Sarcotubular Myopathy Due to Novel TRIM32 Mutation in Association with Multiple Sclerosis
Abstract
:1. Introduction
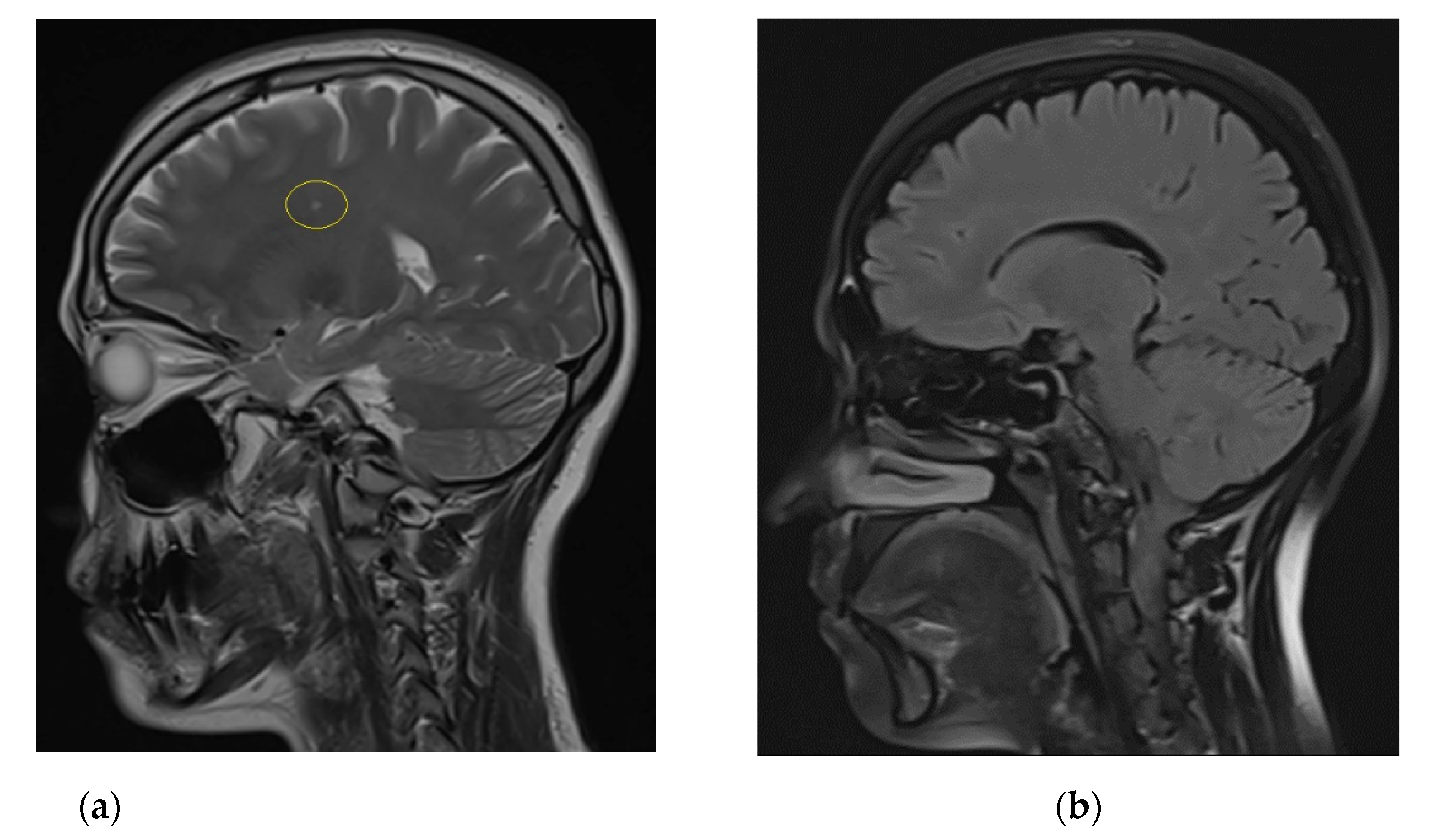
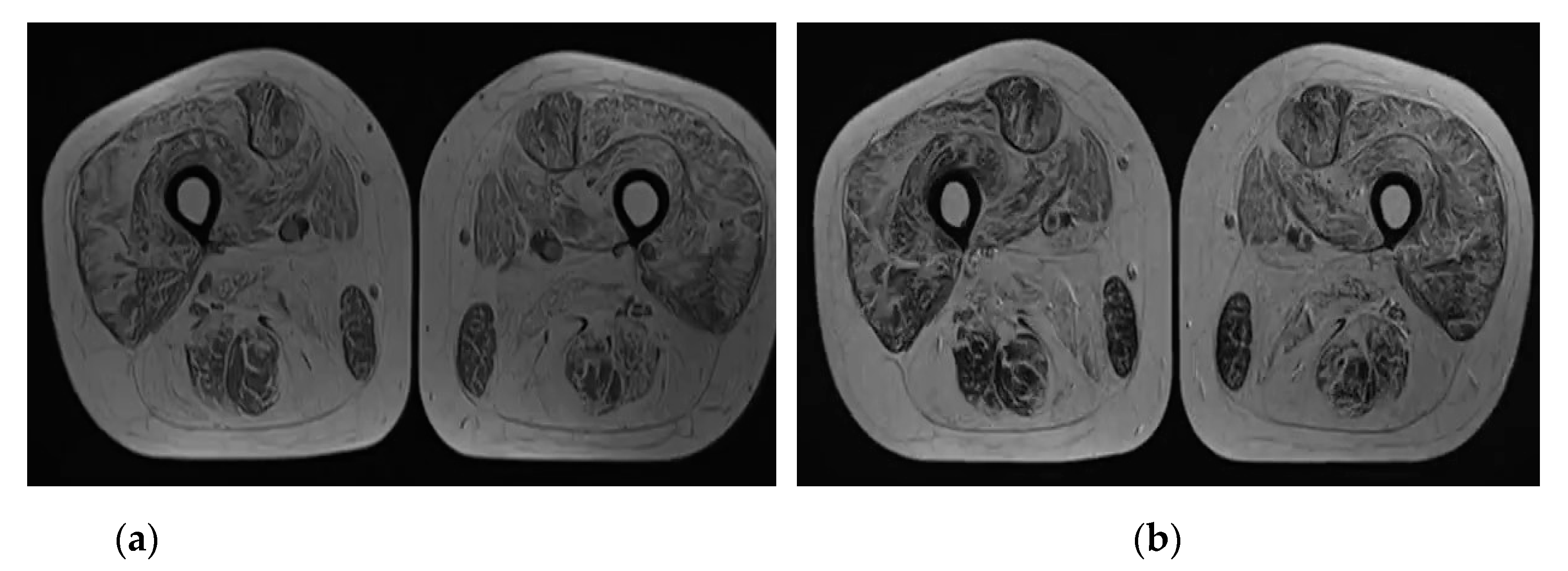
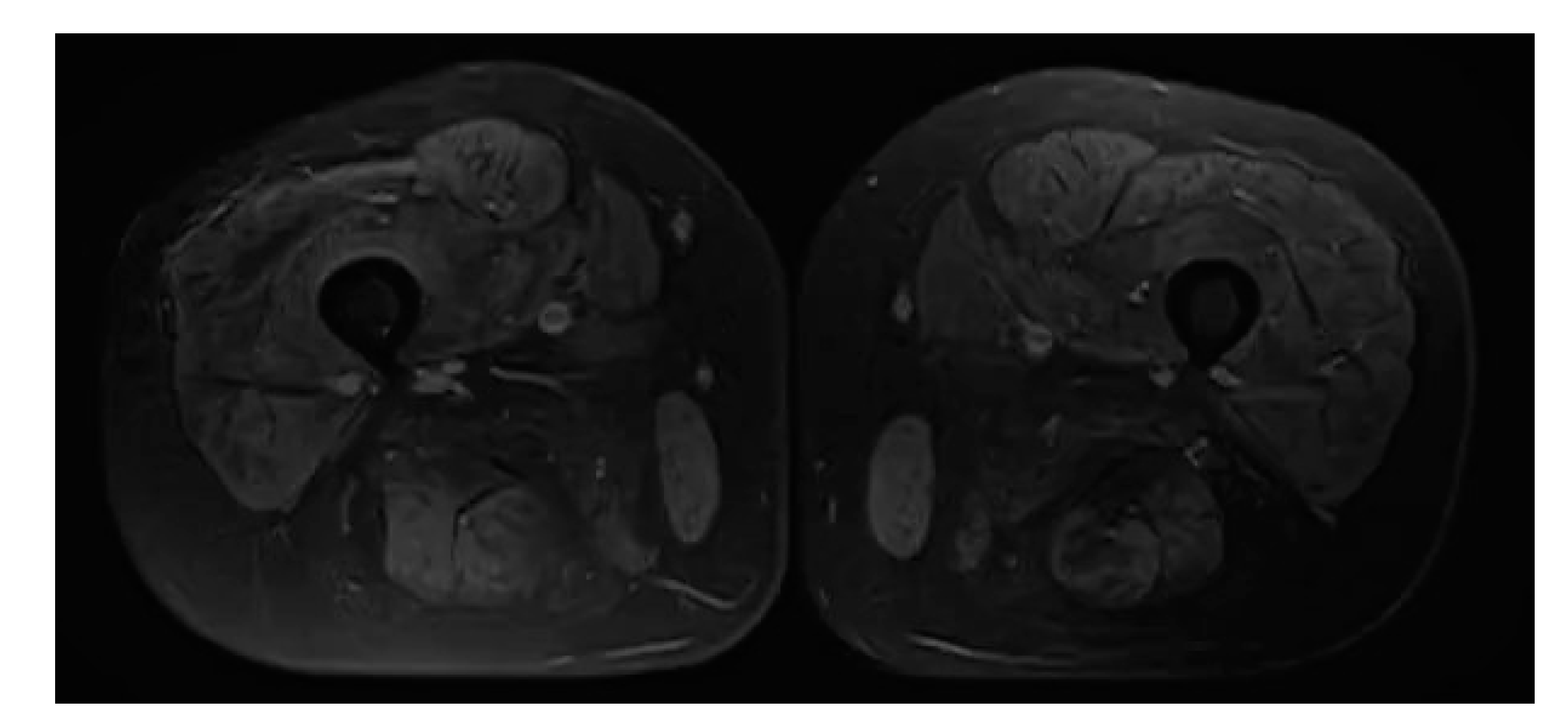
2. Case Report
- performing pulmonary function testing, detection of excessive daytime somnolence, nonrestorative sleep, episodes of syncope, near-syncope, palpitations, symptomatic or asymptomatic tachycardia or arrhythmias, or signs and symptoms of cardiac failure for cardiology evaluation (Level B).
- realization of periodic assessments by a physical and occupational therapist for symptomatic and preventive screening (Level B).
- combination of aerobic exercise with a supervised submaximal strength (Level C).
- practicing gentle, low-impact aerobic exercise (swimming, stationary bicycling) to improve cardiovascular performance, increase muscle efficiency, and lessen fatigue (Level C).
- adequate hydration, no exercise to exhaustion, and avoiding supramaximal, high-intensity exercise (Level C).
- detection of warning signs of overwork weakness and myoglobinuria, which include feeling weaker rather than stronger within 30 minutes after exercise, excessive muscle soreness 24–48 hours following exercise, severe muscle cramping, heaviness in the extremities, and prolonged shortness of breath (Level B) [25].
