Neural Interruption by Unilateral Labyrinthectomy Biases the Directional Preference of Otolith-Related Vestibular Neurons
Abstract
1. Introduction
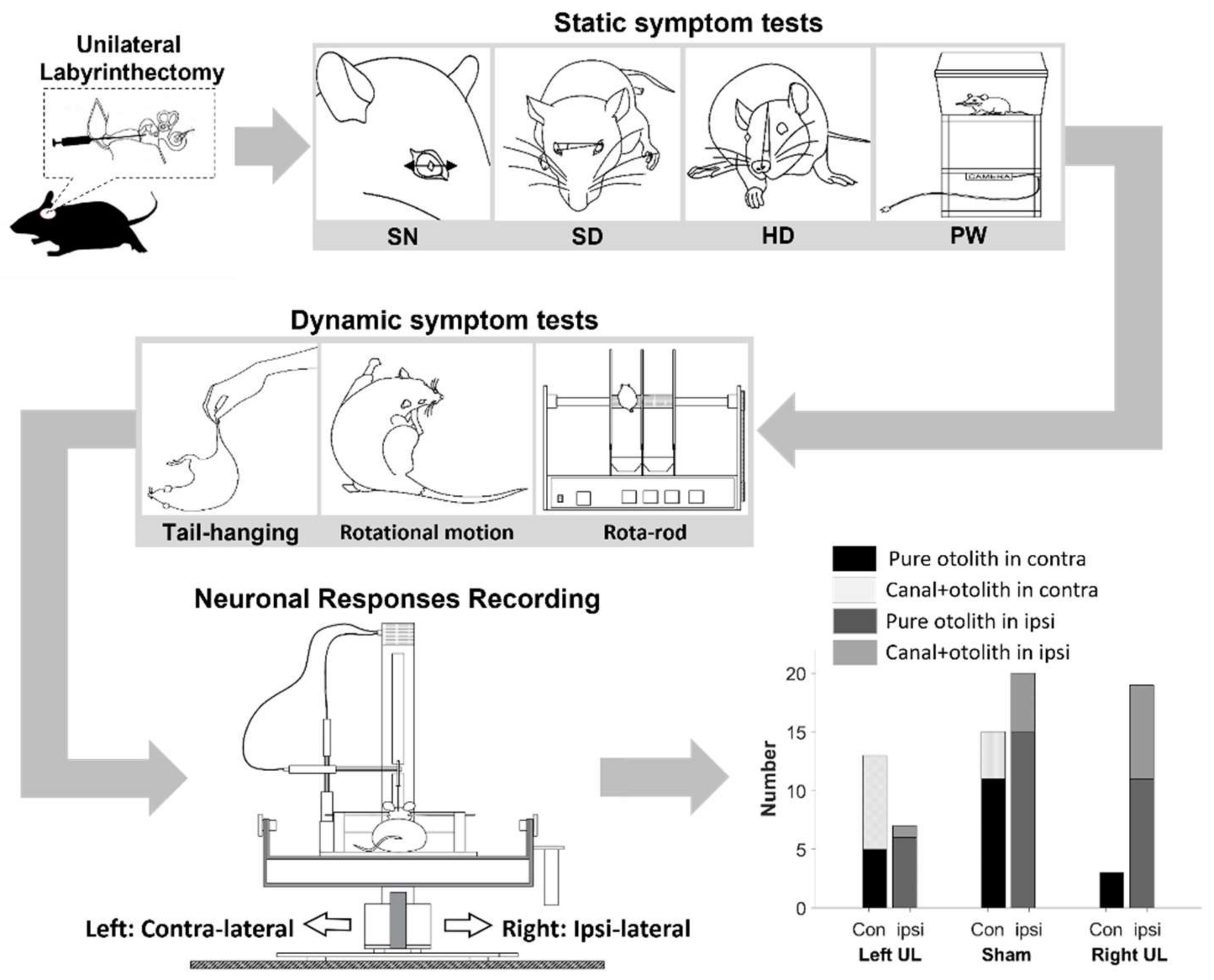
2. Materials and Methods
2.1. Animal Preparation
2.2. Behavioral Tests
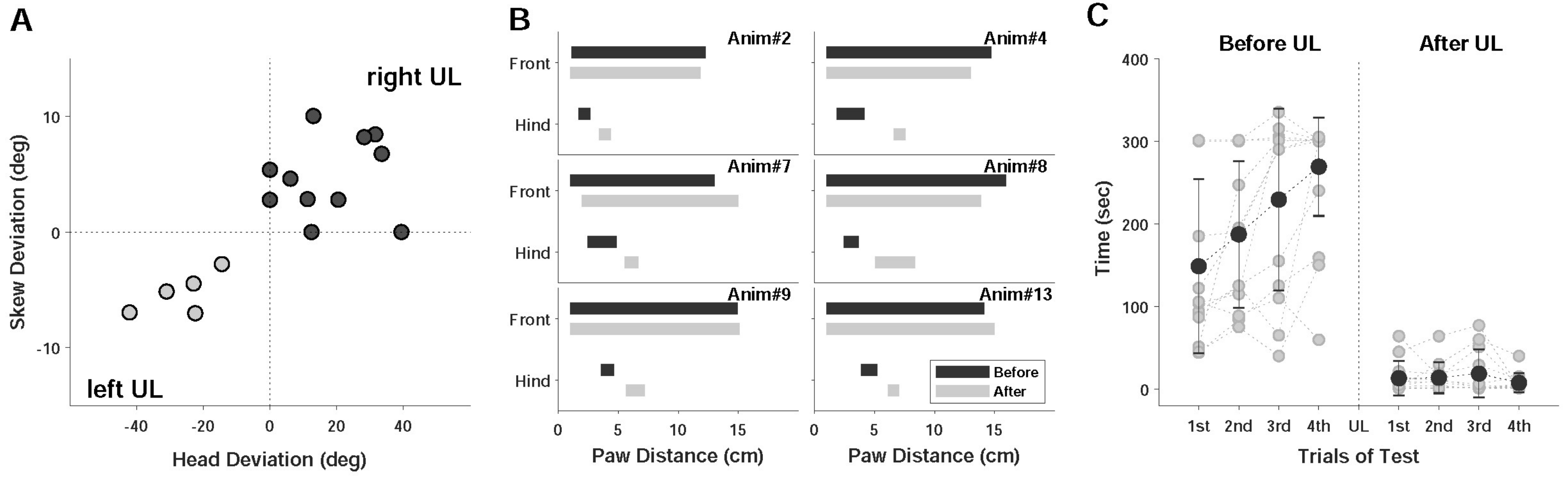
2.2.1. Static Symptoms
2.2.2. Dynamic Symptoms
2.3. Extracellular Neural Recording
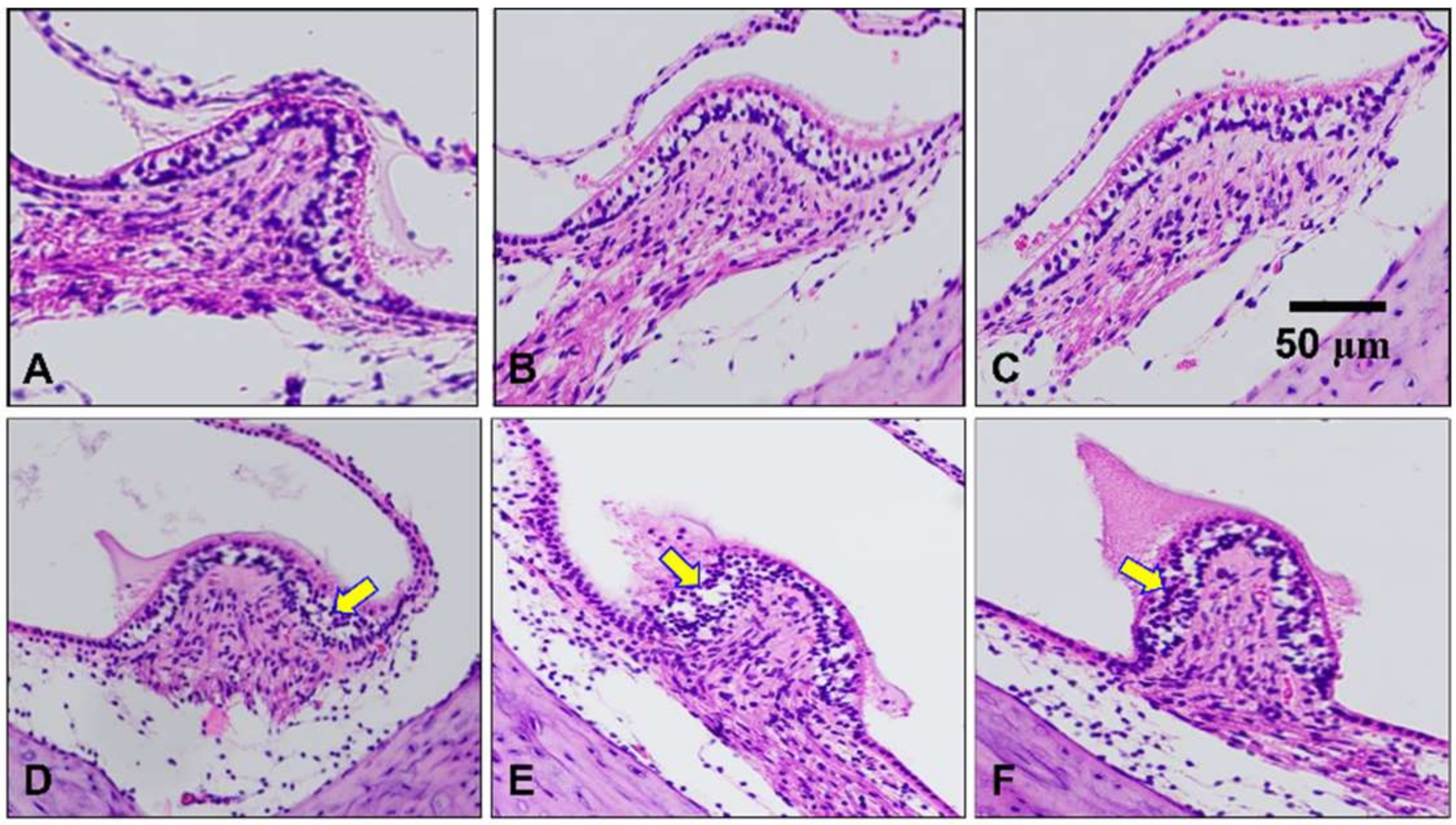
2.4. Histological Assessment of the Peripheral Vestibular Structure
2.5. Data Analysis
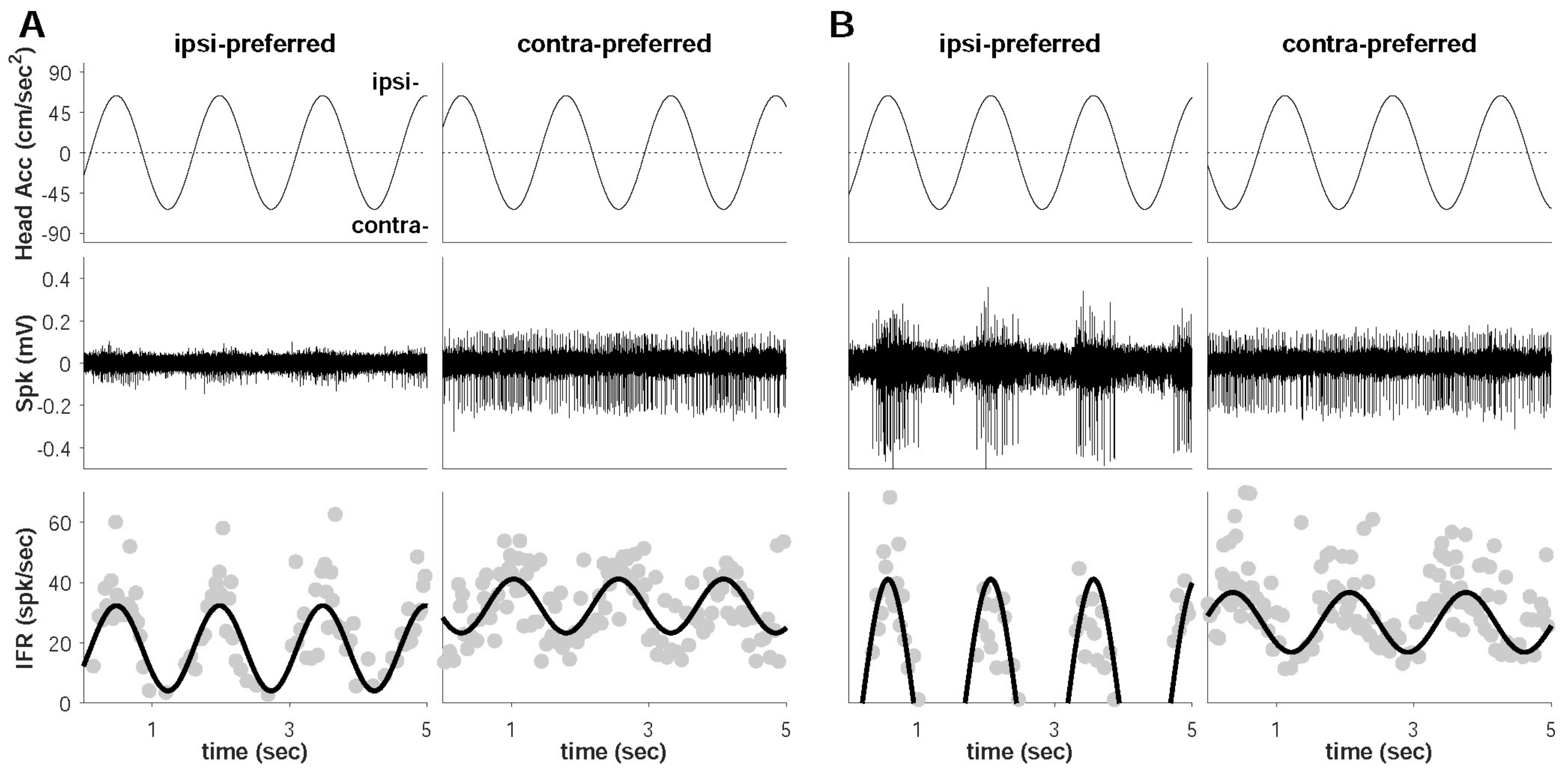
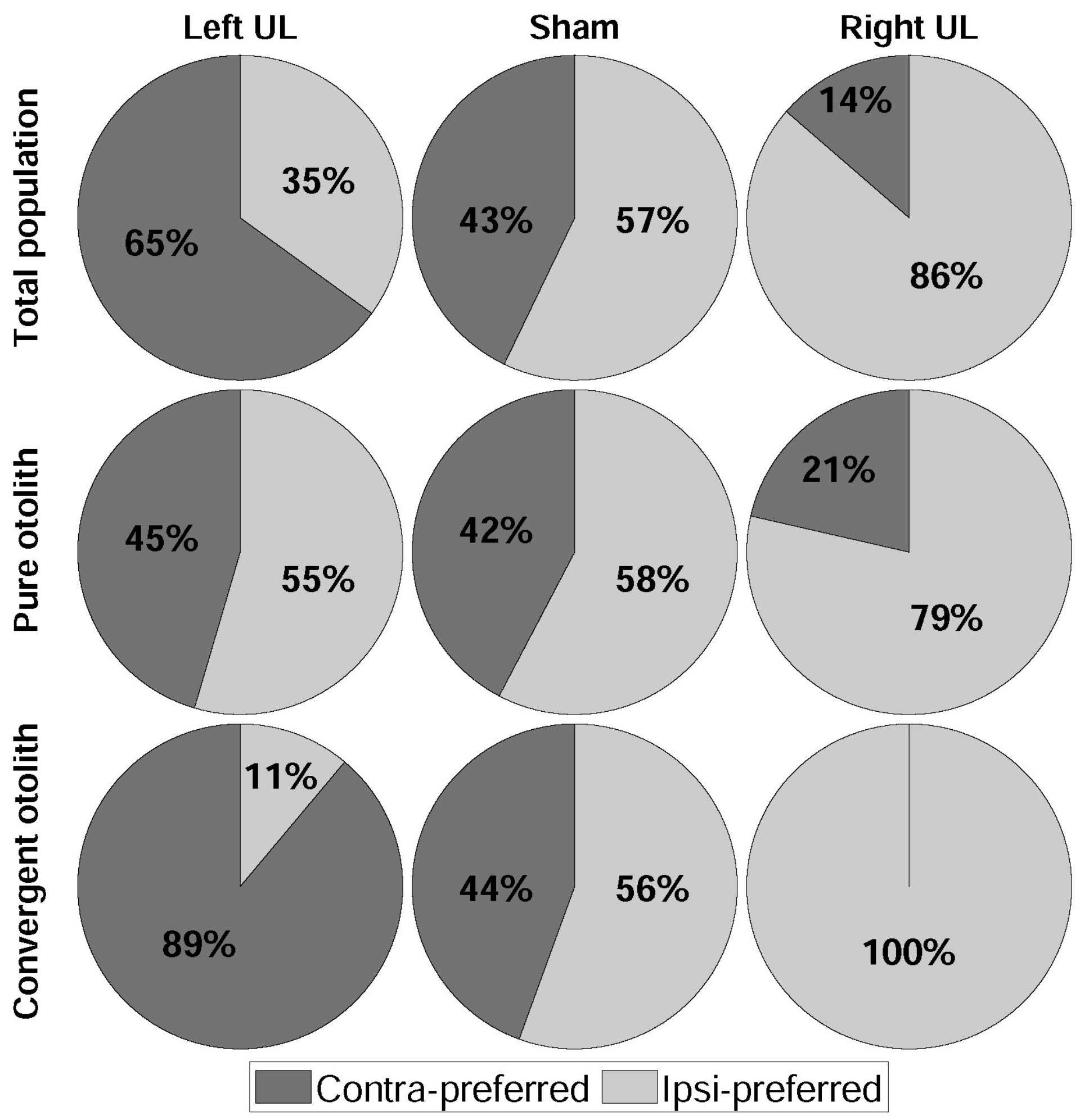
3. Results
4. Discussion
4.1. Dominant Driving Force of Directional Preference
4.2. Model Confirmation Based on Behavioral Responses and Histological Results
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Angelaki, D.E.; Cullen, K.E. Vestibular System: The Many Facets of a Multimodal Sense. Annu. Rev. Neurosci. 2008, 31, 125–150. [Google Scholar] [CrossRef]
- Borel, L.; Lopez, C.; Péruch, P.; Lacour, M. Vestibular syndrome: A change in internal spatial representation. Neurophysiol. Clin. 2008, 38, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Lacour, M.; Dutheil, S.; Tighilet, B.; Lopez, C.; Borel, L. Tell Me Your Vestibular Deficit, and I’ll Tell You How You’ll Compensate. Ann. N. Y. Acad. Sci. 2009, 1164, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Péricat, D.; Farina, A.; Agavnian-Couquiaud, E.; Chabbert, C.; Tighilet, B. Complete