The Assessment of Serum Concentrations of AGEs and Their Soluble Receptor (sRAGE) in Multiple Sclerosis Patients
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Participants
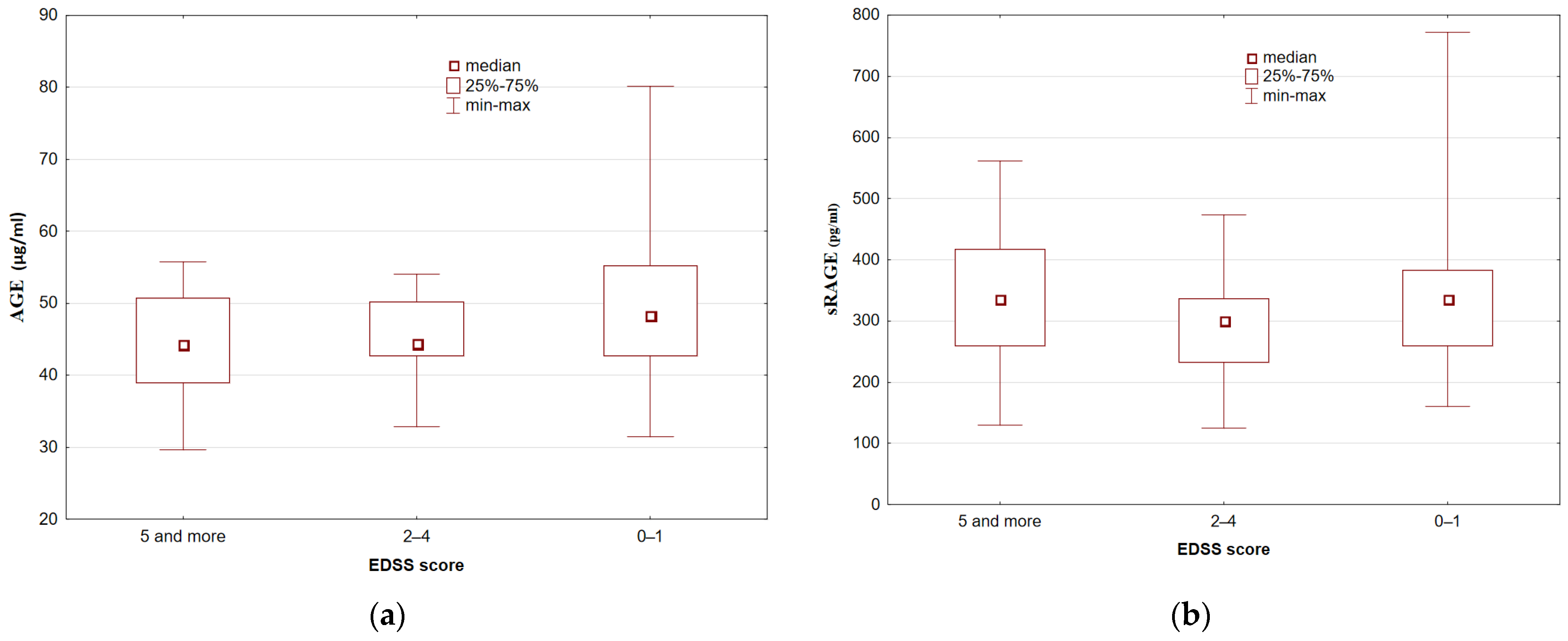
3.2. Serum AGE and sRAGE Concentrations
4. Discussion
5. Conclusions
6. Limitations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pirko, I.; Noseworthy, J.H. Demyelinating Disorders of the Central Nervous System. In Textbook of Clinical Neurology, 3rd ed.; Goetz, C., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2007; pp. 1103–1133. [Google Scholar] [CrossRef]
- Martin, R.; Hohlfeld, R.; McFarland, H.F. Multiple sclerosis. In Neurological Disorders Course and Treatment, 2nd ed.; Brandt, T., Caplan, L.R., Dichgans, J., Diener, H.C., Kennard, C., Eds.; Academic Press: Cambridge, UK, 2003; pp. 677–679. [Google Scholar]
- Bermejo, P.E.; Oreja-Guevara, C.; Díez-Tejedor, E. El dolor en la esclerosis múltiple: Prevalencia, mecanismos, tipos y tratamiento. Rev. Neurol. 2010, 50, 101–108. [Google Scholar] [CrossRef]
- Giorgio, A.; Battaglini, M.; Smith, S.M.; DeStefano, N. Brain atrophy assessment in multiple sclerosis: Importance and limita-tions. Neuroimaging Clin. N. Am. 2008, 18, 675–686. [Google Scholar] [CrossRef]
- Flachenecker, P.; Kümpfel, T.; Kallmann, B.; Gottschalk, M.; Grauer, O.; Rieckmann, P.; Trenkwalder, C.; Toyka, K.V. Fatigue in multiple sclerosis: A comparison of different rating scales and correlation to clinical parameters. Mult. Scler. J. 2002, 8, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Kamińska, J.; Koper, O.M.; Piechal, K.; Kemona, H. Multiple sclerosis-etiology and diagnostic potential. Postepy. Hig. Med. Dosw. 2017, 71, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Lassmann, H. Multiple Sclerosis Pathology. Cold Spring Harb. Perspect. Med. 2018, 8, a028936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faissner, S.; Plemel, J.R.; Gold, R.; Yong, V.W. Progressive multiple sclerosis: From pathophysiology to therapeutic strategies. Nat. Rev. Drug Discov. 2019, 18, 905–922. [Google Scholar] [CrossRef] [PubMed]
- Zahoor, I.; Haq, E. Vitamin D and Multiple Sclerosis: An Update. In Multiple Sclerosis: Perspectives in Treatment and Pathogenesis; Codon Publications: Brisbane, Australia, 2017; pp. 71–84. [Google Scholar]
- Gilden, D.H. Infectious causes of multiple sclerosis. Lancet Neurol. 2005, 4, 195–202. [Google Scholar] [CrossRef]
- Swanborg, R.H.; Whittum-Hudson, J.A.; Hudson, A.P. Infectious agents and multiple sclerosis—Are Chlamydia pneumoniae and human herpes virus 6 involved? J. Neuroimmunol. 2003, 136, 1–8. [Google Scholar] [CrossRef]
- Correale, J.; Farez, M. Association between parasite infection and immune responses in multiple sclerosis. Ann. Neurol. 2007, 61, 97–108. [Google Scholar] [CrossRef]
