Weaker Connectivity of the Cortical Networks Is Linked with the Uncharacteristic Gait in Youth with Cerebral Palsy
Abstract
:1. Introduction
2. Material and Method
2.1. Participants
2.2. MRI Data Acquisition
2.3. Preprocessing Analyses
2.4. Quality Control
2.5. Seed-Based Functional Connectivity Analyses
2.5.1. Definition of the Seed Regions
2.5.2. First-Level Analyses
2.5.3. Second-Level Analyses
2.6. Spatiotemporal Gait Biomechanics
3. Results
3.1. Demographics
3.2. Sensorimotor Network Functional Connectivity
3.2.1. Visual Network Functional Connectivity
3.2.2. Auditory Network Functional Connectivity
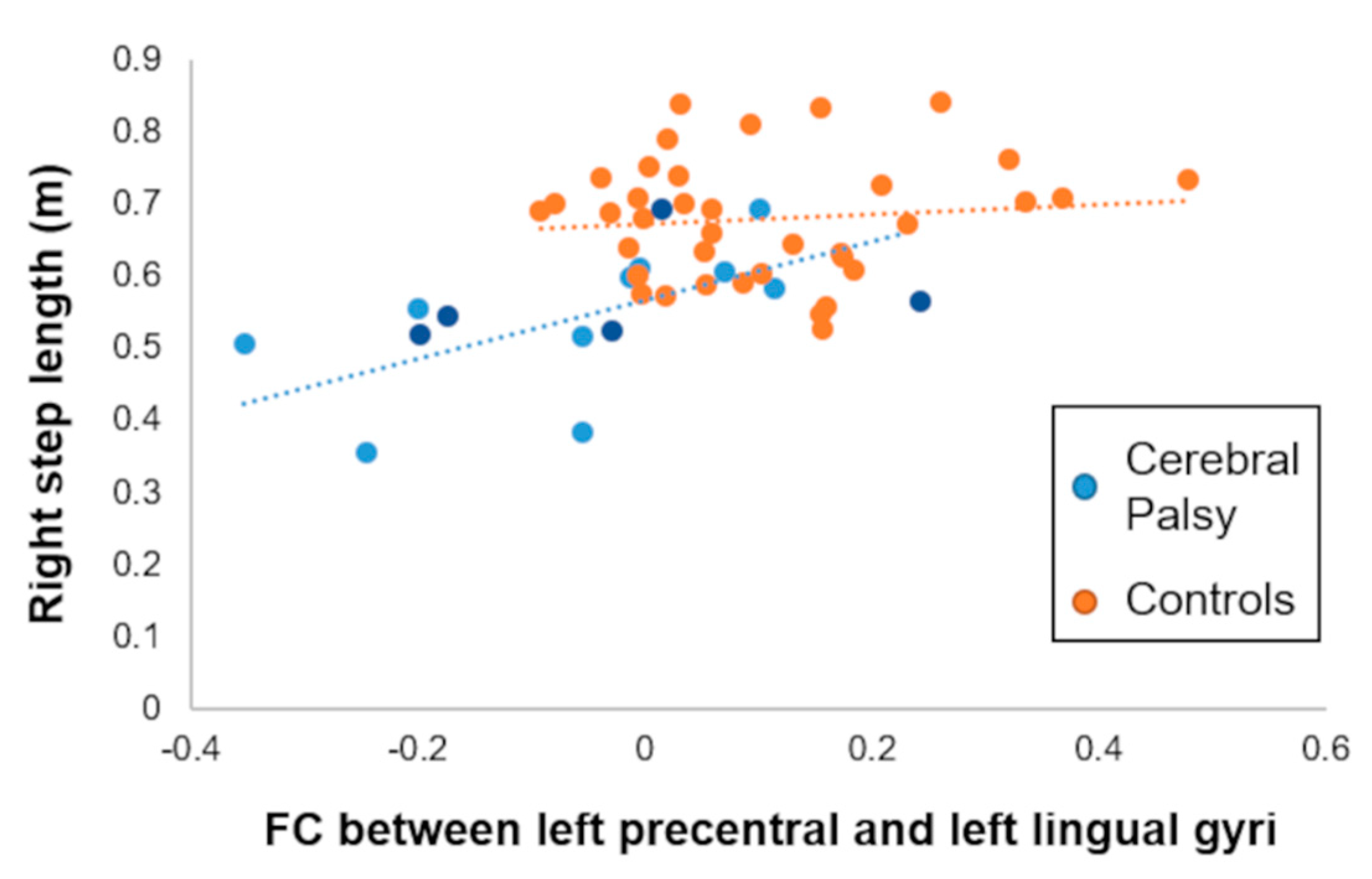
3.3. Association with Spatiotemporal Gait Biomechanics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Christensen, D.; Van Naarden Braun, K.; Doernberg, N.S.; Maenner, M.J.; Arneson, C.L.; Durkin, M.S.; Benedict, R.E.; Kirby, R.S.; Wingate, M.S.; Fitzgerald, R.; et al. Prevalence of cerebral palsy, co-occurring autism spectrum disorders, and motor functioning—Autism and Developmental Disabilities Monitoring Network, USA, 2008. Dev. Med. Child Neurol. 2014, 56, 59–65. [Google Scholar] [CrossRef]
- Kurz, M.J.; Bergwell, H.; Spooner, R.; Baker, S.; Heinrichs-Graham, E.; Wilson, T.W. Motor beta cortical oscillations are related with the gait kinematics of youth with cerebral palsy. Ann. Clin. Transl. Neurol. 2020, 7, 2421–2432. [Google Scholar] [CrossRef]
- Kurz, M.J.; Heinrichs-Graham, E.; Becker, K.M.; Wilson, T.W. The magnitude of the somatosensory cortical activity is related to the mobility and strength impairments seen in children with cerebral palsy. J. Neurophysiol. 2015, 113, 3143–3150. [Google Scholar] [CrossRef] [Green Version]
- Kurz, M.J.; Wilson, T.W.; Arpin, D.J. An fNIRS exploratory investigation of the cortical activity during gait in children with spastic diplegic cerebral palsy. Brain Dev. 2014, 36, 870–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, K.A.; Damiano, D.L.; Kim, Y.; Bulea, T.C. Mu Rhythm during Standing and Walking Is Altered in Children with Unilateral Cerebral Palsy Compared to Children with Typical Development. Dev. Neurorehabil. 2021, 24, 8–17. [Google Scholar] [CrossRef] [PubMed]
- VerMaas, J.R.; Embury, C.M.; Hoffman, R.M.; Trevarrow, M.P.; Wilson, T.W.; Kurz, M.J. Beyond the eye: Cortical differences in primary visual processing in children with cerebral palsy. Neuroimage Clin. 2020, 27, 102318. [Google Scholar] [CrossRef] [PubMed]