and irreversible unilateral vestibular loss: A novel rat model of vestibular pathology. J. Neurosci. Methods 2017, 283, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.E.; Della Santina, C.C.; Cullen, K.E. Plasticity within excitatory and inhibitory pathways of the vestibulo-spinal circuitry guides changes in motor performance. Sci. Rep. 2017, 7, 853. [Google Scholar] [CrossRef] [PubMed]
- Yoder, R.M.; Taube, J.S. Head direction cell activity in mice: Robust directional signal depends on intact otolith organs. J. Neurosci. 2009, 29, 1061–1076. [Google Scholar] [CrossRef]
- Kasri, M.; Picquet, F.; Falempin, M. Effects of unilateral and bilateral labyrinthectomy on rat postural muscle properties: The soleus. Exp. Neurol. 2004, 185, 143–153. [Google Scholar] [CrossRef]
- Dakin, C.J.; Héroux, M.E.; Luu, B.L.; Inglis, J.T.; Blouin, J.S. Vestibular contribution to balance control in the medial gastrocnemius and soleus. J. Neurophysiol. 2004, 115, 1289–1297. [Google Scholar] [CrossRef]
- Chan, Y.S.; Cheung, Y.M. Response of otolith-related neurons in bilateral vestibular nucleus of acute hemilabyrinthectomized cats to off-vertical axis rotationsa. Ann. N. Y. Acad. Sci. 1992, 656, 755–765. [Google Scholar] [CrossRef]
- Hitier, M.; Besnard, S.; Smith, P.F. Vestibular pathways involved in cognition. Front. Integ. Neurosci. 2014, 8, 59. [Google Scholar] [CrossRef]
