Utility of a Short Neuropsychological Protocol for Detecting HIV-Associated Neurocognitive Disorders in Patients with Asymptomatic HIV-1 Infection
Abstract
1. Introduction
2. Subjects and Methods
2.1. Subjects
2.2. Short Neuropsychological Protocol for HAND Detection
| Neurocognitive and Psychological Domain | Test | Conventions |
|---|---|---|
| Attention span | Wechsler Memory Scale Digit Span Subtest [46] | T1 = Total score T2 = Direct Digit score |
| Verbal learning and memory | Rey Auditory Verbal Learning Test [47,48] | T5 = Trial 3 T6 = Trial 5 T7 = Total score in trials 1 to 5 |
| Language Phonemic verbal fluency | Controlled Word Association Test [49] | T12 = Total score T13 = Total score in letter “P” T14 = Total score in letter “M” |
| Vocabulary | Wechsler Intelligence Scale Vocabulary Subtest [50] | T3 |
| Naming | Boston Naming Test [51] | T9 = Phonemic clues |
| Auditory-verbal comprehension | Language Comprehension Subtest of the Brief Neuropsychological Assessment in Spanish [52] | T19 |
| Information processing speed | Digit Symbol-Coding Subtest of the Wechsler Intelligence Scale (WAIS-III) [50] | T4 |
| Visuoconstructive skills | Rey Complex Figure Test [53] | T8 = Total direct score copy |
| Executive functions Inhibition | Stroop Color-Word Test [54] | T10 = Total score (word) T11 = Total score (color) |
| Motor programming | Motor Skills Subtest of the Brief Neuropsychological Assessment in Spanish (NEUROPSI) [52] | T15 = Score in change of hand position T16 = Score in alternating movements of the two hands T17 = Score in opposite reactions T18 = Total score |
| Anxiety | State-Trait Anxiety Inventory (STAI) [55] | T20 = STAI (State) T21 = STAI (Trait) |
2.3. Procedure
2.4. Statistical Analysis
3. Results
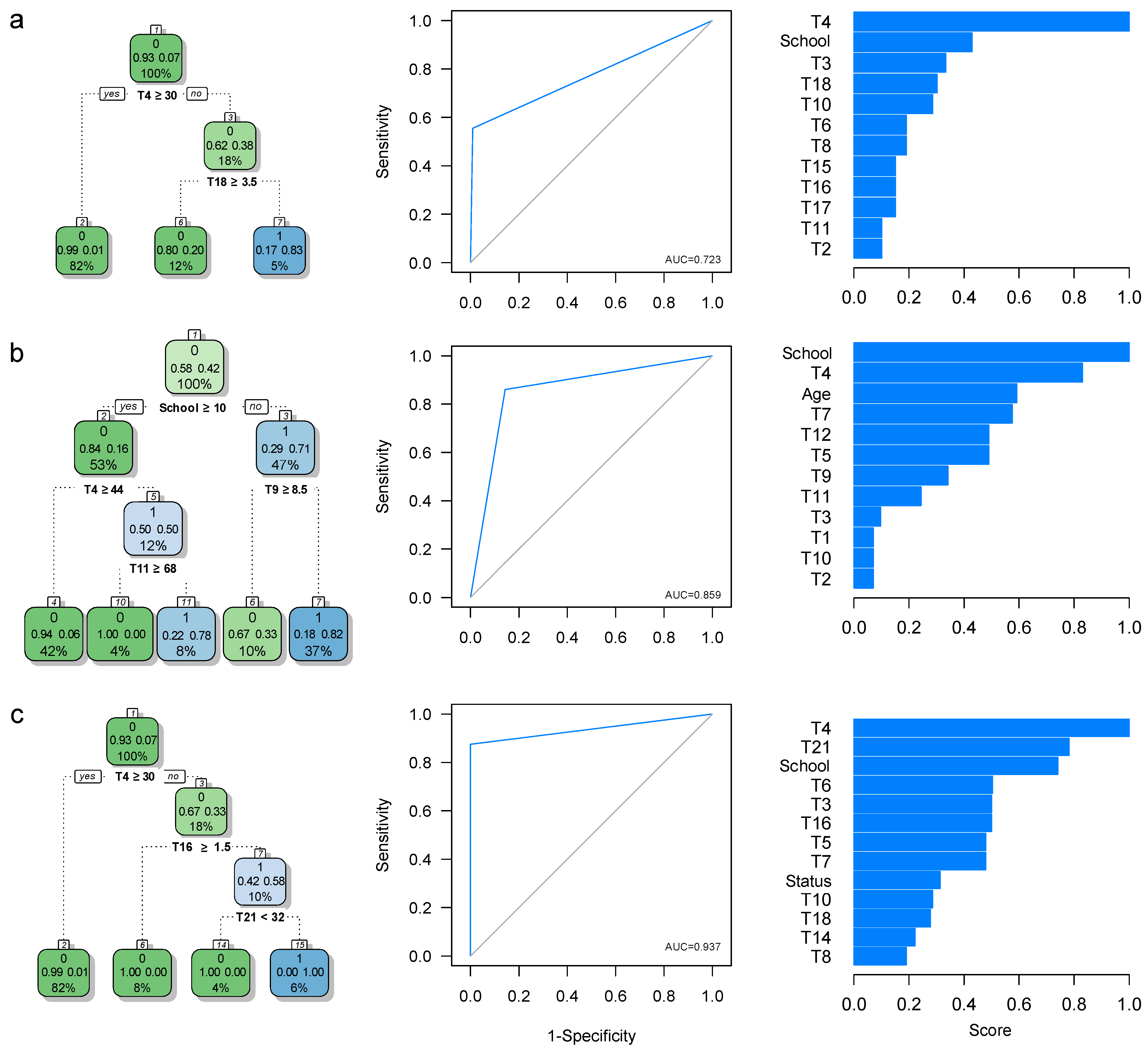
3.1. Predictive Models for HAND Detection
3.1.1. HAND Detection Based on the 1SD Criterion
3.1.2. HAND Detection Based on the MMSE and IHDS Operational Criteria
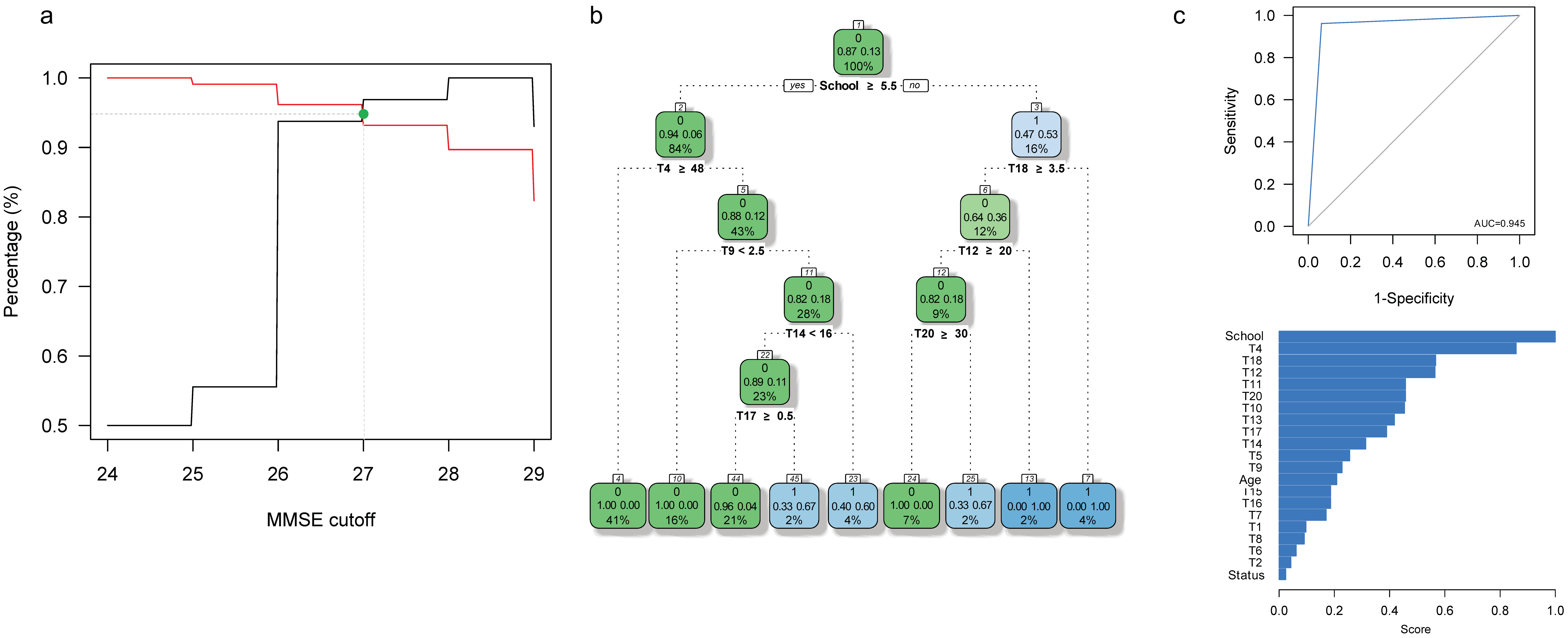
3.2. Specific Cut-Off Values for HAND Screening in This Caribbean Community
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Moral De La Rubia, J.; Segovia Chavez, M. Discrimination Against Women Infected with Human Immunodeficiency Virus (HIV) By Members of Their Own Family. Psicogente 2015, 18, 89–103. [Google Scholar] [CrossRef]
- ONUSIDA. Hoja Informativa—Últimas Estadísticas Sobre el Estado de la Epidemia de Sida. 2019. Available online: https://www.unaids.org/es/resources/fact-sheet (accessed on 30 January 2020).
- Marin-Webb, V.; Jessen, H.; Kopp, U.; Jessen, A.B.; Hahn, K. Validation of the International HIV Dementia Scale as a Screening Tool for HIV-Associated Neurocognitive Disorders in a German-Speaking HIV Outpatient Clinic. PLoS ONE 2016, 11, e0168225. [Google Scholar] [CrossRef] [PubMed]
- Heaton, R.K.; Franklin, D.R.; Ellis, R.J.; McCutchan, J.A.; Letendre, S.L.; Leblanc, S.; Corkran, S.H.; Duarte, N.A.; Clifford, D.B.; Woods, S.P.; et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: Differences in rates, nature, and predictors. J. Neurovirol. 2011, 17, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Woods, S.P.; Moore, D.J.; Weber, E.; Grant, I. Cognitive Neuropsychology of HIV-Associated Neurocognitive Disorders. Neuropsychol. Rev. 2009, 19, 152–168. [Google Scholar] [CrossRef] [PubMed]
- Eggers, C.; Arendt, G.; Hahn, K.; Husstedt, I.W.; Maschke, M.; Neuen-Jacob, E.; Obermann, M.; Rosenkranz, T.; Schielke, E.; Straube, E.; et al. HIV-1-associated neurocognitive disorder: Epidemiology, pathogenesis, diagnosis, and treatment. J. Neurol. 2017, 264, 1715–1727. [Google Scholar] [CrossRef]