3. Discussion
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Panicucci, C.; Traverso, M.; Baratto, S.; Romeo, C.; Iacomino, M.; Gemelli, C.; Tagliafico, A.; Broda, P.; Zara, F.; Bruno, C.; et al. Novel TRIM32 mutation in sarcotubular myopathy. Acta Myol. 2019, 38, 8–12. [Google Scholar]
- Frosk, P.; Weiler, T.; Nylen, E.; Sudha, T.; Greenberg, C.R.; Morgan, K.; Fujiwara, T.M.; Wrogemann, K. Limb-Girdle Muscular Dystrophy Type 2H Associated with Mutation in TRIM32, a Putative E3-Ubiquitin–Ligase Gene. Am. J. Hum. Genet. 2002, 70, 663–672. [Google Scholar] [CrossRef] [Green Version]
- Straub, V.; Murphy, A.; Udd, B.; Corrado, A.; Aymé, S.; Bönneman, C.; de Visser, M.; Hamosh, A.; Jacobs, L.; Khizanishvili, N.; et al. 229th ENMC international workshop: Limb girdle muscular dystrophies—Nomenclature and reformed classification Naarden, the Netherlands, 17–19 March 2017. Neuromuscul. Disord. 2018, 28, 702–710. [Google Scholar] [CrossRef] [Green Version]
- Shokeir, M.H.K.; Kobrinsky, N.L. Autosomal recessive muscular dystrophy in Manitoba Hutterites. Clin. Genet. 2008, 9, 197–202. [Google Scholar] [CrossRef]
- Weiler, T.; Greenberg, C.R.; Zelinski, T.; Nylen, E.; Coghlan, G.; Crumley, M.J.; Fujiwara, T.M.; Morgan, K.; Wrogemann, K. A Gene for Autosomal Recessive Limb-Girdle Muscular Dystrophy in Manitoba Hutterites Maps to Chromosome Region 9q31-q33: Evidence for Another Limb-Girdle Muscular Dystrophy Locus. Am. J. Hum. Genet. 1998, 63, 140–147. [Google Scholar] [CrossRef] [Green Version]
- Bushby, K.M.D. Making sense of the limb-girdle muscular dystrophies. Brain 1999, 122, 1403–1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goizet, C.; Boukhris, A.; Mundwiller, E.; Tallaksen, C.; Forlani, S.; Toutain, A.; Carriere, N.; Depienne, C.; Durr, A.; Stevanin, G.; et al. Complicated forms of autosomal dominant hereditary spastic paraplegia are frequent in SPG10. Hum. Mutat. 2008, 30, E376–E385. [Google Scholar] [CrossRef] [PubMed]
- Crimella, C.; Baschirotto, C.; Arnoldi, A.; Tonelli, A.; Tenderini, E.; Airoldi, G.; Martinuzzi, A.; Trabacca, A.; Losito, L.; Scarlato, M.; et al. Mutations in the motor and stalk domains of KIF5A in spastic paraplegia type 10 and in axonal Charcot-Marie-Tooth type 2. Clin. Genet. 2011, 82, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Duis, J.; Dean, S.; Applegate, C.; Harper, A.; Xiao, R.; He, W.; Dollar, J.D.; Sun, L.R.; Waberski, M.B.; Crawford, T.O.; et al. KIF5A mutations cause an infantile onset phenotype including severe myoclonus with evidence of mitochondrial dysfunction. Ann. Neurol. 2016, 80, 633–637. [Google Scholar] [CrossRef] [Green Version]
- Brenner, D.; Yilmaz, R.; Müller, K.; Grehl, T.; Petri, S.; Meyer, T.; Grosskreutz, J.; Weydt, P.; Ruf, W.; Neuwirth, C.; et al. Hot-spot KIF5A mutations cause familial ALS. Brain 2018, 141, 688–697. [Google Scholar] [CrossRef] [Green Version]
- Lossos, A.; Baala, L.; Soffer, V.; Averbuch-Heller, L.; Dotan, S.; Munnich, A.; Lyonnet, S.; Gomori, J.M.; Genem, A.; Neufeld, M.; et al. A novel autosomal recessive myopathy with external ophthalmoplegia linked to chromosome 17p13.1-p12. Brain 2004, 128, 42–51. [Google Scholar] [CrossRef] [Green Version]
- Tajsharghi, H.; Hammans, S.; Lindberg, C.; Lossos, A.; Clarke, N.F.; Mazanti, I.; Waddell, L.B.; Fellig, Y.; Foulds, N.; Katifi, H.; et al. Recessive myosin myopathy with external ophthalmoplegia associated with MYH2 mutations. Eur. J. Hum. Genet. 2013, 22, 801–808. [Google Scholar] [CrossRef] [Green Version]