- Newlands, S.D.; Abbatematteo, B.; Wei, M.; Carney, L.H.; Luan, H. Convergence of linear acceleration and yaw rotation signals on non-eye movement neurons in the vestibular nucleus of macaques. J. Neurophysiol. 2018, 119, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Lowenstein, O.; Sand, A. The individual and integrated activity of the semicircular canals of the elasmobranch labyrinth. J. Physiol. 1940, 99, 89–101. [Google Scholar] [CrossRef]
- Lindeman, H.H. Studies on the morphology of the sensory regions of the vestibular apparatus. Ergeb. Anatom. Entwick. 1969, 42, 1–113. [Google Scholar]
- Carey, J.P.; Della Santina, C.C. Principles of Applied Vestibular Physiology. In Cummings Otolaryngology-Head and Neck Surgery; Flint, P.W., Haughey, B., Lund, V., Robbins, K., Thomas, J.R., Lesperance, M., Eds.; Elsevier: St. Louis, MO, USA, 2014; pp. 2494–2524. [Google Scholar]
- Fitzpatrick, R.C.; Day, B.L. Probing the human vestibular system with galvanic stimulation. J. Appl. Physiol. 2004, 96, 2301–2316. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, C.; Goldberg, J.M. Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. I. Response to static tilts and to long-duration centrifugal force. J. Neurophysiol. 1976, 39, 970–984. [Google Scholar] [CrossRef]
- Loe, P.R.; Tomko, D.L.; Werner, G. The neural signal of angular head position in primary afferent vestibular nerve axons. J. Physiol. 1973, 230, 29–50. [Google Scholar] [CrossRef]