- VerMaas, J.R.; Gehringer, J.E.; Wilson, T.W.; Kurz, M.J. Children with cerebral palsy display altered neural oscillations within the visual MT/V5 cortices. Neuroimage Clin. 2019, 23, 101876. [Google Scholar] [CrossRef]
- VerMaas, J.R.; Lew, B.J.; Trevarrow, M.P.; Wilson, T.W.; Kurz, M.J. Children with Cerebral Palsy Have Altered Occipital Cortical Oscillations during a Visuospatial Attention Task. Cereb. Cortex 2021. [Google Scholar] [CrossRef]
- Biswal, B.; Yetkin, F.Z.; Haughton, V.M.; Hyde, J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 1995, 34, 537–541. [Google Scholar] [CrossRef]
- Buckner, R.L.; Krienen, F.M.; Yeo, B.T. Opportunities and limitations of intrinsic functional connectivity MRI. Nat. Neurosci. 2013, 16, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Doucet, G.; Naveau, M.; Petit, L.; Delcroix, N.; Zago, L.; Crivello, F.; Jobard, G.; Tzourio-Mazoyer, N.; Mazoyer, B.; Mellet, E.; et al. Brain activity at rest: A multiscale hierarchical functional organization. J. Neurophysiol. 2011, 105, 2753–2763. [Google Scholar] [CrossRef] [Green Version]
- Fox, M.D.; Snyder, A.Z.; Vincent, J.L.; Corbetta, M.; Van Essen, D.C.; Raichle, M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. USA 2005, 102, 9673–9678. [Google Scholar] [CrossRef] [Green Version]
- Golland, Y.; Golland, P.; Bentin, S.; Malach, R. Data-driven clustering reveals a fundamental subdivision of the human cortex into two global systems. Neuropsychologia 2008, 46, 540–553. [Google Scholar] [CrossRef] [Green Version]
- Toosy, A.T.; Ciccarelli, O.; Parker, G.J.; Wheeler-Kingshott, C.A.; Miller, D.H.; Thompson, A.J. Characterizing function-structure relationships in the human visual system with functional MRI and diffusion tensor imaging. Neuroimage 2004, 21, 1452–1463. [Google Scholar] [CrossRef]
- van den Heuvel, M.P.; Mandl, R.C.; Kahn, R.S.; Hulshoff Pol, H.E. Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Hum. Brain Mapp. 2009, 30, 3127–3141. [Google Scholar] [CrossRef]
- Luo, N.; Sui, J.; Abrol, A.; Chen, J.; Turner, J.A.; Damaraju, E.; Fu, Z.; Fan, L.; Lin, D.; Zhuo, C.; et al. Structural Brain Architectures Match Intrinsic Functional Networks and Vary across Domains: A Study from 15 000+ Individuals. Cereb. Cortex 2020. [Google Scholar] [CrossRef] [PubMed]
- Damoiseaux, J.S.; Rombouts, S.A.; Barkhof, F.; Scheltens, P.; Stam, C.J.; Smith, S.M.; Beckmann, C.F. Consistent resting-state networks across healthy subjects. Proc. Natl. Acad. Sci. USA 2006, 103, 13848–13853. [Google Scholar] [CrossRef] [Green Version]
