Restrained Eating Is Associated with Lower Cortical Thickness in the Inferior Frontal Gyrus in Adolescents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Eating Behavior Scales
2.3. Clinical Variables
2.4. Executive Functions
2.5. Personality
2.6. MRI Acquisition
2.7. MRI Processing: Cortical and Subcortical Brain Regions
2.8. Cluster Analysis
2.9. Minimally- and Fully-Adjusted ANCOVA Models
3. Results
3.1. Adolescent Sample (n = 108)
3.1.1. Cluster Analysis
3.1.2. Executive Functions and Personality
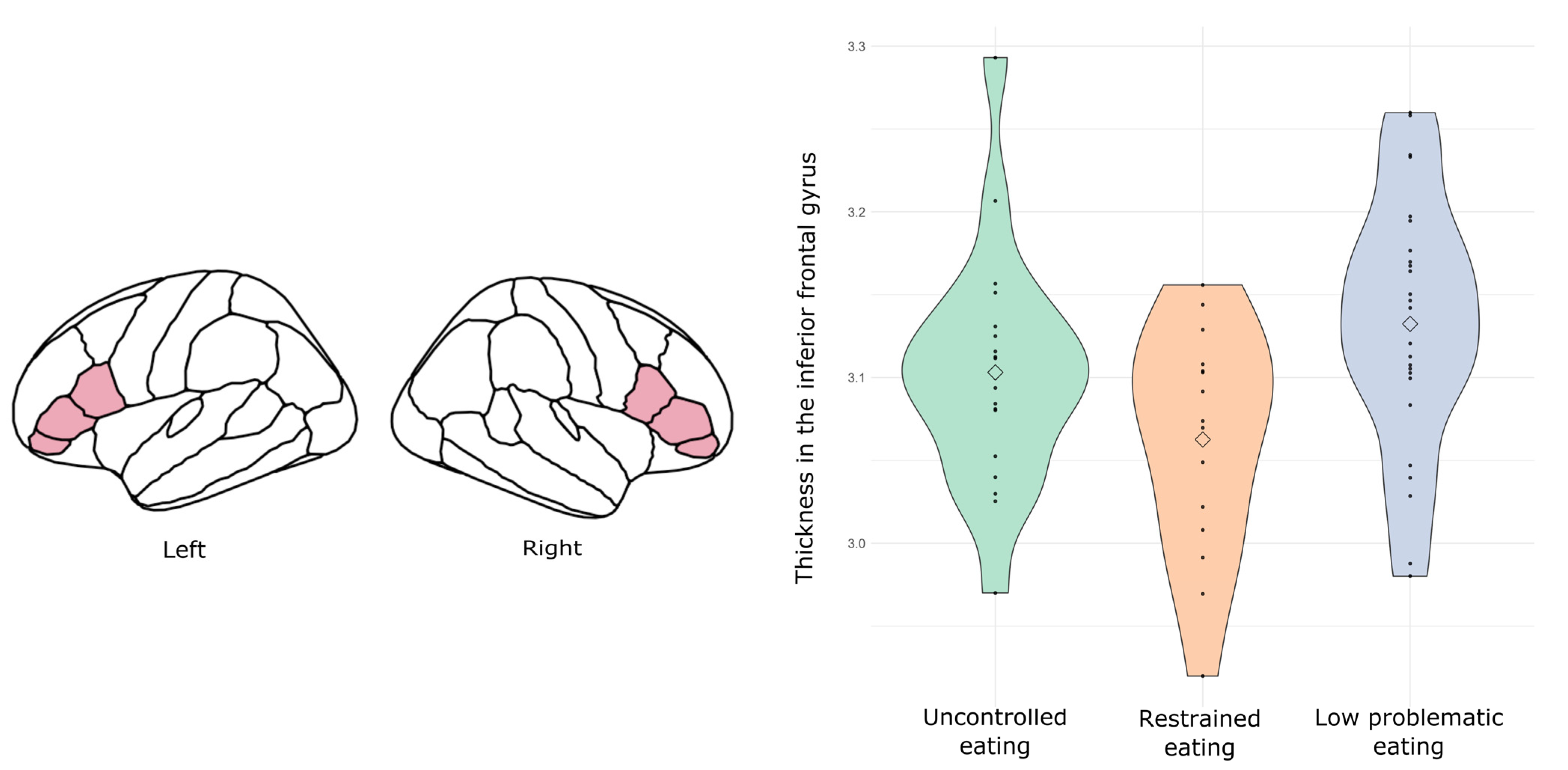
3.1.3. Neuroanatomical Results (Subsample: n = 60)
3.2. Adult Sample (n = 175)
3.2.1. Cluster Analysis
3.2.2. Executive Functions and Personality
3.2.3. Neuroanatomical Results (Subsample n = 106)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Udo, T.; Grilo, C.M. Prevalence and Correlates of DSM-5–Defined Eating Disorders in a Nationally Representative Sample of U.S. Adults. Biol. Psychiatry 2018, 84, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, C.; Hayward, C.; De Zwaan, M.; Kraemer, H.C.; Agras, S. Coming to Terms With Risk Factors for Eating Disorders: Application of Risk Terminology and Suggestions for a General Taxonomy. Psychol. Bull. 2004, 130, 19–65. [Google Scholar] [CrossRef]
- Stice, E.; Gau, J.M.; Rohde, P.; Shaw, H. Risk factors that predict future onset of each DSM–5 eating disorder: Predictive specificity in high-risk adolescent females. J. Abnorm. Psychol. 2017, 126, 38–51. [Google Scholar] [CrossRef]
- Hilbert, A.; Pike, K.M.; Goldschmidt, A.B.; Wilfley, D.E.; Fairburn, C.G.; Dohm, F.-A.; Walsh, B.T.; Weissman, R.S. Risk factors across the eating disorders. Psychiatry Res. 2014, 220, 500–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poínhos, R.; Oliveira, B.M.; Correia, F. Eating behavior patterns and BMI in Portuguese higher education students. Appetite 2013, 71, 314–320. [Google Scholar] [CrossRef]
- Bouhlal, S.; McBride, C.M.; Trivedi, N.S.; Agurs-Collins, T.; Persky, S. Identifying eating behavior phenotypes and their correlates: A novel direction toward improving weight management interventions. Appetite 2017, 111, 142–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lunn, S.; Poulsen, S.; Daniel, S.I. Subtypes in bulimia nervosa: The role of eating disorder symptomatology, negative affect, and interpersonal functioning. Compr. Psychiatry 2012, 53, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Galmiche, M.; Déchelotte, P.; Lambert, G.; Tavolacci, M.P. Prevalence of eating disorders over the 2000–2018 period: A systematic literature review. Am. J. Clin. Nutr. 2019, 109, 1402–1413. [Google Scholar] [CrossRef] [PubMed]