- Ebers, G.C. Genetics and multiple sclerosis. An. Neurol. 1994, 36, 12–16. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, Y.; Sujkowska, D.; Wang, J.; Ramgolam, V.; Sospedra, M.; Adams, J.; Martin, R.; Pinilla, C.; Markovic-Plese, S. Degenerate TCR recognition and dual DR2 restriction of autoreactive T cells: Implications for the initiation of the autoimmune response in multiple sclerosis. Eur. J. Immunol. 2008, 38, 1297–1309. [Google Scholar] [CrossRef]
- Markovic-Plese, S. Degenerate T-Cell Receptor Recognition, Autoreactive Cells, and the Autoimmune Response in Multiple Sclerosis. Neuroscientist 2009, 15, 225–231. [Google Scholar] [CrossRef]
- Antel, J.; Bar-Or, A. Roles of immunoglobulins and B cells in multiple sclerosis: From pathogenesis to treatment. J. Neuroimmunol. 2006, 180, 3–8. [Google Scholar] [CrossRef]
- Disanto, G.; Morahan, J.M.; Barnett, M.H.; Giovannoni, G.; Ramagopalan, S.V. The evidence for a role of B cells in multiple sclerosis. Neurology 2012, 78, 823–832. [Google Scholar] [CrossRef] [Green Version]
- Lazibat, I.; Majdak, M.R.; Županić, S. Multiple Sclerosis: New Aspects of Immunopathogenesis. Acta Clin. Croat. 2018, 57, 352–361. [Google Scholar] [CrossRef]
- Gasperoni, F.; Turini, P.; Agostinelli, E. A novel comprehensive paradigm for the etiopathogenesis of multiple sclerosis: Therapeutic approaches and future perspectives on its treatment. Amino Acids 2019, 51, 745–759. [Google Scholar] [CrossRef]
- Scazzone, C.; Agnello, L.; Ragonese, P.; Lo Sasso, B.; Bellia, C.; Bivona, G.; Schillaci, R.; Salemi, G.; Ciaccio, M. Association of CYP2R1 rs10766197 with MS risk and disease progression. J. Neurosci. Res. 2018, 96, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.; Trevick, S.; Younger, D.S. Epidemiology of Multiple Sclerosis. Neurol. Clin. 2016, 34, 919–939. [Google Scholar] [CrossRef] [PubMed]
- Dobson, R.; Giovannoni, G. Multiple sclerosis – a review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bivona, G.; Gambino, C.M.; Iacolino, G.; Ciaccio, M. Vitamin D and the nervous system. Neurol. Res. 2019, 41, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Bivona, G.; Agnello, L.; Bellia, C.; Iacolino, G.; Scazzone, C.; Lo Sasso, B.; Ciaccio, M. Non-Skeletal Activities of Vitamin D: From Physiology to Brain Pathology. Medicina 2019, 55, 341. [Google Scholar] [CrossRef] [Green Version]
- Bivona, G.; Agnello, L.; Butera, D.; Ciaccio, M. The immunological implications of the new Vitamin D metabolism. Cent. Eur. J. Immunol. 2018, 43, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Scazzone, C.; Agnello, L.; Lo Sasso, B.; Ciaccio, A.M.; Giglio, R.V.; Bivona, G.; Ciaccio, M. Vitamin D and multiple sclerosis: An open-ended story. Open Biochem. J. 2019, 13, 88–98. [Google Scholar] [CrossRef]