- Antinori, A.; Arendt, G.; Becker, J.T.; Brew, B.; Byrd, D.A.; Cherner, M.; Clifford, D.B.; Cinque, P.; Epstein, L.; Goodkin, K.; et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007, 69. [Google Scholar] [CrossRef]
- Joska, J.A.; Witten, J.; Thomas, K.G.; Robertson, C.; Casson-Crook, M.; Roosa, H.; Creighton, J.; Lyons, J.; McArthur, J.; Sacktor, N.C. A Comparison of Five Brief Screening Tools for HIV-Associated Neurocognitive Disorders in the USA and South Africa. AIDS Behav. 2016, 20, 1621–1631. [Google Scholar] [CrossRef]
- Heaton, R.K.; Clifford, D.B.; Franklin, D.R.; Woods, S.P.; Ake, C.; Vaida, F.; Ellis, R.J.; Letendre, S.L.; Marcotte, T.D.; Atkinson, J.H.; et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010, 75, 2087–2096. [Google Scholar] [CrossRef] [PubMed]
- Oshinaike, O.O.; Akinbami, A.A.; Ojo, O.O.; Ojini, I.F.; Okubadejo, N.; Danesi, A.M. Comparison of the Minimental State Examination Scale and the International HIV Dementia Scale in Assessing Cognitive Function in Nigerian HIV Patients on Antiretroviral Therapy. AIDS Res. Treat. 2012, 2012, 581531. [Google Scholar] [CrossRef] [PubMed]
- Kabuba, N.; Menon, J.A.; Franklin, D.R.; Heaton, R.K.; Hestad, K.A. Use of Western Neuropsychological Test Battery in Detecting HIV-Associated Neurocognitive Disorders (HAND) in Zambia. AIDS Behav. 2017, 21, 1717–1727. [Google Scholar] [CrossRef]
- Butters, N.; Grant, I.; Haxby, J.; Judd, L.L.; Martin, A.; McClelland, J.; Pequegnat, W.; Schacter, D.; Stover, E. Assessment of Aids-related cognitive changes: Recommendations of the NIMH workshop on neuropsychological assessment approaches. J. Clin. Exp. Neuropsychol. 1990, 12, 963–978. [Google Scholar] [CrossRef]
- Maj, M.; Starace, F.; Sartourius, N. Neuropsychiatric aspects of HIV-1 infection: Data collection instrument for a WHO cross-cultural study. Organ 1991, 69, 243–245. [Google Scholar]
- Grant, I.; Martin, A. Neuropsychology of HIV Infection; Oxford University Press: Oxford, UK, 1994. [Google Scholar]
- Pugh, K.; Riccio, M.; Jadresic, D.; Burgess, A.P.; Baldeweg, T.; Catalan, J.; Lovett, E.; Hawkins, D.A.; Gruzelier, J.; Thompson, C. A longitudinal study of the neuropsychiatric consequences of HIV-1 infection in gay men. II. Psychological and health status at baseline and at 12-month follow-up. Psychol. Med. 1994, 24, 897–904. [Google Scholar] [CrossRef]
- Ardila-Ardila, A.; Goodkin, K.; Concha-Bartolini, M.; Lecusay-Ruiz, R.; Mellan-Fajardo, S.O.; Suárez-Bustamante, P.; Molina-Vásquez, R.; Lee, D.; Chayeb, G.; Wilkie, F.L. HUMANS: A neuropsychological battery for evaluating HIV 1 infected patients. Rev. Neurol. 2003, 36, 756–762. [Google Scholar] [PubMed]
- De Almeida, M.; Kamat, R.; Cherner, M.; Umlauf, A.; Ribeiro, C.E.; de Pereira, A.P.; Franklin, D.; Heaton, R.K.; Ellis, R. Improving Detection of HIV-Associated Cognitive Impairment: Comparison of the International HIV Dementia Scale and a Brief Screening Battery. J. Acquir. Immune Defic. Syndr. 2017, 74, 332. [Google Scholar] [CrossRef][Green Version]
- Zipursky, A.R.; Gogolishvili, D.; Rueda, S.; Brunetta, J.; Carvalhal, A.; McCombe, J.A.; Gill, M.J.; Rachlis, A.; Rosenes, R.; Arbess, G.; et al. Evaluation of brief screening tools for neurocognitive impairment in HIV/AIDS: A systematic review of the literature. AIDS 2013, 27, 2385. [Google Scholar] [CrossRef]
- Cysique, L.A.; Casaletto, K.B.; Heaton, R.K. Reliably Measuring Cognitive Change in the Era of Chronic HIV Infection and Chronic HIV-Associated Neurocognitive Disorders. In Current Topics in Behavioral Neurosciences; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–28. [Google Scholar] [CrossRef]