- Doucet, G.E.; Lee, W.H.; Frangou, S. Evaluation of the spatial variability in the major resting-state networks across human brain functional atlases. Hum. Brain Mapp. 2019, 40, 4577–4587. [Google Scholar] [CrossRef]
- Elliott, M.L.; Knodt, A.R.; Cooke, M.; Kim, M.J.; Melzer, T.R.; Keenan, R.; Ireland, D.; Ramrakha, S.; Poulton, R.; Caspi, A.; et al. General functional connectivity: Shared features of resting-state and task fMRI drive reliable and heritable individual differences in functional brain networks. Neuroimage 2019, 189, 516–532. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Fox, P.T.; Miller, K.L.; Glahn, D.C.; Fox, P.M.; Mackay, C.E.; Filippini, N.; Watkins, K.E.; Toro, R.; Laird, A.R.; et al. Correspondence of the brain’s functional architecture during activation and rest. Proc. Natl. Acad. Sci. USA 2009, 106, 13040–13045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, L.E.; Andrews, T.J.; Hulette, C.; Richards, A.; Groelle, M.; Paydarfar, J.; Purves, D. Structure of the human sensorimotor system. I: Morphology and cytoarchitecture of the central sulcus. Cereb. Cortex 1997, 7, 18–30. [Google Scholar] [CrossRef]
- Franco, A.R.; Mannell, M.V.; Calhoun, V.D.; Mayer, A.R. Impact of analysis methods on the reproducibility and reliability of resting-state networks. Brain Connect. 2013, 3, 363–374. [Google Scholar] [CrossRef]
- Li, R.; Yin, S.; Zhu, X.; Ren, W.; Yu, J.; Wang, P.; Zheng, Z.; Niu, Y.N.; Huang, X.; Li, J. Linking Inter-Individual Variability in Functional Brain Connectivity to Cognitive Ability in Elderly Individuals. Front. Aging Neurosci. 2017, 9, 385. [Google Scholar] [CrossRef] [Green Version]
- Mueller, S.; Wang, D.; Fox, M.D.; Yeo, B.T.; Sepulcre, J.; Sabuncu, M.R.; Shafee, R.; Lu, J.; Liu, H. Individual variability in functional connectivity architecture of the human brain. Neuron 2013, 77, 586–595. [Google Scholar] [CrossRef] [Green Version]
- Burton, H.; Dixit, S.; Litkowski, P.; Wingert, J.R. Functional connectivity for somatosensory and motor cortex in spastic diplegia. Somatosens. Mot. Res. 2009, 26, 90–104. [Google Scholar] [CrossRef] [Green Version]
- Mu, X.; Wang, Z.; Nie, B.; Duan, S.; Ma, Q.; Dai, G.; Wu, C.; Dong, Y.; Shan, B.; Ma, L. Altered regional and circuit resting-state activity in patients with occult spastic diplegic cerebral palsy. Pediatr. Neonatol. 