- Halmagyi, G.M.; Curthoys, I.S.; Dai, M.J. Diagnosis of Unilateral Otolith Hypofunction. Neurol. Clin. 1990, 8, 313–329. [Google Scholar] [CrossRef]
- Newlands, S.D.; Lin, N.; Wei, M. Responses of non-eye movement central vestibular neurons to sinusoidal horizontal translation in compensated macaques after unilateral labyrinthectomy. J. Neurophysiol. 2014, 112, 9–21. [Google Scholar] [CrossRef][Green Version]
- Smith, P.F.; Curthoys, I.S. Mechanisms of recovery following unilateral labyrinthectomy: A review. Brain Res. Rev. 1989, 14, 155–180. [Google Scholar] [CrossRef]
- Lacour, M.; Helmchen, C.; Vidal, P.P. Vestibular compensation: The neuro-otologist’s best friend. J. Neurol. 2016, 263, 54–64. [Google Scholar] [CrossRef]
- Kim, G.; Kim, K.S.; Lee, S. The integration of neural information by a passive kinetic stimulus and galvanic vestibular stimulation in the lateral vestibular nucleus. Med. Biol. Eng. Comput. 2017, 55, 1621–1633. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Kim, K.S.; Lee, S. Non-associative learning processes in vestibular nucleus. Med. Biol. Eng. Comput. 2018, 56, 1841–1851. [Google Scholar] [CrossRef]
- Kim, G.; Lee, S.; Kim, K.S. Dominant parameter of galvanic vestibular stimulation for the non-associative learning processes. Med. Biol. Eng. Comput. 2020, 58, 701–708. [Google Scholar] [CrossRef]