- Vainik, U.; Garcia, I.G.; Dagher, A. Uncontrolled eating: A unifying heritable trait linked with obesity, overeating, personality and the brain. Eur. J. Neurosci. 2018, 50, 2430–2445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartra, O.; McGuire, J.T.; Kable, J.W. The valuation system: A coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. NeuroImage 2013, 76, 412–427. [Google Scholar] [CrossRef] [Green Version]
- Grace, A.A.; Floresco, S.; Goto, Y.; Lodge, D.J. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007, 30, 220–227. [Google Scholar] [CrossRef]
- Berridge, K.C.; Ho, C.-Y.; Richard, J.; DiFeliceantonio, A. The tempted brain eats: Pleasure and desire circuits in obesity and eating disorders. Brain Res. 2010, 1350, 43–64. [Google Scholar] [CrossRef] [Green Version]
- Han, J.E.; Boachie, N.; Garcia-Garcia, I.; Michaud, A.; Dagher, A. Neural correlates of dietary self-control in healthy adults: A meta-analysis of functional brain imaging studies. Physiol. Behav. 2018, 192, 98–108. [Google Scholar] [CrossRef]
- Sominsky, L.; Spencer, S.J. Eating behavior and stress: A pathway to obesity. Front. Psychol. 2014, 5, 434. [Google Scholar] [CrossRef]
- Kistenmacher, A.; Goetsch, J.; Ullmann, D.; Wardzinski, E.K.; Melchert, U.H.; Jauch-Chara, K.; Oltmanns, K.M. Psychosocial stress promotes food intake and enhances the neuroenergetic level in men. Stress 2018, 21, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, L.; Adolphs, R. Emotion processing and the amygdala: From a ‘low road’ to ‘many roads’ of evaluating biological significance. Nat. Rev. Neurosci. 2010, 11, 773–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-García, I.; Kube, J.; Gaebler, M.; Horstmann, A.; Villringer, A.; Neumann, J. Neural processing of negative emotional stimuli and the influence of age, sex and task-related characteristics. Neurosci. Biobehav. Rev. 2016, 68, 773–793. [Google Scholar] [CrossRef] [PubMed]
- Vuilleumier, P. How brains beware: Neural mechanisms of emotional attention. Trends Cogn. Sci. 2005, 9, 585–594. [Google Scholar] [CrossRef]
- Yao, L.; Li, W.; Dai, Z.; Dong, C. Eating behavior associated with gray matter volume alternations: A voxel based morphometry study. Appetite 2016, 96, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Zhang, Y.; Qiu, J.; Li, X.; Ma, K.; Chen, S.; Chen, H. Brain structures associated with eating behaviors in normal-weight young females. Neuropsychol. 2019, 133, 107171. [Google Scholar] [CrossRef] [PubMed]
- Abdo, N.; Boyd, E.; Baboumian, S.; Pantazatos, S.P.; Geliebter, A. Relationship between binge eating and associated eating behaviors with subcortical brain volumes and cortical thickness. J. Affect. Disord. 2020, 274, 1201–1205. [Google Scholar] [CrossRef] [PubMed]