- Chan, L.G.; Ho, M.J.; Lin, Y.C.; Ong, Y.; Wong, C.S. Development of a neurocognitive test battery for HIV-associated neurocognitive disorder (HAND) screening: Suggested solutions for resource-limited clinical settings. AIDS Res. Ther. 2019, 16, 9. [Google Scholar] [CrossRef]
- Smail, R.C.; Brew, B.J. HIV-Associated Neurocognitive Disorder. In The Human Hypothalamus: Neuropsychiatric Disorders; Elsevier: Amsterdam, The Netherlands, 2018; Volume 152, pp. 75–97. [Google Scholar]
- Power, C.; Selnes, O.A.; Grim, J.A.; McArthur, J.C. HIV Dementia Scale: A Rapid Screening Test. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1995, 8, 273–278. [Google Scholar] [CrossRef]
- Sacktor, N.C.; Wong, M.; Nakasujja, N.; Skolasky, R.; Selnes, O.A.; Musisi, S.; Robertson, K.; McArthur, J.C.; Ronald, A.; Katabira, E. The International HIV Dementia Scale: A new rapid screening test for HIV dementia. AIDS 2005, 19, 1367–1374. [Google Scholar]
- Antinori, A.; Arendt, G.; Grant, I.; The Mind Exchange Working Group; Letendre, S.; Muñoz-Moreno, J.A.; Eggers, C.; Brew, B.; Brouillette, M.-J. Assessment, Diagnosis, and Treatment of HIV-Associated Neurocognitive Disorder: A Consensus Report of the Mind Exchange Program. Clin. Infect. Dis. 2013, 56, 1004–1017. [Google Scholar] [CrossRef]
- Haddow, L.J.; Floyd, S.; Copas, A.; Gilson, R.J.C. A Systematic Review of the Screening Accuracy of the HIV Dementia Scale and International HIV Dementia Scale. PLoS ONE 2013, 8, e61826. [Google Scholar] [CrossRef]
- Moore, D.J.; Roediger, M.J.P.; Eberly, L.E.; Blackstone, K.; Hale, B.; Weintrob, A.; Ganesan, A.; Agan, B.K.; Letendre, S.L.; Crum-Cianflone, N.F. Identification of an Abbreviated Test Battery for Detection of HIV-Associated Neurocognitive Impairment in an Early-Managed HIV-Infected Cohort. PLoS ONE 2012, 7, e47310. [Google Scholar] [CrossRef]
- Kamminga, J.; Lal, L.; Wright, E.J.; Bloch, M.; Brew, B.J.; Cysique, L.A. Monitoring HIV-Associated Neurocognitive Disorder Using Screenings: A Critical Review Including Guidelines for Clinical and Research Use. Curr. HIV/AIDS Rep. 2017, 14, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Pedraza, O.; Mungas, D. Measurement in Cross-Cultural Neuropsychology. Neuropsychol. Rev. 2008, 18, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Robertson, K.; Jiang, H.; Evans, S.R.; Marra, C.M.; Berzins, B.; Hakim, J.; Sacktor, N.; Silva, M.T.; Campbell, T.B.; Nair, A.; et al. International neurocognitive normative study: Neurocognitive comparison data in diverse resource-limited settings: AIDS Clinical Trials Group A5271. J. Neurovirol. 2016, 22, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Parsons, T.D.; Rogers, S.; Hall, C.; Robertson, K. Motor based assessment of neurocognitive functioning in resource-limited international settings. J. Clin. Exp. Neuropsychol. 2007, 29, 59–66. [Google Scholar] [CrossRef]
- McArthur, J.C.; Brew, B. HIV-associated neurocognitive disorders: Is there a hidden epidemic? AIDS 2010, 24, 1367–1370. [Google Scholar] [CrossRef]
- Vélez, J.I. Machine Learning based Psychology: Advocating for A Data-Driven Approach. Int. J. Psychol. Res. 2021, 14, 6–11. [Google Scholar] [CrossRef]
- Henriquez, M.L.C.; Acosta-Lopez, J.; Martínez-Banfi, M.L.; Vélez, J.I.; Mejia-Segura, E.; Lozano-Gutiérrez, S.G.; Sánchez-Rojas, M.; Zurbarán, M.A.; Zurek, E.E.; Arcos-Burgos, M.; et al. ADHD Endophenotypes in Caribbean Families. J. Atten. Disord. 2018. [Google Scholar] [CrossRef]
- Valcour, V.; Pohl, K. Machine Learning to Distinguish HAND from Alzheimer’s Disease in HIV over Age 60. 2020. Available online: http://grantome.com/grant/NIH/R01-MH113406-01 (accessed on 4 February 2020).
