Chronic Fentanyl Self-Administration Generates a Shift toward Negative Affect in Rats during Drug Use
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Catheterization Surgery
2.3. Self-Administration Apparatus
2.4. Self-Administration
2.5. USV Recording and Scoring
2.6. Data Analyses
2.6.1. Escalation of Intake
2.6.2. USV Analysis
3. Results
3.1. Acquisition of Fentanyl SA
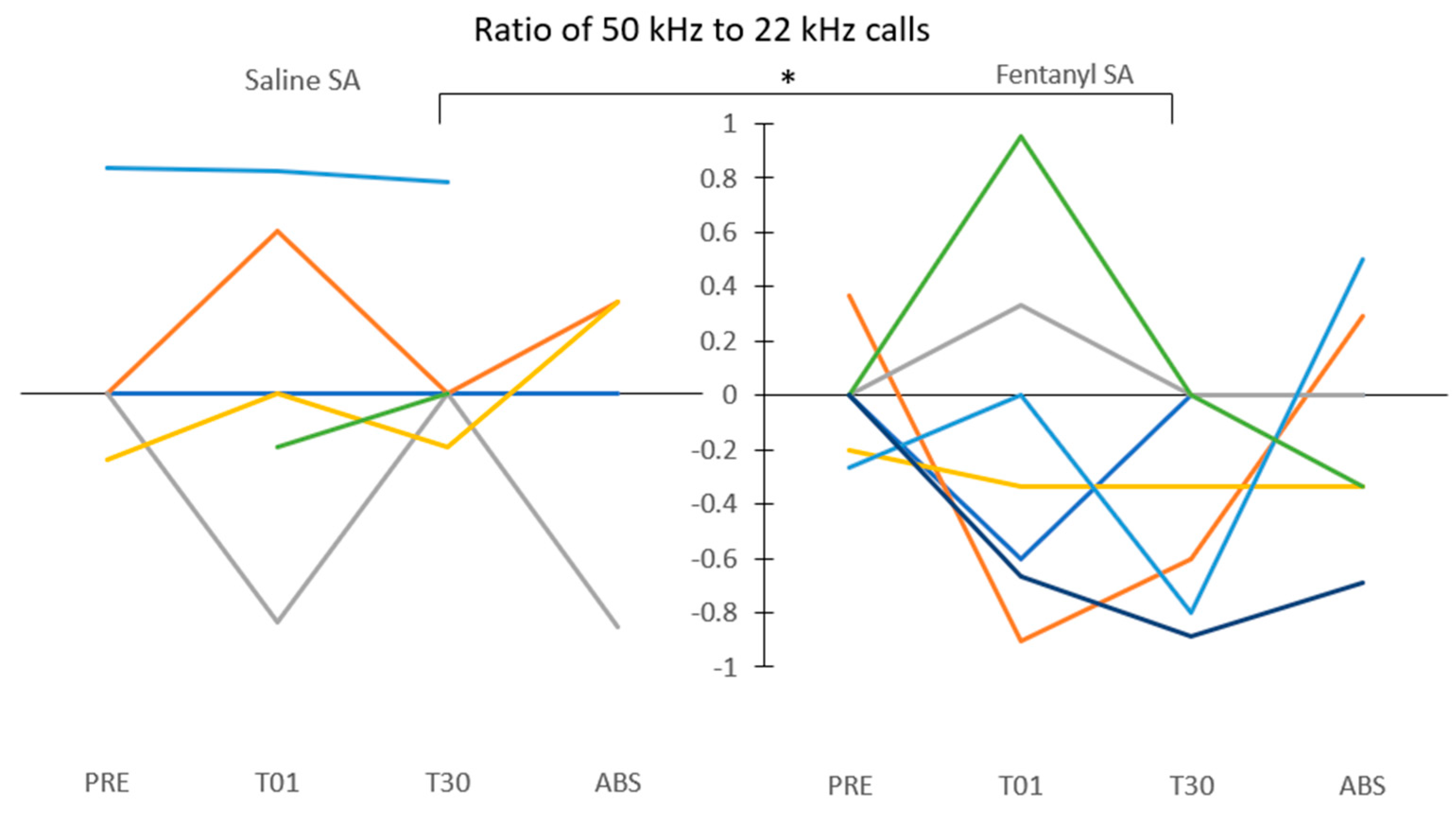
3.2. Shift toward Negative Affect after 30 Days of Fentanyl SA
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knutson, B.; Burgdorf, J.; Panksepp, J. Ultrasonic vocalizations as indices of affective states in rats. Psychol. Bull. 2002, 128, 961. [Google Scholar] [CrossRef]
- Portfors, C.V. Types and functions of ultrasonic vocalizations in laboratory rats and mice. J. Am. Assoc. Lab. Anim. Sci. 2007, 46, 28–34. [Google Scholar]
- Blanchard, R.; Blanchard, D.; Agullana, R.; Weiss, S.M. Twenty-two kHz alarm cries to presentation of a predator, by laboratory rats living in visible burrow systems. Physiol. Behav. 1991, 50, 967–972. [Google Scholar] [CrossRef]
- Brudzynski, S.M.; Bihari, F.; Ociepa, D.; Fu, X.-W. Analysis of 22 kHz ultrasonic vocalization in laboratory rats: Long and short calls. Physiol. Behav. 1993, 54, 215–221. [Google Scholar] [CrossRef]
- Covington, H.E.; Miczek, K.A. Vocalizations during withdrawal from opiates and cocaine: Possible expressions of affective distress. Eur. J. Pharmacol. 2003, 467, 1–13. [Google Scholar] [CrossRef]
- Vivian, J.A.; Miczek, K.A. Ultrasounds during morphine withdrawal in rats. Psychopharmacology 1991, 104, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Tonoue, T.; Ashida, Y.; Makino, H.; Hata, H. Inhibition of shock-elicited ultrasonic vocalization by opioid peptides in the rat: A psychotropic effect. Psychoneuroendocrinology 1986, 11, 177–184. [Google Scholar] [CrossRef]
- Brudzynski, S. Biological Functions of Rat Ultrasonic Vocalizations, Arousal Mechanisms, and Call Initiation. Brain Sci. 2021, 11, 605. [Google Scholar] [CrossRef]
- Knutson, B.; Burgdorf, J.; Panksepp, J. High-Frequency Ultrasonic Vocalizations Index Conditioned Pharmacological Reward in Rats. Physiol. Behav. 1999, 66, 639–643. [Google Scholar] [CrossRef]
- Brudzynski, S.M.; Pniak, A. Social contacts and production of 50-kHz short ultrasonic calls in adult rats. J. Comp. Psychol. 2002, 116, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Burgdorf, J.; Wood, P.L.; Kroes, R.A.; Moskal, J.R.; Panksepp, J. Neurobiology of 50-kHz ultrasonic vocalizations in rats: Electrode mapping, lesion, and pharmacology studies. Behav. Brain Res. 2007, 182, 274–283. [Google Scholar] [CrossRef]