- Maayan, L.; Hoogendoorn, C.; Sweat, V.; Convit, A. Disinhibited Eating in Obese Adolescents Is Associated with Orbitofrontal Volume Reductions and Executive Dysfunction. Obesity 2011, 19, 1382–1387. [Google Scholar] [CrossRef] [PubMed]
- Calvo, D.; Galioto, R.; Gunstad, J.; Spitznagel, M.B. Uncontrolled eating is associated with reduced executive functioning. Clin. Obes. 2014, 4, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Segura-Serralta, M.; Ciscar, S.; Blasco, L.; Oltra-Cucarella, J.; Roncero, M.; Espert, R.; Elvira, V.; Pinedo-Esteban, R.; Perpiñá, C. Contribution of executive functions to eating behaviours in obesity and eating disorders. Behav. Cogn. Psychother. 2020, 48, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Jonker, N.C.; Bennik, E.C.; De Jong, P.J. Reinforcement sensitivity and restrained eating: The moderating role of executive control. Eat. Weight. Disord. 2016, 23, 321–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prunell-Castañé, A.; Jurado, M.; García-García, I. Clinical binge eating, but not uncontrolled eating, is associated with differences in executive functions: Evidence from meta-analytic findings. Addict. Behav. Rep. 2021, 13, 100337. [Google Scholar] [CrossRef] [PubMed]
- Prats-Soteras, X.; Jurado, M.A.; Ottino-González, J.; García-García, I.; Segura, B.; Caldú, X.; Sánchez-Garre, C.; Miró, N.; Tor, C.; Sender-Palacios, M.; et al. Inflammatory agents partially explain associations between cortical thickness, surface area, and body mass in adolescents and young adulthood. Int. J. Obes. 2020, 44, 1487–1496. [Google Scholar] [CrossRef] [PubMed]
- González, J.O.; Jurado, M.; Garcia, I.G.; Caldú, X.; Prats-Soteras, X.; Tor, E.; Sender-Palacios, M.; Garolera, M. Allostatic load and executive functions in overweight adults. Psychoneuroendocrinology 2019, 106, 165–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ottino-González, J.; Jurado, M.A.; Garcia-Garcia, I.; Segura, B.; Marques-Iturria, I.; Sender-Palacios, M.J.; Tor, E.; Prats-Soteras, X.; Caldú, X.; Junqué, C.; et al. Allostatic Load Is Linked to Cortical Thickness Changes Depending on Body-Weight Status. Front. Hum. Neurosci. 2017, 11, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Anglé, S.; Engblom, J.; Eriksson, T.; Kautiainen, S.; Saha, M.-T.; Lindfors, P.; Lehtinen, M.; Rimpelä, A. Three factor eating questionnaire-R18 as a measure of cognitive restraint, uncontrolled eating and emotional eating in a sample of young Finnish females. Int. J. Behav. Nutr. Phys. Act. 2009, 6, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henderson, M.; Freeman, C.P.L. A Self-rating Scale for Bulimia the ‘BITE’. Br. J. Psychiatry 1987, 150, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Browning, L.M.; Hsieh, S.D.; Ashwell, M. A systematic review of waist-to-height ratio as a screening tool for the prediction of cardiovascular disease and diabetes: 0·5 could be a suitable global boundary value. Nutr. Res. Rev. 2010, 23, 247–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, K.; Wong, M.; Khalechelvam, P.; Tam, W. Waist-to-height ratio, body mass index and waist circumference for screening paediatric cardio-metabolic risk factors: A meta-analysis. Obes. Rev. 2016, 17, 1258–1275. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.J.; Bellizzi, M.C.; Flegal, K.; Dietz, W.H. Establishing a standard definition for child overweight and obesity worldwide: International survey. BMJ 2000, 320, 1240. [Google Scholar] [CrossRef] [Green Version]
- Herrero, M.; Blanch, J.; Peri, J.; De Pablo, J.; Pintor, L.; Bulbena, A. A validation study of the hospital anxiety and depression scale (HADS) in a Spanish population. Gen. Hosp. Psychiatry 2003, 25, 277–283. [Google Scholar] [CrossRef]
- Heaton, R.K. Wisconsin Card Sorting Test: Computer; Version 4; Research Edition; Psychological Assessment Resources: Florida, FL, USA, 1999; p. 4. [Google Scholar]
- Reitan, R.M. Validity of the Trail Making Test as an indicator of organic brain damage. Percept. Mot. Ski. 1958, 8, 271–276. [Google Scholar] [CrossRef]
- Diamond, A. Executive Functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef] [Green Version]
- Golden, C.J. STROOP, Test de Colores y Palabras; TEA Ediciones: Madrid, Spain, 1995. [Google Scholar]
- Conners, K.C. Conners’ CPT-II Continuous Performance test II; Version 5; MHS Multi Heath Syst. Inc.: North Tonawanda, NY, USA, 2004. [Google Scholar]
- Cloninger, C.R.; Svrakic, D.M. Integrative Psychobiological Approach to Psychiatric Assessment and Treatment. Psychiatry 1997, 60, 120–141. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Zotes, J.A.; Bayón, C.; Montserrat, C.; Valero, J.; Labad, A.; Cloninger, C.R.; Fernández-Aranda, F. Temperament and Character Inventory-Revised (TCI-R). Standardization and normative data in a general population sample. Actas Esp. Psiquiatr. 2004, 32, 8–15. [Google Scholar] [PubMed]
- Kirby, K.N.; Petry, N.M.; Bickel, W.K. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J. Exp. Psychol. 1999, 128, 78–87. [Google Scholar] [CrossRef]
- Struyf, A.; Hubert, M.; Rousseeuw, P.J. Clustering in an Object-Oriented Environment. J. Stat. Softw. 1996, 1, 1–30. [Google Scholar] [CrossRef] [Green Version]
- Hirst, R.B.; Beard, C.L.; Colby, K.A.; Quittner, Z.; Mills, B.M.; Lavender, J.M. Anorexia nervosa and bulimia nervosa: A meta-analysis of executive functioning. Neurosci. Biobehav. Rev. 2017, 83, 678–690. [Google Scholar] [CrossRef] [PubMed]