- Pluta, A.; Wolak, T.; Sobańska, M.; Gawron, N.; Egbert, A.R.; Szymańska, B.; Horban, A.; Firląg-Burkacka, E.; Bieńkowski, P.; Sienkiewicz-Jarosz, H.; et al. HIV and age underlie specific patterns of brain abnormalities and cognitive changes in high functioning patients. Neuropsychology 2019, 33, 358–369. [Google Scholar] [CrossRef]
- Bandera, A.; Taramasso, L.; Bozzi, G.; Muscatello, A.; Robinson, J.A.; Burdo, T.H.; Gori, A. HIV-Associated Neurocognitive Impairment in the Modern ART Era: Are We Close to Discovering Reliable Biomarkers in the Setting of Virological Suppression? Front. Aging Neurosci. 2019, 11, 187. [Google Scholar] [CrossRef] [PubMed]
- Cysique, L.; Murray, J.; Dunbar, M.; Jeyakumar, V.; Brew, B.; Cysique, L.; Murray, J. A screening algorithm for HIV-associated neurocognitive disorders. HIV Med. 2010, 11, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L.; Friedman, J.H.; Olshen, R.A.; Stone, C.J. Classification and Regression Trees; Wadsworth International Group: Belmont, CA, USA, 1984. [Google Scholar]
- Martínez-Banfi, M.; Vélez, J.I.; Perea, M.V.; García, R.G.; Puentes-Rozo, P.J.; Chams, M.M.; Ladera, V. Neuropsychological performance in patients with asymptomatic HIV-1 infection. AIDS Care Psychol. Socio-Med. Asp. AIDS/HIV 2018, 30, 623–633. [Google Scholar] [CrossRef]
- Betancur, J.; Correa, A.; Estrada; Santiago, E.; Orozco, B.; Velez, H.; Rojas, W.; Borrero, J.; Restrepo, J. Infección Respiratoria en Pacientes con VIH/Sida. En Manual de VIH/SIDA y Otras Infecciones de Transmisión Sexual; Corporación para Investigaciones Biológicas: Medellín, Colombia, 2007. [Google Scholar]
- Becker, J.T.; Kingsley, L.A.; Molsberry, S.; Reynolds, S.; Aronow, A.; Levine, A.J.; Martin, E.; Miller, E.N.; Munro, C.A.; Ragin, A.; et al. Cohort Profile: Recruitment cohorts in the neuropsychological substudy of the Multicenter AIDS Cohort Study. Int. J. Epidemiol. 2015, 44, 1506–1516. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Mungas, D. In-office mental status testing: A practical guide. Geriatrics 1991, 46, 63–66. [Google Scholar]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. Examen Cognoscitivo Minimental; Adaptación Española; TEA: Madrid, Spain, 2002. [Google Scholar]
- Grant, I. The Neurocognitive Complications of HIV Infection. In Encyclopedia of the Human Brain; Ramachandran, V.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2002; pp. 475–489. [Google Scholar]
- Weschler, D. Escala de Memoria de Weschler-III; Pearson: Madrid, Spain, 2004. [Google Scholar]
- Rey, A. L’Examen Clinique en Psychologie (The Clinical Psychological Examination); Presse Universitaires de France, Scientific Research Publishing: Paris, France, 1964; Available online: https://www.scirp.org/(S(vtj3fa45qm1ean45vvffcz55))/reference/referencespapers.aspx?referenceid=1656248 (accessed on 2 June 2021).
- Schmidt, M. Rey Auditory and Verbal Learning Test: A Handbook; Western Psychological Services: Los Angeles, CA, USA, 1996. [Google Scholar]
- Spreen, O.; Strauss, E. A Compendium of Neuropsychological Tests, 2nd ed.; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Weschler, D. Manual de Aplicación y Corrección de la Escala de Inteligencia de Weschler (WAIS-III); Pearson: Madrid, Spain, 2001. [Google Scholar]
- Kaplan, E.; Goddglass, H.; Weintraub, S. Boston Naming Test, 2nd ed.; Pro-Ed: Austin, TX, USA, 2001. [Google Scholar]
- Ostrosky-Solis, F.; Ardila, A.; Rosselli, M. Evaluación Neuropsicológica Breve en Español (Neuropsi) [Brief Neuropsychological Evaluation in Spanish (Neuropsi)]; Pearson: Mexico City, Mexico, 1999. [Google Scholar]
- Rey, A. Test de Copia y de Reproducción de Memoria de Figuras Geométricas Complejas [Test of Copying and Reproduction of Memory of Complex Geometric Figures], 8th ed.; Lea & Febiger: Madrid, Spain, 2003. [Google Scholar]
- Golden, C.J. Test de Colores y Palabras STROOP [STROOP Test of Colors and Words], 4th ed.; TEA Ediciones: Madrid, Spain, 2005. [Google Scholar]
- Spielberger, C.D.; Díaz-Guerrero, R. IDARE. Inventario de Ansiedad: Rasgo-Estado. Manual e Instructivo [Trait-State Anxiety Inventory. Manual and Instruction]; Manual Moderno: Mexico City, Mexico, 1975. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 30 July 2021).