- Agnello, L.; Scazzone, C.; Lo Sasso, B.; Bellia, C.; Bivona, G.; Realmuto, S.; Brighina, F.; Schillaci, R.; Ragonese, P.; Salemi, G.; et al. VDBP, CYP27B1, and 25-Hydroxyvitamin D Gene Polymorphism Analyses in a Group of Sicilian Multiple Sclerosis Patients. Biochem. Genet. 2017, 55, 183–192. [Google Scholar] [CrossRef]
- Feige, J.; Moser, T.; Bieler, L.; Schwenker, K.; Hauer, L.; Sellner, J. Vitamin D Supplementation in Multiple Sclerosis: A Critical Analysis of Potentials and Threats. Nutrients 2020, 12, 783. [Google Scholar] [CrossRef] [Green Version]
- Van Horssen, J.; Witte, M.E.; Schreibelt, G.; de Vries, H.E. Radical changes in multiple sclerosis pathogenesis. Biochim. Biophys. Acta-Mol. Basis Dis. 2011, 1812, 141–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colton, C.A.; Gilbert, D.L. Microglia, an in vivo source of reactive oxygen species in the brain. Adv. Neurol. 1993, 59, 321. [Google Scholar] [PubMed]
- Gray, E.; Thomas, T.L.; Betmouni, S.; Scolding, N.; Love, S. Elevated myeloperoxidase activity in white matter in multiple sclerosis. Neurosci. Lett. 2008, 444, 195–198. [Google Scholar] [CrossRef]
- Ortiz, G.G.; Pacheco-Moisés, F.P.; Bitzer-Quintero, O.K.; Ramírez-Anguiano, A.C.; Flores-Alvarado, L.J.; Ramírez-Ramírez, V.; Macias-Islas, M.A.; Torres-Sanchez, E.D. Immunology and Oxidative Stress in Multiple Sclerosis: Clinical and Basic Approach. Clin. Dev. Immunol. 2013, 2013, 708659. [Google Scholar] [CrossRef] [Green Version]
- Otto, J.; Smith, W. The orientation of prostaglandin endoperoxide synthases-1 and -2 in the endoplasmic reticulum. J. Biol. Chem. 1994, 269, 19868–19875. [Google Scholar] [CrossRef]
- Dringen, R.; Pawlowski, P.G.; Hirrlinger, J. Peroxide detoxification by brain cells. J. Neurosci. Res. 2004, 79, 157–165. [Google Scholar] [CrossRef]
- Kankova, K. Diabetic threesome (hyperglicaemia, renal function and nutrition) and advanced glycation end products: Evidence for the multiplehit agent. Proc. Nutr. Soc. 2008, 67, 60–74. [Google Scholar] [CrossRef] [Green Version]
- Tessier, F.J. The Maillard reaction in the human body. The main discoveries and factors that affect glycation. Pathol. Biol. 2010, 58, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Voyer, E.; Alvarado, C. Maillard reaction. Pathogenic effects. Medicina 2019, 79, 137–143. [Google Scholar] [PubMed]
- Zou, T.; Liu, J.; Song, H.; Liu, Y. Discovery of Amadori-Type Conjugates in a Peptide Maillard Reaction and Their Corresponding Influence on the Formation of Pyrazines. J. Food Sci. 2018, 83, 1588–1595. [Google Scholar] [CrossRef] [PubMed]
