; Agostini, A.; Favieri, F.; Forte, G.; Rea, M.; Ferri, R.; Giordano, V.; et al. Assessing the three attentional networks in children from three to six years: A child-friendly version of the Attentional Network Test for Interaction. Behav. Res. Methods 2021. [Google Scholar] [CrossRef]
- Federico, F.; Marotta, A.; Orsolini, M.; Casagrande, M. Aging in cognitive control of social processing: Evidence from the attention network test. Aging Neuropsychol. Cogn. 2021, 28, 128–142. [Google Scholar] [CrossRef]
- Jenningss, J.M.; Dagenbach, D.; Engle, C.M.; Funke, L.J. Age-related changes and the attention network task: An examination of alerting, orienting, and executive function. Aging Neuropsychol. Cogn. 2007, 14, 353–369. [Google Scholar] [CrossRef]
- Fernandez-Duque, D.; Black, S.E. Attentional networks in normal aging and Alzheimer’s disease. Neuropsychology 2006, 20, 133. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.S.; Fan, J.; Lee, T.M.; Wang, C.Q.; Wang, K. Age-related differences in attentional networks of alerting and executive control in young, middle-aged, and older Chinese adults. Brain Cogn. 2011, 75, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Gamboz, N.; Zamarian, S.; Cavallero, C. Age-related differences in the attention network test (ANT). Exper. Aging Res. 2010, 36, 287–305. [Google Scholar] [CrossRef]
- Williams, R.S.; Biel, A.L.; Wegier, P.; Lapp, L.K.; Dyson, B.J.; Spaniol, J. Age differences in the Attention Network Test: Evidence from behavior and event-related potentials. Brain Cogn. 2016, 102, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, D.A.; Sozda, C.N.; Dotson, V.M.; Perlstein, W.M. An event-related potential investigation of the effects of age on alerting, orienting, and executive function. Front. Aging Neurosci. 2016, 8, 99. [Google Scholar] [CrossRef] [PubMed]
- Bartolomeo, P.; Thiebaut de Schotten, M.; Doricchi, F. Left unilateral neglect as a disconnection syndrome. Cereb. Cortex 2007, 17, 2479–2490. [Google Scholar] [CrossRef]
- Silvetti, M.; Lasaponara, S.; Lecce, F.; Dragone, A.; Macaluso, E.; Doricchi, F. The response of the left ventral attentional system to invalid targets and its implication for the spatial neglect syndrome: A multivariate fMRI investigation. Cereb. Cortex 2016, 26, 4551–4562. [Google Scholar] [CrossRef] [PubMed]
- Bowers, D.; Heilman, K.M. Pseudoneglect: Effects of hemispace on a tactile line bisection task. Neuropsychologia 1980, 18, 491–498. [Google Scholar] [CrossRef]