2018, 59, 345–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saunders, J.; Carlson, H.L.; Cortese, F.; Goodyear, B.G.; Kirton, A. Imaging functional motor connectivity in hemiparetic children with perinatal stroke. Hum. Brain Mapp. 2019, 40, 1632–1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, Y.; Li, Y.; Sun, B.; He, H.; Peng, R.; Zhang, T.; Li, J.; Luo, C.; Sun, C.; Yao, D. Functional Connectivity Alterations in Children with Spastic and Dyskinetic Cerebral Palsy. Neural Plast. 2018, 2018, 7058953. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Sun, B.; Zhang, H.; Li, Y.; Zhang, T.; Luo, C.; Sun, C.; Yao, D. Aberrant Interhemispheric Functional Organization in Children with Dyskinetic Cerebral Palsy. Biomed Res. Int. 2019, 2019, 4362539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.D.; Park, H.J.; Park, E.S.; Oh, M.K.; Park, B.; Rha, D.W.; Cho, S.R.; Kim, E.Y.; Park, J.Y.; Kim, C.H.; et al. Motor pathway injury in patients with periventricular leucomalacia and spastic diplegia. Brain 2011, 134, 1199–1210. [Google Scholar] [CrossRef] [Green Version]
- Papadelis, C.; Ahtam, B.; Nazarova, M.; Nimec, D.; Snyder, B.; Grant, P.E.; Okada, Y. Cortical somatosensory reorganization in children with spastic cerebral palsy: A multimodal neuroimaging study. Front. Hum. Neurosci. 2014, 8, 725. [Google Scholar] [CrossRef]
- Ilves, N.; Ilves, P.; Laugesaar, R.; Juurmaa, J.; Mannamaa, M.; Loo, S.; Loorits, D.; Tomberg, T.; Kolk, A.; Talvik, I.; et al. Resting-State Functional Connectivity and Cognitive Impairment in Children with Perinatal Stroke. Neural Plast. 2016, 2016, 2306406. [Google Scholar] [CrossRef]
- Manning, K.Y.; Fehlings, D.; Mesterman, R.; Gorter, J.W.; Switzer, L.; Campbell, C.; Menon, R.S. Resting State and Diffusion Neuroimaging Predictors of Clinical Improvements Following Constraint-Induced Movement Therapy in Children With Hemiplegic Cerebral Palsy. J. Child Neurol. 2015, 30, 1507–1514. [Google Scholar] [CrossRef] [Green Version]
- Yan, C.G.; Wang, X.D.; Zuo, X.N.; Zang, Y.F. DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics 2016, 14, 339–351. [Google Scholar] [CrossRef]
- Friston, K.J.; Williams, S.; Howard, R.; Frackowiak, R.S.; Turner, R. Movement-related effects in fMRI time-series. Magn. Reson. Med. 1996, 35, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, Y.; Restom, K.; Liau, J.; Liu, T.T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 2007, 37, 90–101. [Google Scholar] [CrossRef] [Green Version]
- Cordes, D.; Haughton, V.M.; Arfanakis, K.; Carew, J.D.; Turski, P.A.; Moritz, C.H.; Quigley, M.A.; Meyerand, M.E. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am. J. Neuroradiol. 2001, 22, 1326–1333. [Google Scholar]