- Hamed, A.; Taracha, E.; Szyndler, J.; Krząścik, P.; Lehner, M.; Maciejak, P.; Skórzewska, A.; Plaznik, A. The effects of morphine and morphine conditioned context on 50 kHz ultrasonic vocalisation in rats. Behav. Brain Res. 2012, 229, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Liu, Y.; Li, N.; Wang, C.-X.; Zhang, H.; Jiang, X.-F.; Xu, H.-S.; Fu, X.-M.; Hu, X.; Zhang, D.-R. Addiction related alteration in resting-state brain connectivity. NeuroImage 2010, 49, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F. Negative reinforcement in drug addiction: The darkness within. Curr. Opin. Neurobiol. 2013, 23, 559–563. [Google Scholar] [CrossRef]
- Baker, T.B.; Piper, M.E.; McCarthy, D.E.; Majeskie, M.R.; Fiore, M.C. Addiction Motivation Reformulated: An Affective Processing Model of Negative Reinforcement. Psychol. Rev. 2004, 111, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Beacher, N.J.; Kulik, J.M.; Estrin, D.J.; Pawlak, A.P.; West, M.O. Emergence of negative affect as motivation for drug taking in rats chronically self-administering cocaine. Psychopharmacology 2020, 237, 1407–1420. [Google Scholar] [CrossRef]
- Koob, G.F. Drug Addiction: The Yin and Yang of Hedonic Homeostasis. Neuron 1996, 16, 893–896. [Google Scholar] [CrossRef][Green Version]
- Van Der Poel, A.M.; Noach, E.J.K.; Miczek, K.A. Temporal patterning of ultrasonic distress calls in the adult rat: Effects of morphine and benzodiazepines. Psychopharmacology 1989, 97, 147–148. [Google Scholar] [CrossRef]
- Badiani, A.; Belin, D.; Epstein, D.; Calu, D.; Shaham, Y. Opiate versus psychostimulant addiction: The differences do matter. Nat. Rev. Neurosci. 2011, 12, 685–700. [Google Scholar] [CrossRef]
- Koob, G.F.; Volkow, N.D. Neurocircuitry of Addiction. Neuropsychopharmacology 2009, 35, 217–238. [Google Scholar] [CrossRef]
- Root, D.H.; Barker, D.J.; Ma, S.; Coffey, K.R.; Fabbricatore, A.T.; West, M.O. Evidence for learned skill during cocaine self-administration in rats. Psychopharmacology 2011, 217, 91–100. [Google Scholar] [CrossRef]
- Ahmed, S.H.; Koob, G. Transition from moderate to excessive drug intake: Change in hedonic set point. Science 1998, 282, 298–300. [Google Scholar] [CrossRef]
- Ahmed, S.H.; Walker, J.R.; Koob, G.F. Persistent Increase in the Motivation to Take Heroin in Rats with a History of Drug Escalation. Neuropsychopharmacology 2000, 22, 413–421. [Google Scholar] [CrossRef]
- Lenoir, M.; Guillem, K.; Koob, G.F.; Ahmed, S.H.; Guillem, K. Drug specificity in extended access cocaine and heroin self-administration. Addict. Biol. 2011, 17, 964–976. [Google Scholar] [CrossRef] [PubMed]
- Coffey, K.R.; Marx, R.G.; Neumaier, J.F. DeepSqueak: A deep learning-based system for detection and analysis of ultrasonic vocalizations. Neuropsychopharmacology 2019, 44, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.H. Escalation of Drug Use, in Animal Models of Drug Addiction; Springer: Berlin/Heidelberg, Germany, 2011; pp. 267–292. [Google Scholar]