- Therneau, B.T.; Atkinson, B.R. Recursive Partitioning and Regression Trees (R Package R Part Version 4.1-15). 2019. Available online: https://cran.r-project.org/web/packages/rpart/index.html (accessed on 27 May 2020).
- Rao, D.C. CAT scans, PET scans, and genomic scans. Genet. Epidemiol. 1998, 15, 1–18. [Google Scholar] [CrossRef]
- Metz, C.E. Basic principles of ROC analysis. Semin. Nucl. Med. 1978, 8, 238–239. [Google Scholar] [CrossRef]
- Brin, S.; Motwani, R.; Ullman, J.D.; Tsur, S. Dynamic itemset counting and implication rules for market basket data. ACM SIGMOD Rec. 1997, 26, 255–264. [Google Scholar] [CrossRef]
- McNicholas, P.; Murphy, T.B.; O’Regan, M. Standardising the lift of an association rule. Comput. Stat. Data Anal. 2008, 52, 4712–4721. [Google Scholar] [CrossRef]
- Moore, R.C.; Paolillo, E.W.; Heaton, A.; Fazeli, P.L.; Jeste, D.V.; Moore, D.J. Clinical utility of the UCSD Performance-Based Skills Assessment—Brief (UPSA-B) in adults living with HIV: Associations with neuropsychological impairment and patient-reported everyday functioning difficulties. PLoS ONE 2017, 12, e0183614. [Google Scholar] [CrossRef] [PubMed]
- Goodkin, K.; Hardy, D.J.; Singh, D.; Lopez, E. Diagnostic Utility of the International HIV Dementia Scale for HIV-Associated Neurocognitive Impairment and Disorder in South Africa. J. Neuropsychiatry Clin. Neurosci. 2014, 26, 352–358. [Google Scholar] [CrossRef]
- Haddow, L.J.; Laverick, R.; Daskalopoulou, M.; McDonnell, J.; Lampe, F.; Gilson, R.; Speakman, A.; Antinori, A.; Balestra, P.; Bruun, T.; et al. Multicenter European Prevalence Study of Neurocognitive Impairment and Associated Factors in HIV Positive Patients. AIDS Behav. 2017, 22, 1573–1583. [Google Scholar] [CrossRef] [PubMed]
- Haynes, B.I.; Pitkanen, M.; Kulasegaram, R.; Casey, S.J.; Schutte, M.; Towgood, K.; Peters, B.; Barker, G.; Kopelman, M.D. HIV: Ageing, cognition and neuroimaging at 4-year follow-up. HIV Med. 2018, 19, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Ipser, J.C.; Brown, G.G.; Bischoff-Grethe, A.; Connolly, C.; Ellis, R.; Heaton, R.K.; Grant, I.; Translational Methamphetamine AIDS Research Center (TMARC) Group. HIV Infection Is Associated with Attenuated Frontostriatal Intrinsic Connectivity: A Preliminary Study. J. Int. Neuropsychol. Soc. 2015, 21, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Kamat, R.; Doyle, K.L.; Iudicello, J.E.; Morgan, E.E.; Morris, S.; Smith, D.; Little, S.J.; Grant, I.; Woods, S.P. Neurobehavioral Disturbances During Acute and Early HIV Infection. Cogn. Behav. Neurol. 2016, 29, 1–10. [Google Scholar] [CrossRef]
- Nabha, L.; Duong, L.; Timpone, J. HIV-associated neurocognitive disorders: Perspective on management strategies. Drugs 2013, 73, 893–905. [Google Scholar] [CrossRef]
- Mehta, S.R.; Pérez-Santiago, J.; Hulgan, T.; Day, T.R.C.; Barnholtz-Sloan, J.; Gittleman, H.; Letendre, S.; Ellis, R.; Heaton, R.; Patton, S.; et al. Cerebrospinal fluid cell-free mitochondrial DNA is associated with HIV replication, iron transport, and mild HIV-associated neurocognitive impairment. J. Neuroinflamm. 2017, 14, 72. [Google Scholar] [CrossRef]
- Gurgel Fernandes Távora, L.; Martins Figueiredo, T.; Moitas Krammer de Mesquita, R.; Ricarte Bezerra, F.; Pinheiro Aquino, B.; de Baima Colares, J.K. HIV and Dementia: Prevalence and Risk Factors (HIV e Demência: Prevalência e Fatores de Risco VIH y Demencia: Prevalencia y Factores de Riesgo). Rev. Bras. Promoção Saúde 2016, 29, 212–218. [Google Scholar] [CrossRef]
- Arango-Lasprilla, J.C.; Rogers, H.; Lengenfelder, J.; DeLuca, J.; Moreno, S.; Lopera, F. Cortical and subcortical diseases: Do true neuropsychological differences exist? Arch. Clin. Neuropsychol. 2006, 21, 29–40. [Google Scholar] [CrossRef][Green Version]
- Dwyer, R.; Wenhui, L.; Cysique, L.; Brew, B.; Lal, L.; Bain, P.; Wesselingh, S.; Wright, E. Symptoms of depression and rates of neurocognitive impairment in HIV positive patients in Beijing, China. J. Affect. Disord. 2014, 162, 89–95. [Google Scholar] [CrossRef]
- Maruff, P.; Thomas, E.; Cysique, L.; Brew, B.; Collie, A.; Snyder, P.; Pietrzak, R.H. Validity of the CogState Brief Battery: Relationship to Standardized Tests and Sensitivity to Cognitive Impairment in Mild Traumatic Brain Injury, Schizophrenia, and AIDS Dementia Complex. Arch. Clin. Neuropsychol. 2009, 24, 165–178. [Google Scholar] [CrossRef]
- Lopardo, G.D.; Bissio, E.; Iannella, M.D.C.; Crespo, A.D.; Garone, D.B.; Cassetti, L.I. Good Neurocognitive Performance Measured by the International HIV Dementia Scale in Early HIV-1 Infection. J. Acquir. Immune Defic. Syndr. 2009, 52, 488–492. [Google Scholar] [CrossRef]
- Abusamra, V.; Abusamra, L.; Sampedro, B.; Difalcis, M.; Martínez, G.; Dávolos, J.M.; Ferreres, A. Trastornos cognitivos en pacientes VIH-1: La dimensión pragmática de la comunicación verbal. Rev. Neuropsicol. Latinoam. 2014, 6, 22–30. [Google Scholar] [CrossRef]
- Bloch, M.; Kamminga, J.; Jayewardene, A.; Bailey, M.; Carberry, A.; Vincent, T.; Quan, D.; Maruff, P.; Brew, B.; Cysique, L. A Screening Strategy for HIV-Associated Neurocognitive Disorders That Accurately Identifies Patients Requiring Neurological Review. Clin. Infect. Dis. 2016, 63, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Robbins, R.N.; Joska, J.; Thomas, K.G.F.; Stein, D.; Linda, T.; Mellins, C.A.; Remien, R.H. Exploring the Utility of the Montreal Cognitive Assessment to Detect HIV-Associated Neurocognitive Disorder: The Challenge and Need for Culturally Valid Screening Tests in South Africa. Clin. Neuropsychol. 2013, 27, 437–454. [Google Scholar] [CrossRef]