- Lannou, J.; Cazin, L.; Hamann, K.F. Response of central vestibular neurons to horizontal linear acceleration in the rat. Pflügers Arch. 1980, 385, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Bergstrom, R.A.; You, Y.; Erway, L.C.; Lyon, M.F.; Schimenti, J.C. Deletion mapping of the head tilt (het) gene in mice: A vestibular mutation causing specific absence of otoliths. Genetics 1998, 150, 815–822. [Google Scholar] [CrossRef]
- Cassel, R.; Bordiga, P.; Carcaud, J.; Simon, F.; Beraneck, M.; Le Gall, A.; Benoit, A.; Bouet, V.; Philoxene, B.; Besnard, S.; et al. Morphological and functional correlates of vestibular synaptic deafferentation and repair in a mouse model of acute-onset vertigo. Dis. Models Mech. 2019, 12, dmm039115. [Google Scholar] [CrossRef]
- Aust, S.D.; Morehouse, L.A.; Thomas, C.E. Role of metals in oxygen radical reactions. J. Free Radic. Biol. Med. 1985, 1, 3–25. [Google Scholar] [CrossRef]
- Bostanci, M.Ö.; Bagirici, F. Blocking of L-type calcium channels protects hippocampal and nigral neurons against iron neurotoxicity: The role of L-type calcium channels in iron-induced neurotoxicity. Int. J. Neurosci. 2013, 123, 876–882. [Google Scholar] [CrossRef]
- Willmore, L.J.; Hiramatsu, M.; Kochi, H.; Mori, A. Formation of superoxide radicals after FeCl3 injection into rat isocortex. Brain Res. 1983, 277, 393–396. [Google Scholar] [CrossRef]