- Dobyns, W.; Pagon, R.A.; Armstrong, D.; Curry, C.J.R.; Greenberg, F.; Grix, A.; Holmes, L.B.; Laxova, R.; Michels, V.V.; Robinow, M.; et al. Diagnostic criteria for Walker-Warburg syndrome. Am. J. Med Genet. 1989, 32, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, C.; Clement, E.; Mein, R.; Brockington, M.; Smith, J.; Talim, B.; Straub, V.; Robb, S.; Quinlivan, R.; Feng, L.; et al. Refining genotype phenotype correlations in muscular dystrophies with defective glycosylation of dystroglycan. Brain 2007, 130, 2725–2735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villanova, M.; Mercuri, E.; Bertini, E.; Sabatelli, P.; Morandi, L.; Mora, M.; Sewry, C.; Brockington, M.; Brown, S.C.; Ferreiro, A.; et al. Congenital muscular dystrophy associated with calf hypertrophy, microcephaly and severe mental retardation in three Italian families: Evidence for a novel CMD syndrome. Neuromuscul. Disord. 2000, 10, 541–547. [Google Scholar] [CrossRef]
- de Stefano, N.; Dotti, M.T.; Villanova, M.; Scarano, G.; Federico, A. Merosin positive congenital muscular dystrophy with severe involvement of the central nervous system. Brain Dev. 1996, 18, 323–326. [Google Scholar] [CrossRef]
- D’Amico, A.; Tessa, A.; Bruno, C.; Petrini, S.; Biancheri, R.; Pane, M.; Pedemonte, M.; Ricci, E.; Falace, A.; Rossi, A.; et al. Expanding the clinical spectrum of POMT1 phenotype. Neurology 2006, 66, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Bello, L.; Melacini, P.; Pezzani, R.; D’Amico, A.; Piva, L.; Leonardi, E.; Torella, A.; Soraru, G.; Palmieri, A.; Smaniotto, G.; et al. Cardiomyopathy in patients with POMT1-related congenital and limb-girdle muscular dystrophy. Eur. J. Hum. Genet. 2012, 20, 1234–1239. [Google Scholar] [CrossRef] [Green Version]
- Dinçer, P.; Balcı, B.; Yuva, Y.; Talim, B.; Brockington, M.; Dinçel, D.; Torelli, S.; Brown, S.; Kale, G.; Haliloğlu, G.; et al. A novel form of recessive limb girdle muscular dystrophy with mental retardation and abnormal expression of α-dystroglycan. Neuromuscul. Disord. 2003, 13, 771–778. [Google Scholar] [CrossRef] [Green Version]
- Chiang, A.P.; Beck, J.S.; Yen, H.-J.; Tayeh, M.K.; Scheetz, T.; Swiderski, R.E.; Nishimura, D.Y.; Braun, T.A.; Kim, K.-Y.; Huang, J.; et al. Homozygosity mapping with SNP arrays identifies TRIM32, an E3 ubiquitin ligase, as a Bardet-Biedl syndrome gene (BBS11). Proc. Natl. Acad. Sci. USA 2006, 103, 6287–6292. [Google Scholar] [CrossRef] [Green Version]
- Jerusalem, F.; Engel, A.G.; Gomez, M.R. Sarcotubular myopathy: A newly recognized, benign, congenital, familial muscle disease. Neurology 1973, 23, 897. [Google Scholar] [CrossRef] [PubMed]
- Müller-Felber, W.; Schlotter, B.; Töpfer, M.; Ketelsen, U.-P.; Müller-Höcker, J.; Pongratz, D. Phenotypic variability in two brothers with sarcotubular myopathy. J. Neurol. 1999, 246, 408–411. [Google Scholar] [CrossRef]
- Saccone, V.; Palmieri, M.; Passamano, L.; Piluso, G.; Meroni, G.; Politano, L.; Nigro, V. Mutations that impair interaction properties of TRIM32 associated with limb-girdle muscular dystrophy 2H. Hum. Mutat. 2008, 29, 240–247. [Google Scholar] [CrossRef] [PubMed]
- The Human Phenotype Ontology. Available online: https://hpo.jax.org/app/browse/disease/ORPHA:1878 (accessed on 15 February 2021).