- Amusan, P.; Power, C.; Gill, M.J.; Gomez, D.; Johnson, E.; Rubin, L.H.; Fujiwara, E. Lifetime antiretroviral exposure and neurocognitive impairment in HIV. J. Neurovirol. 2020, 26, 743–753. [Google Scholar] [CrossRef]
- Nightingale, S.; Winston, A.; Letendre, S.; Michael, B.; McArthur, J.C.; Khoo, S.; Solomon, T. Controversies in HIV-associated neurocognitive disorders. Lancet Neurol. 2014, 13, 1139–1151. [Google Scholar] [CrossRef]
- Tedaldi, E.M.; Minniti, N.L.; Fischer, T. HIV-Associated Neurocognitive Disorders: The Relationship of HIV Infection with Physical and Social Comorbidities. BioMed Res. Int. 2015, 2015, 641913. [Google Scholar] [CrossRef]
- Fogel, G.B.; Lamers, S.L.; Levine, A.J.; Valdes-Sueiras, M.; McGrath, M.S.; Shapshak, P.; Singer, E.J. Factors related to HIV-associated neurocognitive impairment differ with age. J. Neurovirol. 2014, 21, 56–65. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jia, P.; Zhao, Z.; Hulgan, T.; Bush, W.; Samuels, D.; Bloss, C.; Heaton, R.K.; Ellis, R.; Schork, N.; Marra, C.M.; et al. Genome-wide association study of HIV-associated neurocognitive disorder (HAND): A CHARTER group study. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2017, 174, 413–426. [Google Scholar] [CrossRef]
- McDonnell, J.; Haddow, L.; Daskalopoulou, M.; Lampe, F.; Speakman, A.; Gilson, R.; Phillips, A.; Sherr, L.; Wayal, S.; Harrison, J.; et al. Minimal Cognitive Impairment in UK HIV-Positive Men Who Have Sex with Men: Effect of Case Definitions and Comparison with the General Population and HIV-Negative Men for the Cognitive Impairment in People with HIV in the European Region (CIPHER) Study Group. J. Acquir. Immune Defic. Syndr. 2014, 67, 120–127. [Google Scholar] [CrossRef]
- Sacktor, N.; Robertson, K. Evolving clinical phenotypes in HIV-associated neurocognitive disorders. Curr. Opin. HIV AIDS 2014, 9, 517–520. [Google Scholar] [CrossRef]
- Focà, E.; Magro, P.; Motta, D.; Compostella, S.; Casari, S.; Bonito, A.; Brianese, N.; Ferraresi, A.; Rodari, P.; Pezzoli, M.C.; et al. Screening for Neurocognitive Impairment in HIV-Infected Individuals at First Contact after HIV Diagnosis: The Experience of a Large Clinical Center in Northern Italy. Int. J. Mol. Sci. 2016, 17, 434. [Google Scholar] [CrossRef]
- Sanmarti, M.; Ibáñez, L.; Huertas, S.; Badenes, D.; Dalmau, D.; Slevin, M.; Krupinski, J.; Popa-Wagner, A.; Jaen, A. HIV-associated neurocognitive disorders. J. Mol. Psychiatry 2014, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- García-Torres, A.; Vergara-Moragues, E.; Piñón-Blanco, A.; Pérez-García, M. Alteraciones neuropsicológicas en pacientes con VIH e historia previa de consumo de sustancias. Un estudio preliminar. Rev. Latinoam. Psicol. 2015, 47, 213–221. [Google Scholar] [CrossRef]
- Nichols, M.J.; Gates, T.M.; Soares, J.R.; Moffat, K.J.; Rae, C.; Brew, B.J.; Cysique, L.A. Atrophic brain signatures of mild forms of neurocognitive impairment in virally suppressed HIV infection. AIDS 2019, 33, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Chalermchai, T.; Valcour, V.; Sithinamsuwan, P.; Pinyakorn, S.; Clifford, D.; Paul, R.H.; Tipsuk, S.; Fletcher, J.L.K.; DeGruttola, V.; Ratto-Kim, S.; et al. Trail Making Test A improves performance characteristics of the International HIV Dementia Scale to identify symptomatic HAND. J. Neurovirol. 2013, 19, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Skinner, S.; Adewale, A.; DeBlock, L.; Gill, M.J.; Power, C. Neurocognitive screening tools in HIV/AIDS: Comparative performance among patients exposed to antiretroviral therapy. HIV Med. 2009, 10, 246–252. [Google Scholar] [CrossRef]
- De Souza, E.M.; Buoniconti, C.S.; Valim, F.; Moura, A.S. Risk factors for neurocognitive impairment in HIV-infected patients and comparison of different screening tools. Dement. Neuropsychol. 2016, 10, 42–46. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arentoft, A.; Byrd, D.; Robbins, R.N.; Monzones, J.; Miranda, C.; Rosario, A.; Coulehan, K.; Fuentes, A.; Germano, K.K.; D’Aquila, E.; et al. Multidimensional effects of acculturation on English-language neuropsychological test performance among HIV+ Caribbean Latinas/os. J. Clin. Exp. Neuropsychol. 2012, 34, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Alford, K.; Banerjee, S.; Nixon, E.; O’Brien, C.; Pounds, O.; Butler, A.; Elphick, C.; Henshaw, P.; Anderson, S.; Vera, J.H. Assessment and Management of HIV-Associated Cognitive Impairment: Experience from a Multidisciplinary Memory Service for People Living with HIV. Brain Sci. 2019, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Clifford, D.B.; Ances, B.M. HIV-Associated Neurocognitive Disorder (HAND). Lancet Infect. Dis. 2013, 13, 976–986. [Google Scholar] [CrossRef]
- Croucher, A.; Winston, A. Neurological complications of HIV. Medicine 2013, 41, 450–455. [Google Scholar] [CrossRef]
- Barragan Duarte, J.L. Mapa Genético de los Colombianos. Unimedios. 2007. Available online: http://historico.unperiodico.unal.edu.co/ediciones/105/15.html (accessed on 20 April 2021).