- Xerri, C.; Gianni, S.; Manzoni, D.; Pompeiano, O. Central compensation of vestibular deficits. I. Response characteristics of lateral vestibular neurons to roll tilt after ipsilateral labyrinth deafferentation. J. Neurophysiol. 1983, 50, 428–448. [Google Scholar] [CrossRef]
- Curthoys, I.S. Vestibular compensation and substitution. Curr. Opin. Neurol. 2000, 13, 27–30. [Google Scholar] [CrossRef]
- Darlington, C.L.; Lawlor, P.; Smith, P.F.; Dragunow, M. Temporal relationship between the expression of Fos, Jun and Krox-24 in the guinea pig vestibular nuclei during the development of vestibular compensation for unilateral vestibular deafferentation. Brain Res. 1996, 735, 173–176. [Google Scholar] [CrossRef]
- Shaabani, M.; Lotfi, Y.; Karimian, S.M.; Rahgozar, M.; Hooshmandi, M. Short-term galvanic vestibular stimulation promotes functional recovery and neurogenesis in unilaterally labyrinthectomized rats. Brain Res. 2016, 1648, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Vignaux, G.; Chabbert, C.; Gaboyard-Niay, S.; Travo, C.; Machado, M.L.; Denise, P.; Comoz, F.; Hitier, M.; Landemore, G.; Philoxène, B.; et al. Evaluation of the chemical model of vestibular lesions induced by arsanilate in rats. Toxicol. Appl. Pharmacol. 2012, 258, 61–71. [Google Scholar] [CrossRef]
- Lee, J.; Kim, M.; Park, B. Vestibular end organ injury induced by middle ear treatment with ferric chloride in rats. Hum. Exp. Toxicol. 2017, 36, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Kim, J.H.; Jin, Y.Z.; Kry, D.; Park, B.R. Temporal changes of cFos-like protein expression in medial vestibular nuclei following arsanilate-induced unilateral labyrinthectomy in rats. Neurosci. Lett. 2002, 319, 9–12. [Google Scholar] [CrossRef]