- Casagrande, M.; Martella, D.; Di Pace, E.; Pirri, F.; Guadalupi, F. Orienting and alerting: Effects of 24 hours of prolonged wakefulness. Exper. Brain Res. 2006, 171, 184–193. [Google Scholar] [CrossRef]
- Bartolomeo, P.; Malkinson, T.S. Hemispheric lateralization of attention processes in the human brain. Curr. Opin. Psychol. 2019, 29, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Hatin, B.; Tottenham, L.S.; Oriet, C. The relationship between collisions and pseudoneglect: Is it right? Cortex 2012, 48, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Marotta, A.; Lupiáñez, J.; Casagrande, M. Investigating hemispheric lateralization of reflexive attention to gaze and arrow cues. Brain Cogn. 2012, 80, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Casagrande, M.; Bertini, M. Laterality of sleep onset process: Which hemisphere goes to sleep first? Biol. Psychol. 2008, 77, 76–80. [Google Scholar] [CrossRef]
- Casagrande, M.; Bertini, M. Right hemisphere superiority for the night and left hemisphere superiority for the day: A re-patterning of laterality across wake-sleep-wake states. Biol. Psychol. 2008, 77, 337–342. [Google Scholar] [CrossRef]
- Casagrande, M.; Violani, C.; De Gennaro, L.; Braibanti, P.; Bertini, M. Which hemisphere falls asleep first? Neuropsychologia 1995, 33, 815–822. [Google Scholar] [CrossRef]
- Greene, D.J.; Barnea, A.; Herzberg, K.; Rassis, A.; Neta, M.; Raz, A.; Zaidel, E. Measuring attention in the hemispheres: The lateralized attention network test (LANT). Brain Cogn. 2008, 66, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Konrad, K.; Neufang, S.; Hanisch, C.; Fink, G.R.; Herpertz-Dahlmann, B. Dysfunctional attentional networks in children with attention deficit/hyperactivity disorder: Evidence from an event-related functional magnetic resonance imaging study. Bio. Psychiatry 2006, 59, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Poynter, W.; Ingram, P.; Minor, S. Visual field asymmetries in attention vary with self-reported attention deficits. Brain Cogn. 2010, 72, 355–361. [Google Scholar] [CrossRef]
- Chica, A.B.; Bartolomeo, P.; Valero-Cabré, A. Dorsal and ventral parietal contributions to spatial orienting in the human brain. J. Neurosci. 2011, 31, 8143–8149. [Google Scholar] [CrossRef]