- Tenconi, E.; Santonastaso, P.; Degortes, D.; Bosello, R.; Titton, F.; Mapelli, D.; Favaro, A. Set-shifting abilities, central coherence, and handedness in anorexia nervosa patients, their unaffected siblings and healthy controls: Exploring putative endophenotypes. World J. Biol. Psychiatry 2010, 11, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Tchanturia, K.; Davies, H.; Roberts, M.; Harrison, A.; Nakazato, M.; Schmidt, U.; Treasure, J.; Morris, R. Poor Cognitive Flexibility in Eating Disorders: Examining the Evidence using the Wisconsin Card Sorting Task. PLoS ONE 2012, 7, e28331. [Google Scholar] [CrossRef] [PubMed]
- Seidel, M.; Brooker, H.; Lauenborg, K.; Wesnes, K.; Sjögren, M. Cognitive Function in Adults with Enduring Anorexia Nervosa. Nutrients 2021, 13, 859. [Google Scholar] [CrossRef]
- Udo, T.; Grilo, C.M. Psychiatric and medical correlates of DSM-5 eating disorders in a nationally representative sample of adults in the United States. Int. J. Eat. Disord. 2019, 52, 42–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamnes, C.K.; Herting, M.M.; Goddings, A.-L.; Meuwese, R.; Blakemore, S.-J.; Dahl, R.E.; Güroğlu, B.; Raznahan, A.; Sowell, E.R.; Crone, E.; et al. Development of the Cerebral Cortex across Adolescence: A Multisample Study of Inter-Related Longitudinal Changes in Cortical Volume, Surface Area, and Thickness. J. Neurosci. 2017, 37, 3402–3412. [Google Scholar] [CrossRef] [PubMed]
- Aron, A.R.; Robbins, T.; Poldrack, R.A. Inhibition and the right inferior frontal cortex: One decade on. Trends Cogn. Sci. 2014, 18, 177–185. [Google Scholar] [CrossRef] [PubMed]
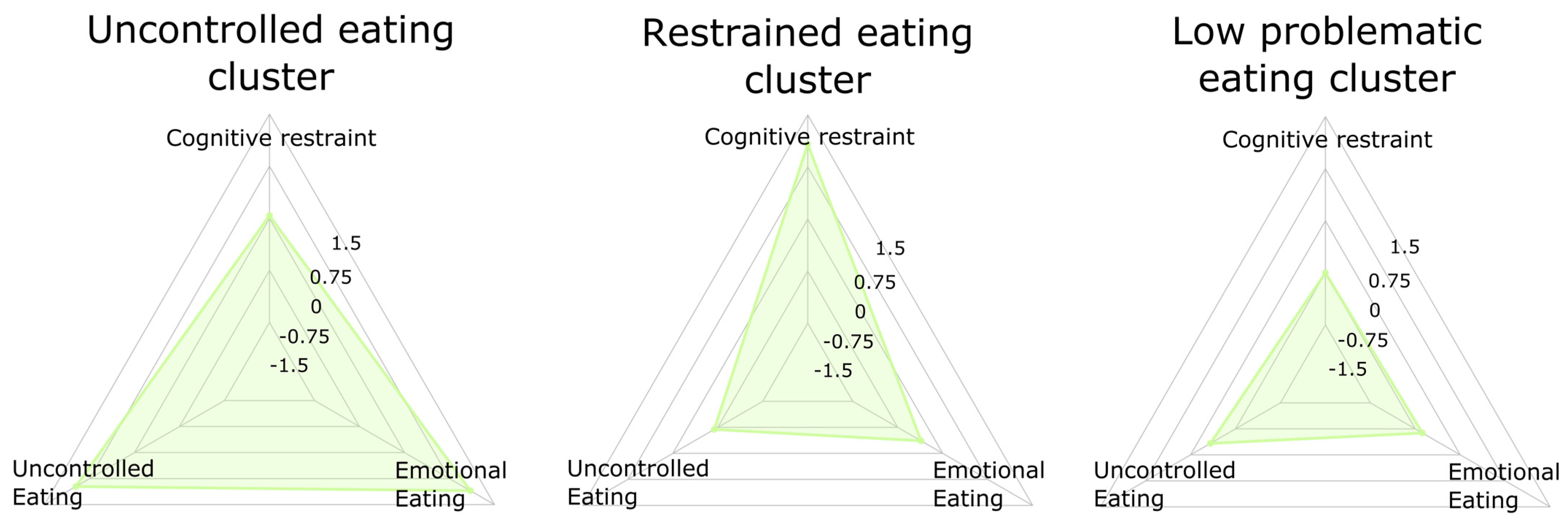

| Domains | Variables | Uncontrolled Eating (n = 35) | Restrained Eating (n = 29) | Low Problematic Eating (n = 44) | F | p |
|---|---|---|---|---|---|---|
| Intra-cluster variables (TFEQ; centered) | Cognitive restraint | 0.04 (0.87) | 1.08 (0.57) | −0.75 (0.56) | 63.54 | <0.001 |
| Disinhibited eating | 0.98 (0.77) | −0.69 (0.68) | −0.33 (0.70) | 51.15 | <0.001 | |
| Emotional eating | 1.10 (0.84) | −0.36 (0.64) | −0.63 (0.43) | 76.71 | <0.001 | |
| Extra-cluster validation (BITE) | BITE symptoms | 6.65 (4.26) | 2.71 (2.19) | 2.66 (2.07) | 19.93 | <0.001 |
| Demographic and clinical variables | Age | 14.63 (1.70) | 14.27 (1.50) | 14.28 (1.60) | 0.55 | 0.556 |
| Sex | 19 females (54%) 16 males (46%) | 14 females (48%) 15 males (52%) | 21 females (48%) 23 males (52%) | X2(2) = 0.3825 | 0.826 | |
| Waist (cm)/Height (cm) | 0.53 (0.08) | 0.55 (0.09) | 0.48 (0.08) | 12.17 | <0.001 | |
| BMI | 28.11 (5.91) | 28.82 (5.47) | 23.5 (5.59) | 9.60 | <0.001 | |