- Horiike, O.; Shimogori, H.; Yamashita, H. Effect of edaravone on streptomycin-induced vestibulotoxicity in the guinea pig. Laryngoscope 2004, 114, 1630–1632. [Google Scholar] [CrossRef]
- Shimogori, H.; Yamashita, H. Peripheral vestibular disorder induced by (±)-α-amino-3-hydroxy-5-methyl-isoxazole-4-propionic acid (AMPA). Neurosci. Lett. 2004, 371, 69–72. [Google Scholar] [CrossRef]
- Brodsky, M.C.; Donahue, S.P.; Vaphiades, M.; Brandt, T. Skew deviation revisited. Surv. Ophthalmol. 2006, 51, 105–128. [Google Scholar] [CrossRef]
- Hitier, M.; Besnard, S.; Vignaux, G.; Denise, P.; Moreau, S. The ventrolateral surgical approach to labyrinthectomy in rats: Anatomical description and clinical consequences. Surg. Radiol. Anat. 2010, 32, 835–842. [Google Scholar] [CrossRef]
- Sirkin, D.W.; Precht, W.; Courjon, J.H. Initial, rapid phase of recovery from unilateral vestibular lesion in rat not dependent on survival of central portion of vestibular nerve. Brain Res. 1984, 302, 245–256. [Google Scholar] [CrossRef]
| Symptom | Right UL Model | Left UL Model | Total Number (%) |
|---|---|---|---|
| Spontaneous Nystagmus | 7 | 3 | 10 (52.6) |
| Skew Deviation | 9 | 5 | 14 (73.7) |
| Head Deviation | 9 | 5 | 14 (73.7) |