- Da Costa, S.; van der Zwaag, W.; Marques, J.P.; Frackowiak, R.S.; Clarke, S.; Saenz, M. Human primary auditory cortex follows the shape of Heschl’s gyrus. J. Neurosci. 2011, 31, 14067–14075. [Google Scholar] [CrossRef] [PubMed]
- Yeo, B.T.; Krienen, F.M.; Sepulcre, J.; Sabuncu, M.R.; Lashkari, D.; Hollinshead, M.; Roffman, J.L.; Smoller, J.W.; Zollei, L.; Polimeni, J.R.; et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011, 106, 1125–1165. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.; Hay, P.; Campbell, L.; Trollor, J.N. A review of the association between obesity and cognitive function across the lifespan: Implications for novel approaches to prevention and treatment. Obes. Rev. 2011, 12, 740–755. [Google Scholar] [CrossRef]
- Qin, S.; Duan, X.; Supekar, K.; Chen, H.; Chen, T.; Menon, V. Large-scale intrinsic functional network organization along the long axis of the human medial temporal lobe. Brain Struct. Funct. 2016, 221, 3237–3258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manning, K.Y.; Menon, R.S.; Gorter, J.W.; Mesterman, R.; Campbell, C.; Switzer, L.; Fehlings, D. Neuroplastic Sensorimotor Resting State Network Reorganization in Children With Hemiplegic Cerebral Palsy Treated With Constraint-Induced Movement Therapy. J. Child Neurol. 2016, 31, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Hilderley, A.J.; Taylor, M.J.; Fehlings, D.; Chen, J.L.; Wright, F.V. Optimization of fMRI methods to determine laterality of cortical activation during ankle movements of children with unilateral cerebral palsy. Int. J. Dev. Neurosci. 2018, 66, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Lee, D.R.; Shin, Y.K.; Lee, N.G.; Han, B.S.; You, S.J. Comparative neuroimaging in children with cerebral palsy using fMRI and a novel EEG-based brain mapping during a motor task—A preliminary investigation. NeuroRehabilitation 2013, 32, 279–285. [Google Scholar] [CrossRef]
- Lee, D.; Pae, C.; Lee, J.D.; Park, E.S.; Cho, S.R.; Um, M.H.; Lee, S.K.; Oh, M.K.; Park, H.J. Analysis of structure-function network decoupling in the brain systems of spastic diplegic cerebral palsy. Hum. Brain Mapp. 2017, 38, 5292–5306. [Google Scholar] [CrossRef] [Green Version]