- Asanowicz, D.; Marzecová, A.; Jaśkowski, P.; Wolski, P. Hemispheric asymmetry in the efficiency of attentional networks. Brain Cogn. 2012, 79, 117–128. [Google Scholar] [CrossRef][Green Version]
- Spagna, A.; He, G.; Jin, S.; Gao, L.; Mackie, M.A.; Tian, Y.; Fan, J. Deficit of supramodal executive control of attention in schizophrenia. J. Psychiatr. Res. 2018, 97, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Corbetta, M.; Patel, G.; Shulman, G.L. The reorienting system of the human brain: From environment to theory of mind. Neuron 2008, 58, 306–324. [Google Scholar] [CrossRef] [PubMed]
- Corbetta, M.; Shulman, G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002, 3, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; McCandliss, B.D.; Fossella, J.; Flombaum, J.I.; Posner, M.I. The activation of attentional networks. Neuroimage 2005, 26, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Larson, E.; Lee, A.K.C. Switching auditory attention using spatial and non-spatial features recruits different cortical networks. Neuroimage 2014, 84, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Ofek, E.; Pratt, H. Ear advantage and attention: An ERP study of auditory cued attention. Hear. Res. 2004, 189, 107–118. [Google Scholar] [CrossRef]
- Fjell, A.M.; Walhovd, K.B. Structural brain changes in aging: Courses, causes and cognitive consequences. Rev. Neurosci. 2010, 21, 187–222. [Google Scholar] [CrossRef]
- Tomasi, D.; Volkow, N.D. Aging and functional brain networks. Mol. Psychiatry 2012, 17, 549–558. [Google Scholar] [CrossRef]
- Raz, N. Aging of the brain and its impact on cognitive performance: Integration of structural and functional findings. In The Handbook of Aging and Cognition; Craik, F.I.M., Salthouse, T.A., Eds.; Lawrence Erlbaum Associates Publishers: Mahwah, NJ, USA, 2000. [Google Scholar]
- Friedrich, T.E.; Hunter, P.V.; Elias, L.J. The trajectory of pseudoneglect in adults: A systematic review. Neuropsychol. Rev. 2018, 28, 436–452. [Google Scholar] [CrossRef]
- Benwell, C.S.; Thut, G.; Grant, A.; Harvey, M. A rightward shift in the visuospatial attention vector with healthy aging. Front. Aging Neurosci. 2014, 6, 113. [Google Scholar] [CrossRef]
- Learmonth, G.; Benwell, C.S.; Thut, G.; Harvey, M. Age-related reduction of hemispheric lateralisation for spatial attention: An EEG study. NeuroImage 2017, 153, 139–151. [Google Scholar] [CrossRef]