- Best, L.M.; Zhao, L.L.; Scardochio, T.; Clarke, P.B. Effects of repeated morphine on ultrasonic vocalizations in adult rats: Increased 50 kHz call rate and altered subtype profile. Psychopharmacology 2017, 234, 155–165. [Google Scholar] [CrossRef]
- Wright, J.M.; Deng, L.; Clarke, P.B.S. Failure of rewarding and locomotor stimulant doses of morphine to promote adult rat 50-kHz ultrasonic vocalizations. Psychopharmacology 2012, 224, 477–487. [Google Scholar] [CrossRef]
- McAuliffe, W.E. A Second Look at First Effects: The Subjective Effects of Opiates on Nonaddicts. J. Drug Issues 1975, 5, 369–397. [Google Scholar] [CrossRef]
- Verendeev, A.; Riley, A.L. Relationship between the rewarding and aversive effects of morphine and amphetamine in individual subjects. Learn. Behav. 2011, 39, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Verendeev, A.; Riley, A.L. Conditioned taste aversion and drugs of abuse: History and interpretation. Neurosci. Biobehav. Rev. 2012, 36, 2193–2205. [Google Scholar] [CrossRef]
- Ahrens, A.M.; Ma, S.T.; Maier, E.; Duvauchelle, C.; Schallert, T. Repeated intravenous amphetamine exposure: Rapid and persistent sensitization of 50-kHz ultrasonic trill calls in rats. Behav. Brain Res. 2009, 197, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Kuchniak, K.; Wyszogrodzka, E.; Chrapusta, S.J.; Czarna, M.; Michalak, M.; Płaźnik, A.; Krząścik, P.; Mierzejewski, P.; Taracha, E. Using anticipatory and drug-evoked appetitive ultrasonic vocalization for monitoring the rewarding effect of amphetamine in a rat model of drug self-administration. Behav. Brain Res. 2019, 376, 112187. [Google Scholar] [CrossRef]
- Simola, N.; Serra, M.; Marongiu, J.; Costa, G.; Morelli, M. Increased emissions of 50-kHz ultrasonic vocalizations in hemiparkinsonian rats repeatedly treated with dopaminomimetic drugs: A potential preclinical model for studying the affective properties of dopamine replacement therapy in Parkinson’s disease. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 108, 110184. [Google Scholar] [CrossRef]
- Barker, D.J.; Root, D.; Ma, S.; Jha, S.; Megehee, L.; Pawlak, A.P.; West, M.O. Dose-dependent differences in short ultrasonic vocalizations emitted by rats during cocaine self-administration. Psychopharmacology 2010, 211, 435–442. [Google Scholar] [CrossRef][Green Version]
- Fabbricatore, A.T.; Ghitza, U.E.; Prokopenko, V.F.; West, M.O. Electrophysiological evidence of mediolateral functional dichotomy in the rat accumbens during cocaine self-administration: Tonic firing patterns. Eur. J. Neurosci. 2009, 30, 2387–2400. [Google Scholar] [CrossRef]