- Narayanaswami, P.; Weiss, M.; Selcen, D.; David, W.; Raynor, E.; Carter, G.; Wicklund, M.; Barohn, R.J.; Ensrud, E.; Griggs, R.; et al. Evidence-based guideline summary: Diagnosis and treatment of limb-girdle and distal dystrophies: Report of the Guideline Development Subcommittee of the American Academy of Neurology and the Practice Issues Review Panel of the American Association of Neuromuscular & Electrodiagnostic Medicine. Neurology 2014, 83, 1453–1463. [Google Scholar] [CrossRef]
- Thompson, A.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Ellison, P.M.; Goodall, S.; Kennedy, N.; Dawes, H.; Clark, A.; Pomeroy, V.; Duddy, M.; Baker, M.R.; Saxton, J.M. Neurostructural and Neurophysiological Correlates of Multiple Sclerosis Physical Fatigue: Systematic Review and Meta-Analysis of Cross-Sectional Studies. Neuropsychol. Rev. 2021, 1–14. [Google Scholar] [CrossRef]
- Fedota, O.M.; Lysenko, N.G.; Ruban, S.; Kolisnyk, O.I.; Goraichuk, I. The effects of polymorphisms in growth hormone and growth hormone receptor genes on production and reproduction traits in Aberdeen-Angus cattle (Bos taurus L., 1758). Cytol. Genet. 2017, 51, 352–360. [Google Scholar] [CrossRef]
| Gene | Variant | Zygosity | Variant Classification |
|---|---|---|---|
| KIF5A | c.2300C > G (p.Thr767Arg) | heterozygous | Uncertain Significance |
| MYH2 | c.2840G > A (p.Arg947Lys) | heterozygous | Uncertain Significance |
| POMT1 | c.1502G > C (p.Gly501Ala) | heterozygous | Uncertain Significance |
| TRIM32 | c.1855C > T (p.Pro619Ser) | homozygous | Uncertain Significance |
| Gene | Diagnosis | Phenotype |
|---|---|---|
| KIF5A (Kinesin heavy chain isoform 5A) | Autosomal dominant hereditary spastic paraplegia 10 (SPG10) (MedGen UID: 349003) |
|
| Intractable neonatal myoclonus (MedGen UID: 934625) |
| |
| Amyotrophic lateralsclerosis 25 (ALS25) (MedGen UID: 1534540) |
| |
| MYH2 (Myosin-2) | Autosomal dominant and recessive inclusion body myopathy type 3 (MYPOP) (MedGen UID: 381340) |
|
| POMT1(Protein O-mannosyl-transferase 1) | Autosomal recessive muscular dystrophy-dystroglycanopathy type A1 (MDDGA1) (MedGen UID: 75553) |
|
| Autosomal recessive muscular dystrophy-dystroglycanopathy type B1 (MDDGB1) (MedGen UID: 461765) |
| |
| Autosomal recessive muscular dystrophy-dystroglycanopathy type C1 (MDDGC1) (MedGen UID: 332193) |
| |
| TRIM32 (Tripartite motif containing 32) | Autosomal recessive Bardet-Biedl syndrome (BBS) (MedGen UID: 395295) |
|
| Limb-girdle muscular dystrophy type 2H (LGMD2H) or Limb-girdle Muscular Dystrophy R8 (LGMDR8) (MedGen UID:78750) |
|
| Term Name | Frequency | Presence in the Patient |
|---|---|---|
| Tall stature | Frequent | Not detected |
| Mask-like facies | Very frequent | Not detected |
| EMG abnormality | Very frequent | Detected |
| Increased variability in muscle fiber diameter | Very frequent | Not conducted |
| Myopathy | Very frequent | Detected |
| Proximal muscle weakness in lower limbs | Very frequent | Detected |
| Gait disturbance | Very frequent | Detected |
| Waddling gait | Very frequent | Detected |
| Elevated serum creatine kinase | Very frequent | Detected |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchuk, M.; Dovbonos, T.; Makukh, H.; Semeryak, O.; Sharhorodska, Y. Sarcotubular Myopathy Due to Novel TRIM32 Mutation in Association with Multiple Sclerosis. Brain Sci. 2021, 11, 1020. https://doi.org/10.3390/brainsci11081020
Marchuk M, Dovbonos T, Makukh H, Semeryak O, Sharhorodska Y. Sarcotubular Myopathy Due to Novel TRIM32 Mutation in Association with Multiple Sclerosis. Brain Sciences. 2021; 11(8):1020. https://doi.org/10.3390/brainsci11081020
Chicago/Turabian StyleMarchuk, Margarita, Tetiana Dovbonos, Halyna Makukh, Orest Semeryak, and Yevheniya Sharhorodska. 2021. "Sarcotubular Myopathy Due to Novel TRIM32 Mutation in Association with Multiple Sclerosis" Brain Sciences 11, no. 8: 1020. https://doi.org/10.3390/brainsci11081020
APA StyleMarchuk, M., Dovbonos, T., Makukh, H., Semeryak, O., & Sharhorodska, Y. (2021). Sarcotubular Myopathy Due to Novel TRIM32 Mutation in Association with Multiple Sclerosis. Brain Sciences, 11(8), 1020. https://doi.org/10.3390/brainsci11081020