| Body weight status | 10 lean (29%) 5 overweight (14%) 20 obese (57%) | 5 lean (17%) 4 overweight (14%) 20 obese (69%) | 26 lean (59%) 7 overweight (16%) 11 obese (25%) | X2(2) = 17.57 | 0.002 | |
| Anxiety and depression (HADS) | 8.18 (4.23) | 7.34 (4.24) | 6.31 (4.35) | 1.81 | 0.170 |
| Domains | Variables | Uncontrolled Eating (n = 54) | Restrained Eating (n = 61) | Low Problematic Eating (n = 60) | F | p |
|---|---|---|---|---|---|---|
| Intra-cluster variables (TFEQ; centered) | Cognitive restraint | 0.27 (0.88) | 0.70 (0.68) | −0.96 (0.53) | 89.18 | <0.001 |
| Disinhibited eating | 1.01 (0.88) | −0.22 (0.47) | −0.10 (0.75) | 85.22 | <0.001 | |
| Emotional eating | 1.20 (0.62) | −0.19 (0.55) | −0.88 (0.39) | 227.4 | <0.001 | |
| Extra-cluster validation (BITE) | BITE symptoms | 10.45 (5.80) | 4.77 (3.29) | 2.49 (2.61) | 55.45 | <0.001 |
| Demographic and clinical variables | Age | 31.15 (7.60) | 30.69 (9.61) | 30.67 (18.14) | 0.06 | 0.945 |
| Sex | 37 females (68.5%) 17 males (31.5%) | 39 females (64%) 22 males (36%) | 28 females (47%) 32 males (53%) | X2 = 6.42 | 0.040 | |
| Waist (cm)/Height (cm) | 0.60 (0.11) | 0.56 (0.10) | 0.51 (0.10) | 12.17 | <0.001 | |
| BMI | 31.60 (7.68) | 29.80 (7.15) | 25.81 (7.74) | 8.97 | <0.001 | |
| Body weight status | 12 lean (22%) 9 overweight (17%) 33 obese (61%) | 16 lean (26%) 24 overweight (39%) 21 obese (34%) | 41 lean (68%) 5 overweight (23%) 14 obese (8%) | X2 = 44.68 | <0.001 | |
| Anxiety and depression (HADS) | 7.54 (4.79) | 5.39 (3.55) | 5.80 (3.78) | 7.19 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-García, I.; Garolera, M.; Ottino-González, J.; Prats-Soteras, X.; Prunell-Castañé, A.; Jurado, M.Á. Restrained Eating Is Associated with Lower Cortical Thickness in the Inferior Frontal Gyrus in Adolescents. Brain Sci. 2021, 11, 978. https://doi.org/10.3390/brainsci11080978
García-García I, Garolera M, Ottino-González J, Prats-Soteras X, Prunell-Castañé A, Jurado MÁ. Restrained Eating Is Associated with Lower Cortical Thickness in the Inferior Frontal Gyrus in Adolescents. Brain Sciences. 2021; 11(8):978. https://doi.org/10.3390/brainsci11080978
Chicago/Turabian StyleGarcía-García, Isabel, Maite Garolera, Jonatan Ottino-González, Xavier Prats-Soteras, Anna Prunell-Castañé, and María Ángeles Jurado. 2021. "Restrained Eating Is Associated with Lower Cortical Thickness in the Inferior Frontal Gyrus in Adolescents" Brain Sciences 11, no. 8: 978. https://doi.org/10.3390/brainsci11080978
APA StyleGarcía-García, I., Garolera, M., Ottino-González, J., Prats-Soteras, X., Prunell-Castañé, A., & Jurado, M. Á. (2021). Restrained Eating Is Associated with Lower Cortical Thickness in the Inferior Frontal Gyrus in Adolescents. Brain Sciences, 11(8), 978. https://doi.org/10.3390/brainsci11080978