- Barker, D.J.; Simmons, S.J.; Servilio, L.C.; Bercovicz, D.; Ma, S.; Root, D.H.; Pawlak, A.P.; West, M.O. Ultrasonic vocalizations: Evidence for an affective opponent process during cocaine self-administration. Psychopharmacology 2013, 231, 909–918. [Google Scholar] [CrossRef]
- Avvisati, R.; Contu, L.; Stendardo, E.; Michetti, C.; Montanari, C.; Scattoni, M.L.; Badiani, A. Ultrasonic vocalization in rats self-administering heroin and cocaine in different settings: Evidence of substance-specific interactions between drug and setting. Psychopharmacology 2016, 233, 1501–1511. [Google Scholar] [CrossRef]
- Berridge, K.C.; Robinson, T.E. Liking, wanting, and the incentive-sensitization theory of addiction. Am. Psychol. 2016, 71, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Zacny, J.P.; Lichtor, J.L.; Flemming, D.; Coalson, D.W.; Thompson, W.K. A dose-response analysis of the subjective, psychomotor and physiological effects of intravenous morphine in healthy volunteers. J. Pharmacol. Exp. Ther. 1994, 268, 1–9. [Google Scholar]
- Hu, H.; Cui, Y.; Yang, Y. Circuits and functions of the lateral habenula in health and in disease. Nat. Rev. Neurosci. 2020, 21, 277–295. [Google Scholar] [CrossRef]
- Clerke, J.A.; Congiu, M.; Mameli, M. Neuronal adaptations in the lateral habenula during drug withdrawal: Preclinical evidence for addiction therapy. Neuropharmacology 2021, 192, 108617. [Google Scholar] [CrossRef]
- Zhu, Y.; Wienecke, C.F.R.; Nachtrab, G.; Chen, Y.Z.C.F.R.W.G.N.X. A thalamic input to the nucleus accumbens mediates opiate dependence. Nat. Cell Biol. 2016, 530, 219–222. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dao, A.N.; Beacher, N.J.; Mayr, V.; Montemarano, A.; Hammer, S.; West, M.O. Chronic Fentanyl Self-Administration Generates a Shift toward Negative Affect in Rats during Drug Use. Brain Sci. 2021, 11, 1064. https://doi.org/10.3390/brainsci11081064
Dao AN, Beacher NJ, Mayr V, Montemarano A, Hammer S, West MO. Chronic Fentanyl Self-Administration Generates a Shift toward Negative Affect in Rats during Drug Use. Brain Sciences. 2021; 11(8):1064. https://doi.org/10.3390/brainsci11081064
Chicago/Turabian StyleDao, Angela N., Nicholas J. Beacher, Vivian Mayr, Annalisa Montemarano, Sam Hammer, and Mark O. West. 2021. "Chronic Fentanyl Self-Administration Generates a Shift toward Negative Affect in Rats during Drug Use" Brain Sciences 11, no. 8: 1064. https://doi.org/10.3390/brainsci11081064
APA StyleDao, A. N., Beacher, N. J., Mayr, V., Montemarano, A., Hammer, S., & West, M. O. (2021). Chronic Fentanyl Self-Administration Generates a Shift toward Negative Affect in Rats during Drug Use. Brain Sciences, 11(8), 1064. https://doi.org/10.3390/brainsci11081064