- World Health Organization. Men, Ageing and Health: Achieving Health across the Life Span (No. WHO/NMH/NPH/01.2); World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Raven, J.C.; Court, J.H. Raven’s Progressive Matrices; Western Psychological Services: Los Angeles, CA, USA, 1938. [Google Scholar]
- Weissman, D.H.; Banich, M.T. The cerebral hemispheres cooperate to perform complex but not simple tasks. Neuropsychology 2000, 14, 41. [Google Scholar] [CrossRef] [PubMed]
- Guarino, A.; Forte, G.; Giovannoli, J.; Casagrande, M. Executive functions in the elderly with mild cognitive impairment: A systematicreview on motor and cognitive inhibition, conflict control and cognitive flexibility. Aging Ment. Health 2020, 24, 1028–1045. [Google Scholar] [CrossRef]
- Erel, H.; Levy, D.A. Orienting of visual attention in aging. Neurosci. Biobehav. Rev. 2016, 69, 357–380. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Gu, X.; Guise, K.G.; Liu, X.; Fossella, J.; Wang, H.; Posner, M.I. Testing the behavioral interaction and integration of attentional networks. Brain Cogn. 2009, 70, 209–220. [Google Scholar] [CrossRef]
- Fernandez-Duque, D.; Posner, M.I. Relating the mechanisms of orienting and alerting. Neuropsychologia 1997, 35, 477–486. [Google Scholar] [CrossRef]
- Ishigami, Y.; Klein, R.M. Repeated measurement of the components of attention using two versions of the Attention Network Test (ANT): Stability, isolability, robustness, and reliability. J. Neurosci. Methods 1968, 190, 117–128. [Google Scholar] [CrossRef]
- Shulman, G.L.; Pope, D.L.; Astafiev, S.V.; McAvoy, M.P.; Snyder, A.Z.; Corbetta, M. Right hemisphere dominance during spatial selective attention and target detection occurs outside the dorsal frontoparietal network. J. Neurosci. 2010, 30, 3640–3651. [Google Scholar] [CrossRef] [PubMed]
- Chandrakumar, D.; Keage, H.A.; Gutteridge, D.; Dorrian, J.; Banks, S.; Loetscher, T. Interactions between spatial attention and alertness in healthy adults: A meta-analysis. Cortex 2019, 119, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, R.; Grady, C.L.; Nyberg, L.; McIntosh, A.R.; Tulving, E.; Kapur, S.; Craik, F.I. Age-related differences in neural activity during memory encoding and retrieval: A positron emission tomography study. J. Neurosci. 1996, 17, 391–400. [Google Scholar] [CrossRef]
- Berlingeri, M.; Bottini, G.; Danelli, L.; Ferri, F.; Traficante, D.; Sacheli, L.; Paulesu, E. With time on our side? Task-dependent compensatory processes in graceful aging. Exp. Brain Res. 2010, 205, 307–324. [Google Scholar] [CrossRef] [PubMed]