- Ego, A.; Lidzba, K.; Brovedani, P.; Belmonti, V.; Gonzalez-Monge, S.; Boudia, B.; Ritz, A.; Cans, C. Visual-perceptual impairment in children with cerebral palsy: A systematic review. Dev. Med. Child Neurol. 2015, 57, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Fazzi, E.; Signorini, S.G.; LA Piana, R.; Bertone, C.; Misefari, W.; Galli, J.; Balottin, U.; Bianchi, P.E. Neuro-ophthalmological disorders in cerebral palsy: Ophthalmological, oculomotor, and visual aspects. Dev. Med. Child Neurol. 2012, 54, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Pueyo, R.; Junque, C.; Vendrell, P.; Narberhaus, A.; Segarra, D. Neuropsychologic impairment in bilateral cerebral palsy. Pediatr. Neurol. 2009, 40, 19–26. [Google Scholar] [CrossRef]
- Surkar, S.M.; Hoffman, R.M.; Davies, B.; Harbourne, R.; Kurz, M.J. Impaired anticipatory vision and visuomotor coordination affects action planning and execution in children with hemiplegic cerebral palsy. Res. Dev. Disabil. 2018, 80, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Staudt, M.; Pavlova, M.; Bohm, S.; Grodd, W.; Krageloh-Mann, I. Pyramidal tract damage correlates with motor dysfunction in bilateral periventricular leukomalacia (PVL). Neuropediatrics 2003, 34, 182–188. [Google Scholar] [CrossRef]
- Betzel, R.F.; Byrge, L.; He, Y.; Goni, J.; Zuo, X.N.; Sporns, O. Changes in structural and functional connectivity among resting-state networks across the human lifespan. Neuroimage 2014, 102 Pt 2, 345–357. [Google Scholar] [CrossRef]
- Frangou, S.; Modabbernia, A.; Doucet, G.E.; Papachristou, E.; Williams, S.C.R.; Agartz, I.; Aghajani, M.; Akudjedu, T.N.; Albajes-Eizagirre, A.; Alnæs, D.; et al. Cortical Thickness across the Lifespan: Data from 17,075 healthy individuals aged 3–90 years. Hum. Bain Mapp. 2021. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Satterthwaite, T.D.; Medaglia, J.D.; Yang, M.; Gur, R.E.; Gur, R.C.; Bassett, D.S. Emergence of system roles in normative neurodevelopment. Proc. Natl. Acad. Sci. USA 2015, 112, 13681–13686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuyama, H.; Ouchi, Y.; Matsuzaki, S.; Nagahama, Y.; Yamauchi, H.; Ogawa, M.; Kimura, J.; Shibasaki, H. Brain functional activity during gait in normal subjects: A SPECT study. Neurosci. Lett. 1997, 228, 183–186. [Google Scholar] [CrossRef]
- Kurth, F.; Zilles, K.; Fox, P.T.; Laird, A.R.; Eickhoff, S.B. A link between the systems: Functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct. Funct. 2010, 214, 519–534. [Google Scholar] [CrossRef]
- la Fougere, C.; Zwergal, A.; Rominger, A.; Forster, S.; Fesl, G.; Dieterich, M.; Brandt, T.; Strupp, M.; Bartenstein, P.; Jahn, K. Real versus imagined locomotion: A [18F]-FDG PET-fMRI comparison. Neuroimage 2010, 50, 1589–1598. [Google Scholar] [CrossRef]