- Cysique, L.A.; Heaton, R.K.; Kamminga, J.; Lane, T.; Gates, T.M.; Moore, D.M.; Hubner, E.; Carr, A.; Brew, B. HIV-associated neurocognitive disorder in Australia: A case of a high-functioning and optimally treated cohort and implications for international neuroHIV research. J. Neurovirol. 2014, 20, 258–268. [Google Scholar] [CrossRef]
- Mebarak, M.; De Castro, A.; Salamanca, M.P.; Quintero, M.F. Salud mental: Un abordaje desde la perspectiva actual de la psicología de la salud. Psicol. desde el Caribe 2009, 23, 83–112. [Google Scholar]
- Marquine, M.J.; Heaton, A.; Johnson, N.; Rivera-Mindt, M.; Cherner, M.; Bloss, C.; Hulgan, T.; Umlauf, A.; Moore, D.J.; Fazeli, P.; et al. Differences in Neurocognitive Impairment among HIV-Infected Latinos in the United States. J. Int. Neuropsychol. Soc. 2018, 24, 163–175. [Google Scholar] [CrossRef]
- Saylor, D.; Dickens, A.; Sacktor, N.; Haughey, N.; Slusher, B.; Pletnikov, M.; Mankowski, J.L.; Brown, A.; Volsky, D.J.; McArthur, D.S.; et al. HIV-associated neurocognitive disorder—Pathogenesis and prospects for treatment. Nat. Rev. Neurol. 2016, 12, 234–248. [Google Scholar] [CrossRef]
- Parry, S.; Zetler, S.; Kentridge, A.; Petrak, J.; Barber, T.; Kentridge, A. Simple screening for neurocognitive impairment in routine HIV outpatient care: Is it deliverable? AIDS Care 2017, 29, 1275–1279. [Google Scholar] [CrossRef]

| Variable | Category | All Individuals (n = 120) | Patients with Asymptomatic HIV-1 (n = 60) | Control Group (n = 60) | Statistic | p |
|---|---|---|---|---|---|---|
| Mean ± S.D. | Mean ± S.D. | Mean ± S.D. | t (df) | |||
| Age (years) | 36.07 ± 10.98 | 38.60 ± 9.48 | 33.53 ± 11.84 | 2.59 (118) | 0.011 | |
| Years of education | 9.02 ± 2.65 | 8.07 ± 2.85 | 9.97 ± 2.07 | 4.18 (118) | <0.0001 | |
| n (%) | n (%) | n (%) | (df) | |||
| Gender | Female | 74 (61.7) | 32 (53.3) | 42 (70) | 3.52 (1) | 0.060 |
| Male | 46 (38.3) | 28 (46.7) | 18 (30) | |||
| Sexual orientation | Heterosexual | 111 (92.5) | 53 (88.3) | 58 (96.7) | 4.42 (2) | 0.109 |
| Homosexual | 5 (4.2) | 3 (5) | 2 (3.3) | |||
| Bisexual | 4 (3.3) | 4 (6.7) | 0 (0) | |||
| Hand preference | Left | 6 (5) | 0 (0) | 6 (10) | 6.32 (1) | 0.012 |
| Right | 114 (95) | 60 (100) | 54 (90) |
| Asymptomatic HIV-1 Infection | ARPA-Based Predicted HAND | HAND Diagnosis | Total | |
|---|---|---|---|---|
| No | Yes | |||
| No | No | 56 | 0 | 56 |
| Yes | 4 | 0 | 4 | |
| Yes | No | 0 | 5 | 5 |
| Yes | 0 | 55 | 55 | |
| Instrument | a | b | c | d | Se | Sp | PPV | NPV | FDR | FPR | CCR | Lift | AUC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
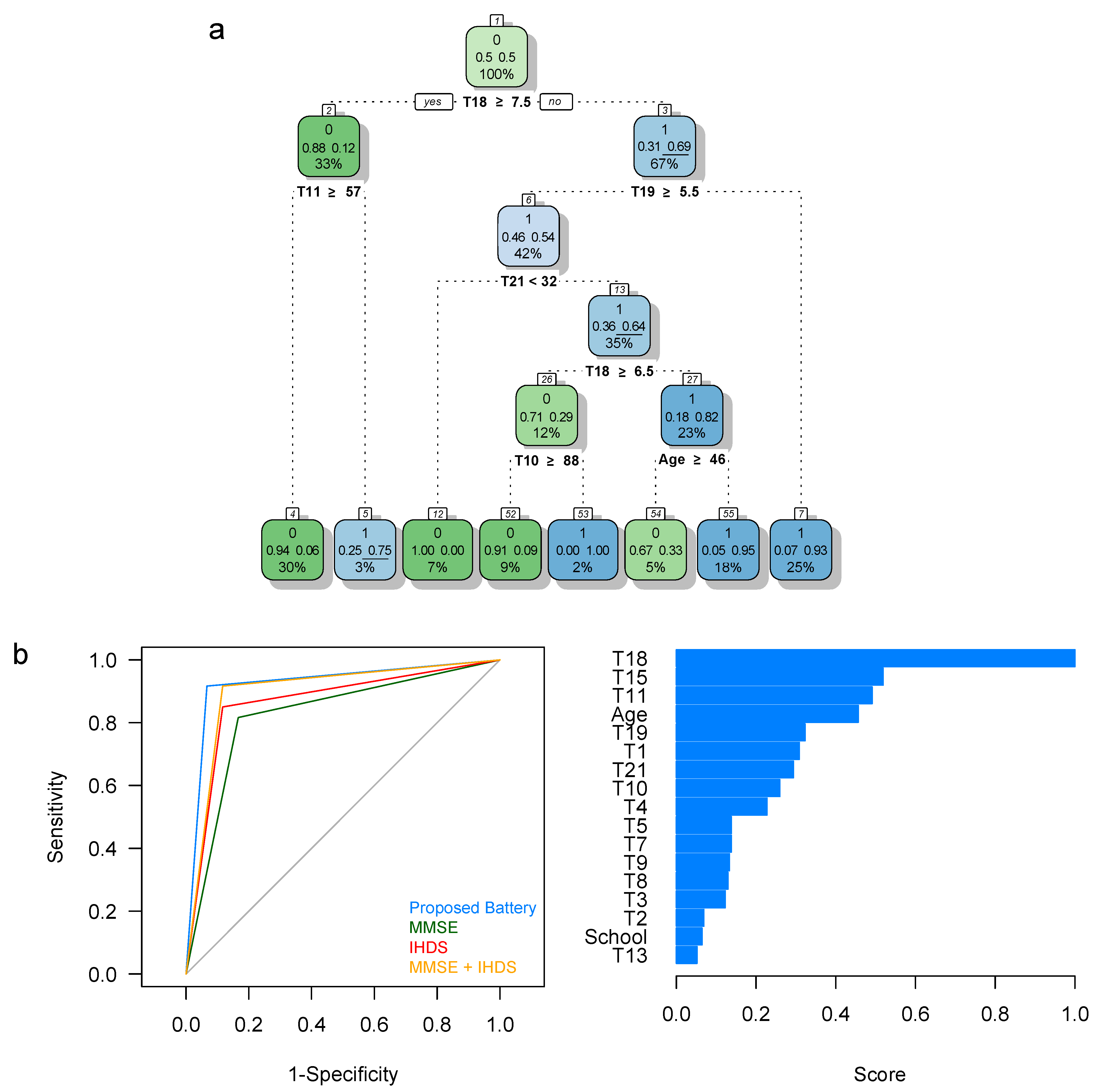
| Short Protocol | 55 | 4 | 5 | 56 | 0.917 | 0.933 | 0.932 | 0.918 | 0.068 | 0.067 | 0.925 | 1.864 | 0.925 |