- Dolcos, F.; Rice, H.J.; Cabeza, R. Hemispheric asymmetry and aging: Right hemisphere decline or asymmetry reduction. Neurosci. Biobehav. Rev. 2002, 26, 819–825. [Google Scholar] [CrossRef]
- Jenkins, L.; Myerson, J.; Joerding, J.A.; Hale, S. Converging evidence that visuospatial cognition is more age-sensitive than verbal cognition. Psychol. Aging 2000, 15, 157. [Google Scholar] [CrossRef]
- Guarino, A.; Favieri, F.; Boncompagni, I.; Agostini, F.; Cantone, M.; Casagrande, M. Executive Functions in Alzheimer Disease: A Systematic Review. Front. Aging Neurosci. 2019, 10, 437. [Google Scholar] [CrossRef]
| Group | Age | Years of Education | MMSE | IQ |
|---|---|---|---|---|
| Youth | 23.46 ± 2.08 | 16.39 ± 1.53 | 27.66 ± 0.74 | 114.30 ± 13.06 |
| Adults | 55.26 ±7.54 | 14.35 ± 4.30 | 27.85 ± 1.34 | 118.65 ± 10.09 |
| Elderly/Older | 71.82 ± 5.88 | 13.16 ± 5.11 | 26.80 ± 1.55 | 113.37 ± 13.37 |
| Youth | Adults | Elderly/Older | ||||||
|---|---|---|---|---|---|---|---|---|
| RVF | LVF | RVF | LVF | RVF | LVF | |||
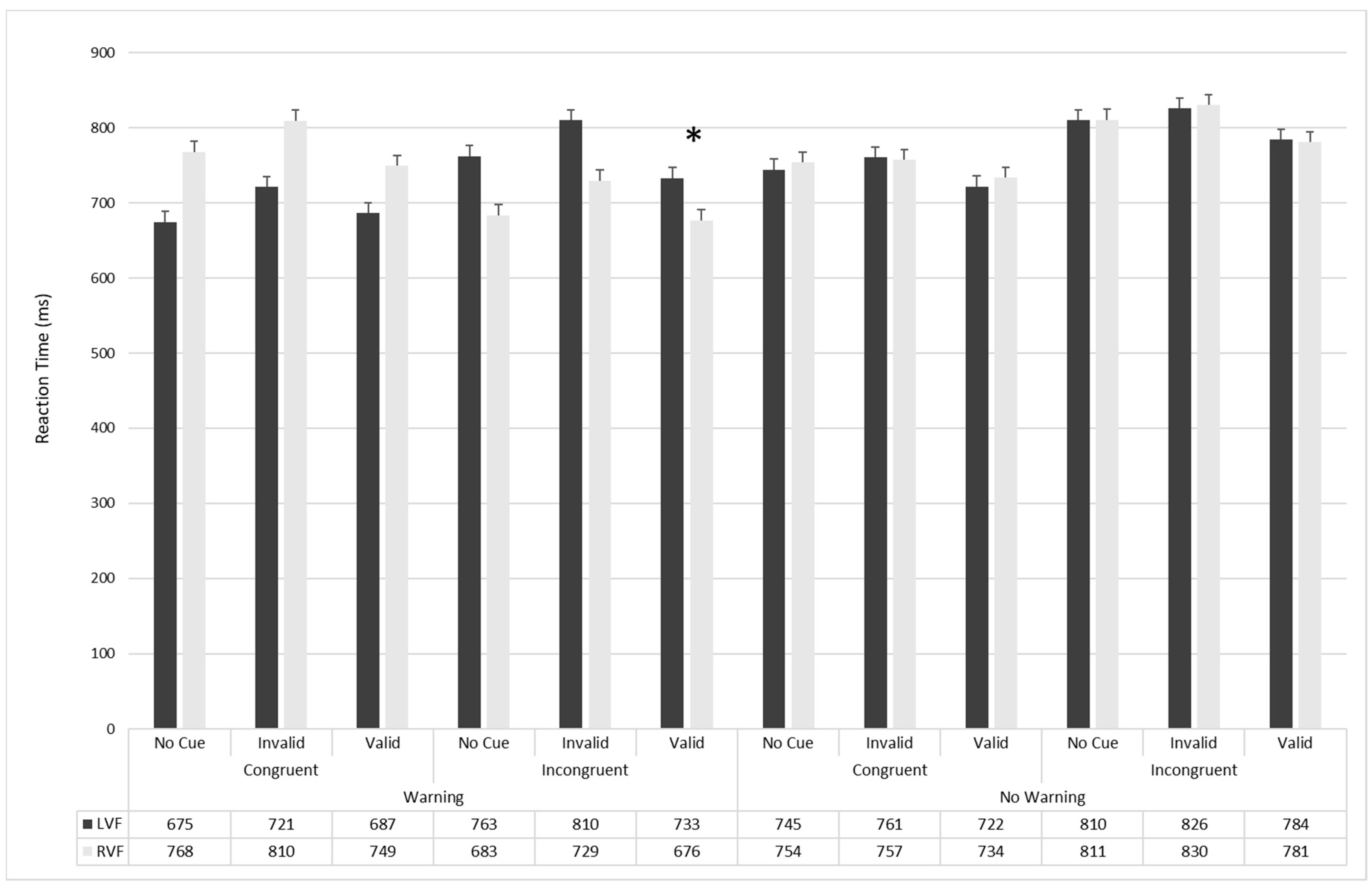
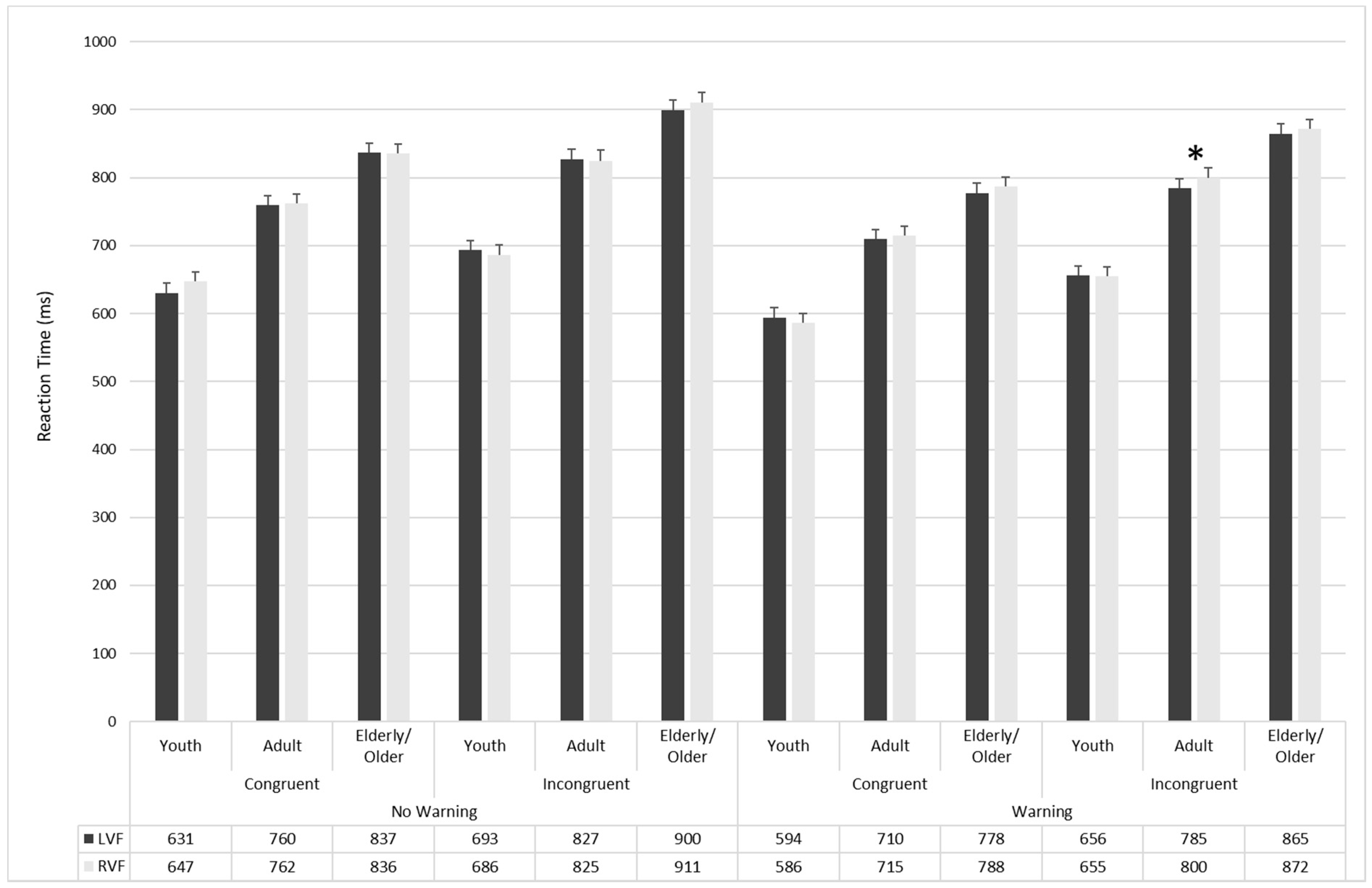
| No-Warning | Congruent | Valid | 643.04 17.9 | 610.9 16.15 | 742.89 17.9 | 736.58 16.15 | 815.36 17.9 | 817.95 16.15 |
| Invalid | 650.03 15.04 | 645.13 14.79 | 765.56 15.04 | 779.87 14.79 | 855.51 15.04 | 857.44 14.8 | ||
| No-cue | 648.64 14.86 | 635.56 15.57 | 776.61 14.86 | 763.4 15.57 | 838.16 14.8 | 834.87 15.57 | ||
| Incongruent | Valid | 656.06 16.02 | 668.05 15.71 | 803.84 16.02 | 809.71 15.71 | 882.91 16.01 | 875.07 15.71 | |
| Invalid | 708.5 16.85 | 705.71 16.51 | 844.68 16.85 | 847.53 16.52 | 938.19 16.85 | 924.01 16.52 | ||
| No-cue | 693.67 17.65 | 706.66 15.98 | 826.95 17.65 | 823.68 15.99 | 912.13 17.65 | 899.79 15.99 | ||
| Warning | Congruent | Valid | 573.28 15.07 | 585.23 16.06 | 696.09 15.07 | 713.58 16.05 | 759.4 15.07 | 761.34 16.05 |
| Invalid | 608.13 14.88 | 617.36 15.22 | 754.77 14.88 | 730.55 15.23 | 824.2 14.8 | 815.33 15.23 | ||
| No-cue | 577.61 16.39 | 580.80 15.17 | 693.28 16.39 | 686.44 15.17 | 779.1 16.39 | 756.6 15.17 | ||
| Incongruent | Valid | 633.73 15.44 | 624 13.71 | 782.53 15.44 | 760.05 13.71 | 831.46 15.44 | 814.86 13.71 | |
| Invalid | 689.98 16.6 | 697.49 16.48 | 825.7 16.6 | 814.42 16.48 | 913.7 16.6 | 917.88 16.48 | ||