- Miyai, I.; Suzuki, M.; Hatakenaka, M.; Kubota, K. Effect of body weight support on cortical activation during gait in patients with stroke. Exp. Brain Res. 2006, 169, 85–91. [Google Scholar] [CrossRef]
- Kurz, M.J.; Wilson, T.W.; Arpin, D.J. Stride-time variability and sensorimotor cortical activation during walking. Neuroimage 2012, 59, 1602–1607. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.; Trevarrow, M.; Gehringer, J.; Bergwell, H.; Arpin, D.; Heinrichs-Graham, E.; Wilson, T.W.; Kurz, M.J. Gamma somatosensory cortical oscillations are attenuated during the stance phase of human walking. Neurosci. Lett. 2020, 732, 135090. [Google Scholar] [CrossRef]
- Strigaro, G.; Ruge, D.; Chen, J.C.; Marshall, L.; Desikan, M.; Cantello, R.; Rothwell, J.C. Interaction between visual and motor cortex: A transcranial magnetic stimulation study. J. Physiol. 2015, 593, 2365–2377. [Google Scholar] [CrossRef] [Green Version]
- Kurz, M.J.; Proskovec, A.L.; Gehringer, J.E.; Heinrichs-Graham, E.; Wilson, T.W. Children with cerebral palsy have altered oscillatory activity in the motor and visual cortices during a knee motor task. Neuroimage Clin. 2017, 15, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Kurz, M.J.; Becker, K.M.; Heinrichs-Graham, E.; Wilson, T.W. Neurophysiological abnormalities in the sensorimotor cortices during the motor planning and movement execution stages of children with cerebral palsy. Dev. Med. Child. Neurol. 2014, 56, 1072–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinomais, M.; Groeschel, S.; Staudt, M.; Krageloh-Mann, I.; Wilke, M. Relationship between functional connectivity and sensory impairment: Red flag or red herring? Hum. Brain Mapp. 2012, 33, 628–638. [Google Scholar] [CrossRef] [PubMed]

| Patients with Cerebral Palsy (n = 27) | Healthy Controls (n = 38) | Case-Control Differences (p-Value) | |
|---|---|---|---|
| Age, mean (SD) | 16.39 (4.90) | 14.44 (2.35) | n.s. |
| Sex (%, N of females) | 44.44 (12) | 36.84 (14) | n.s. |
| Preferred Average Velocity, mean (SD, m/s) | 0.93 (0.28) | 1.17 (0.18) | <0.001 |
| Preferred Average Cadence, mean (SD, steps/min) | 100.64 (23.86) | 103.73 (9.96) | n.s. |
| Preferred Average Left step length, mean (SD, m) | 0.56 (0.12) | 0.67 (0.08) | <0.001 |
| Preferred Average Right step length, mean (SD, m) | 0.55 (0.11) | 0.68 (0.08) | <0.001 |
| Preferred average width, mean (SD, m) | 0.13 (0.06) | 0.09 (0.03) | <0.001 |
| Spastic diplegic CP (n, %) | 17 (62.96) | - | |
| Hemiplegic CP (n, %) | 10 (37.04) | - |
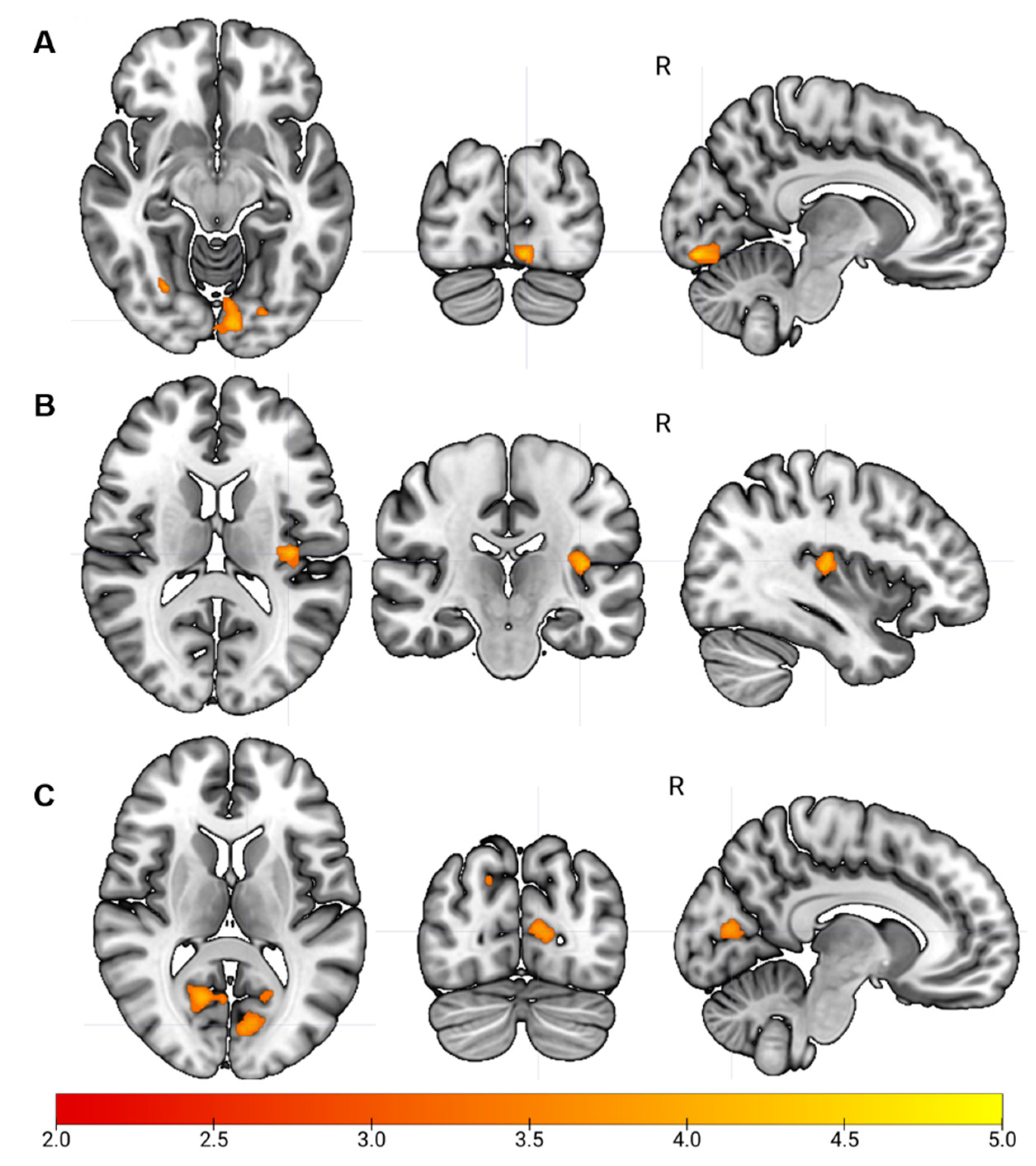