| MMSE | 50 | 11 | 10 | 49 | 0.833 | 0.817 | 0.820 | 0.831 | 0.180 | 0.183 | 0.825 | 1.639 | 0.825 |
| IHDS | 53 | 9 | 7 | 51 | 0.883 | 0.850 | 0.855 | 0.879 | 0.145 | 0.150 | 0.867 | 1.710 | 0.867 |
| MMSE + IHDS | 53 | 5 | 7 | 55 | 0.883 | 0.917 | 0.914 | 0.887 | 0.086 | 0.083 | 0.900 | 1.828 | 0.900 |
| Criterion 1: MMSE in (10,25] | ||||
| Asymptomatic HIV-1 Infection | Predicted HAND | HAND Diagnosis | Total | |
| No | Yes | |||
| No | No | 60 | 0 | 60 |
| Yes | 0 | 0 | 0 | |
| Yes | No | 50 | 4 | 54 |
| Yes | 1 | 5 | 6 | |
| Criterion 2: IHDS < 11 | ||||
| No | No | 38 | 2 | 40 |
| Yes | 8 | 12 | 20 | |
| Yes | No | 22 | 5 | 27 |
| Yes | 2 | 31 | 33 | |
| Criterion 3: MMSE in (10,25] and IHDS < 11 | ||||
| No | No | 60 | 0 | 60 |
| Yes | 0 | 0 | 0 | |
| Yes | No | 52 | 1 | 53 |
| Yes | 0 | 7 | 7 | |
| MMSE < 27 | ||||
|---|---|---|---|---|
| Asymptomatic HIV-1 Infection | Predicted HAND | HAND Diagnosis | Total | |
| No | Yes | |||
| No | No | 57 | 0 | 57 |
| Yes | 1 | 2 | 3 | |
| Yes | No | 43 | 1 | 44 |
| Yes | 3 | 13 | 16 | |
| IHDS < 10 | ||||
| No | No | 51 | 1 | 52 |
| Yes | 1 | 7 | 8 | |
| Yes | No | 30 | 2 | 32 |
| Yes | 4 | 24 | 28 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Banfi, M.; Vélez, J.I.; Mebarak Chams, M.R.; Arcos-Holzinger, M.; Acosta-López, J.E.; García, R.; Perea, M.V.; Arcos-Burgos, M.; Ladera, V. Utility of a Short Neuropsychological Protocol for Detecting HIV-Associated Neurocognitive Disorders in Patients with Asymptomatic HIV-1 Infection. Brain Sci. 2021, 11, 1037. https://doi.org/10.3390/brainsci11081037
Martinez-Banfi M, Vélez JI, Mebarak Chams MR, Arcos-Holzinger M, Acosta-López JE, García R, Perea MV, Arcos-Burgos M, Ladera V. Utility of a Short Neuropsychological Protocol for Detecting HIV-Associated Neurocognitive Disorders in Patients with Asymptomatic HIV-1 Infection. Brain Sciences. 2021; 11(8):1037. https://doi.org/10.3390/brainsci11081037
Chicago/Turabian StyleMartinez-Banfi, Martha, Jorge I. Vélez, Moisés R. Mebarak Chams, Mauricio Arcos-Holzinger, Johan E. Acosta-López, Ricardo García, María Victoria Perea, Mauricio Arcos-Burgos, and Valentina Ladera. 2021. "Utility of a Short Neuropsychological Protocol for Detecting HIV-Associated Neurocognitive Disorders in Patients with Asymptomatic HIV-1 Infection" Brain Sciences 11, no. 8: 1037. https://doi.org/10.3390/brainsci11081037
APA StyleMartinez-Banfi, M., Vélez, J. I., Mebarak Chams, M. R., Arcos-Holzinger, M., Acosta-López, J. E., García, R., Perea, M. V., Arcos-Burgos, M., & Ladera, V. (2021). Utility of a Short Neuropsychological Protocol for Detecting HIV-Associated Neurocognitive Disorders in Patients with Asymptomatic HIV-1 Infection. Brain Sciences, 11(8), 1037. https://doi.org/10.3390/brainsci11081037