| No-cue | 641.57 16.05 | 645 16.88 | 791.2 16.05 | 779.29 16.88 | 870.44 16.05 | 862.25 16.88 | ||
| Left Visual Field | Right Visual Field | |||||||
|---|---|---|---|---|---|---|---|---|
| Age | Alerting | Conflict | Orienting | Alerting | Conflict | Orienting | ||
| Left Visual Field | Alerting | 0.20 ** | - | |||||
| Conflict | −0.05 | −0.40 ** | - | |||||
| Orienting | 0.01 | −0.14 | 0.31 ** | - | ||||
| Right Visual Field | Alerting | –0.04 | 0.15 | −0.01 | −0.01 | - | ||
| Conflict | 0.16 * | −0.05 | 0.30 ** | 0.09 | −0.22 * | - | ||
| Orienting | 0.12 | 0.06 | 0.14 | 0.15 * | −0.30 ** | 0.21 ** | - | |
| Model | B | Standard Error | Beta | t | Sign (p) | 95% CI Lower | 95% CI Upper | ||
|---|---|---|---|---|---|---|---|---|---|
| LVF | Alerting | Age | 0.58 | 0.23 | 0.19 | 2.55 | 0.01 | 0.13 | 1.03 |
| Conflict | Age | −0.12 | 0.26 | −0.03 | −0.46 | −65 | −0.63 | 0.39 | |
| Orienting | Age | −0.04 | 0.31 | −0.01 | −0.12 | 0.91 | −0.64 | 0.57 | |
| RVF | Alerting | Age | −0.16 | 0.24 | −0.51 | −0.66 | 0.51 | −0.64 | 0.32 |
| Conflict | Age | 0.84 | 0.27 | 0.23 | 3.04 | 0.003 | 0.29 | 1.38 | |
| Orienting | Age | 0.48 | 0.40 | 0.09 | 1.20 | 0.23 | −0.31 | 1.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casagrande, M.; Agostini, F.; Favieri, F.; Forte, G.; Giovannoli, J.; Guarino, A.; Marotta, A.; Doricchi, F.; Martella, D. Age-Related Changes in Hemispherical Specialization for Attentional Networks. Brain Sci. 2021, 11, 1115. https://doi.org/10.3390/brainsci11091115
Casagrande M, Agostini F, Favieri F, Forte G, Giovannoli J, Guarino A, Marotta A, Doricchi F, Martella D. Age-Related Changes in Hemispherical Specialization for Attentional Networks. Brain Sciences. 2021; 11(9):1115. https://doi.org/10.3390/brainsci11091115
Chicago/Turabian StyleCasagrande, Maria, Francesca Agostini, Francesca Favieri, Giuseppe Forte, Jasmine Giovannoli, Angela Guarino, Andrea Marotta, Fabrizio Doricchi, and Diana Martella. 2021. "Age-Related Changes in Hemispherical Specialization for Attentional Networks" Brain Sciences 11, no. 9: 1115. https://doi.org/10.3390/brainsci11091115
APA StyleCasagrande, M., Agostini, F., Favieri, F., Forte, G., Giovannoli, J., Guarino, A., Marotta, A., Doricchi, F., & Martella, D. (2021). Age-Related Changes in Hemispherical Specialization for Attentional Networks. Brain Sciences, 11(9), 1115. https://doi.org/10.3390/brainsci11091115