| Paw Distance | 8 | 5 | 13 (68.4) |
| Rota-Rod | 7 | 4 | 11 (57.9) |
| Rotational Motion | 8 | 4 | 12 (63.2) |
| Tail Hanging | 13 | 2 | 15 (78.9) |
| Symptom | Left UL 1 | Sham | Right UL | |||
|---|---|---|---|---|---|---|
| Preferred Direction | Cont. 2 | Ipsi. 3 | Cont. | Ipsi. | Cont. | Ipsi. |
| Pure otolith | 5 | 6 | 11 | 15 | 3 | 11 |
| Canal + otolith | 8 | 1 | 4 | 5 | 0 | 8 |
| Total | 13 | 7 | 15 | 20 | 3 | 19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, N.; Kim, K.-S.; Kim, G. Neural Interruption by Unilateral Labyrinthectomy Biases the Directional Preference of Otolith-Related Vestibular Neurons. Brain Sci. 2021, 11, 987. https://doi.org/10.3390/brainsci11080987
Nguyen N, Kim K-S, Kim G. Neural Interruption by Unilateral Labyrinthectomy Biases the Directional Preference of Otolith-Related Vestibular Neurons. Brain Sciences. 2021; 11(8):987. https://doi.org/10.3390/brainsci11080987
Chicago/Turabian StyleNguyen, Nguyen, Kyu-Sung Kim, and Gyutae Kim. 2021. "Neural Interruption by Unilateral Labyrinthectomy Biases the Directional Preference of Otolith-Related Vestibular Neurons" Brain Sciences 11, no. 8: 987. https://doi.org/10.3390/brainsci11080987
APA StyleNguyen, N., Kim, K.-S., & Kim, G. (2021). Neural Interruption by Unilateral Labyrinthectomy Biases the Directional Preference of Otolith-Related Vestibular Neurons. Brain Sciences, 11(8), 987. https://doi.org/10.3390/brainsci11080987