| Regions | Hemisphere | FC in CP | FC in Controls | Z | Cluster Size | x | y | z |
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | |||||||
| Seed: Left Precentral Gyrus (Sensorimotor Network) | ||||||||
| Contrast: Controls—CP | ||||||||
| Lingual gyrus | R | −0.05 (0.16) | 0.10 (0.13) | 4.25 | 110 | 24 | −72 | 0 |
| Calcarine | L | −0.06 (0.17) | 0.08 (0.11) | 4.21 | 224 | −8 | −88 | −12 |
| Superior Occipital gyrus | R | −0.03 (0.20) | 0.13 (0.17) | 4.07 | 25 | 2 | −40 | −46 |
| Cuneus | R | −0.05 (0.21) | 0.07 (0.15) | 3.87 | 37 | 2 | −94 | 16 |
| Seed: Left Lingual Gyrus (Visual Network) | ||||||||
| Contrast: Controls—CP | ||||||||
| Heschl gyrus | L | −0.02 (0.15) | 0.17 (0.13) | 4.00 | 105 | −38 | −22 | 12 |
| Rolandic Operculum | R | −0.06 (0.16) | 0.12 (0.15) | 3.85 | 22 | 40 | −14 | 24 |
| Seed: Left Heschl Gyrus (Auditory Network) | ||||||||
| Contrast: Controls—CP | ||||||||
| Inferior parietal lobule | R | 0.02 (0.15) | 0.24 (0.15) | 4.04 | 37 | 42 | 40 | 4 |
| Cerebellum | L | 0.03 (0.14) | 0.25 (0.14) | 3.90 | 30 | −36 | −36 | −30 |
| Calcarine | R | −0.02 (0.14) | 0.12 (0.15) | 3.88 | 141 | 16 | −62 | 10 |
| Frontal superior gyrus | R | −0.06 (0.09) | 0.13 (0.17) | 3.85 | 34 | 14 | −4 | 78 |
| Calcarine | L | 0.01 (0.15) | 0.19 (0.14) | 3.84 | 315 | −18 | −62 | 8 |
| Superior occipital gyrus | R | −0.08 (0.15) | 0.06 (0.12) | 3.49 | 24 | 18 | −80 | 38 |
| Superior occipital gyrus | L | −0.05 (0.18) | 0.13 (0.14) | 3.38 | 26 | −22 | −84 | 32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doucet, G.E.; Baker, S.; Wilson, T.W.; Kurz, M.J. Weaker Connectivity of the Cortical Networks Is Linked with the Uncharacteristic Gait in Youth with Cerebral Palsy. Brain Sci. 2021, 11, 1065. https://doi.org/10.3390/brainsci11081065
Doucet GE, Baker S, Wilson TW, Kurz MJ. Weaker Connectivity of the Cortical Networks Is Linked with the Uncharacteristic Gait in Youth with Cerebral Palsy. Brain Sciences. 2021; 11(8):1065. https://doi.org/10.3390/brainsci11081065
Chicago/Turabian StyleDoucet, Gaelle E., Sarah Baker, Tony W. Wilson, and Max J. Kurz. 2021. "Weaker Connectivity of the Cortical Networks Is Linked with the Uncharacteristic Gait in Youth with Cerebral Palsy" Brain Sciences 11, no. 8: 1065. https://doi.org/10.3390/brainsci11081065
APA StyleDoucet, G. E., Baker, S., Wilson, T. W., & Kurz, M. J. (2021). Weaker Connectivity of the Cortical Networks Is Linked with the Uncharacteristic Gait in Youth with Cerebral Palsy. Brain Sciences, 11(8), 1065. https://doi.org/10.3390/brainsci11081065