- Lushington, G.H.; Barnes, A.C. Protein Glycation: An Old Villain is Shedding Secrets. Comb. Chem. High Throughput Screen. 2019, 22, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Barden, A.; Mori, T.; Beilin, L. Advanced glycation end-products: A review. Diabetologia 2001, 44, 129–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsukahara, H.; Sekine, K.; Uchiyama, M.; Kawakami, H.; Hata, I.; Todoroki, Y.; Hiraoka, M.; Kaji, M.; Yorifuji, T.; Momoi, T.; et al. Formation of Advanced Glycosylation End Products and Oxidative Stress in Young Patients with Type 1 Diabetes. Pediatr. Res. 2003, 54, 419–424. [Google Scholar] [CrossRef] [Green Version]
- Teerlink, T.; Barto, R.; Brink, H.J.T.; Schalkwijk, C.G. Measurement of Nε-(Carboxymethyl)lysine and Nε-(Carboxyethyl)lysine in Human Plasma Protein by Stable-Isotope-Dilution Tandem Mass Spectrometry. Clin. Chem. 2004, 50, 1222–1228. [Google Scholar] [CrossRef] [Green Version]
- Ulrich, P.; Cerami, A. Protein Glycation, Diabetes, and Aging. Recent Prog. Horm. Res. 2001, 56, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlassara, H. The AGE-receptor in the pathogenesis of diabetic complications. Diabetes/Metab. Res. Rev. 2001, 17, 436–443. [Google Scholar] [CrossRef]
- Daroux, M.; Prévost, G.; Maillard-Lefebvre, H.; Gaxatte, C.; D’Agati, V.; Schmidt, A.; Boulanger, É. Advanced glycation end-products: Implications for diabetic and non-diabetic nephropathies. Diabetes Metab. 2010, 36, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Basta, G. Receptor for advanced glycation endproducts and atherosclerosis: From basic mechanisms to clinical implications. Atherosclerosis 2008, 196, 9–21. [Google Scholar] [CrossRef]
- Yan, S.F.; Ramasamy, R.; Schmidt, A.M. Mechanisms of Disease: Advanced glycation end-products and their receptor in inflammation and diabetes complications. Nat. Clin. Pract. Endocrinol. Metab. 2008, 4, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; Kapoor, R.; Felts, P.A. Demyelination: The Role of Reactive Oxygen and Nitrogen Species. Brain Pathol. 1999, 9, 69–92. [Google Scholar] [CrossRef] [PubMed]
- Zuwała-Jagiełło, J. Terapia chorób z udziałem końcowych produktów zaawansowanej glikacji w ich patogenezie. Pol. Merk. Lek. 2009, 27, 152–155. [Google Scholar] [PubMed]
- Raucci, A.; Cugusi, S.; Antonelli, A.; Barabino, S.M.; Monti, L.; Bierhaus, A.; Reiss, K.; Saftig, P.; Bianchi, M.E. A soluble form of the receptor for advanced glycation endproducts (RAGE) is produced by proteolytic cleavage of the membrane-bound form by the sheddase a disintegrin and metalloprotease 10 (ADAM10). FASEB J. 2008, 22, 3716–3727. [Google Scholar] [CrossRef]
- Zeng, S.; Feirt, N.; Goldstein, M.; Guarrera, J.; Ippagunta, N.; Ekong, U.; Dun, H.; Lu, Y.; Qu, W.; Schmidt, A.M.; et al. Blockade of receptor for advanced glycation end product (RAGE) attenuates ischemia and reperfusion injury to the liver in mice. Hepatol. 2004, 39, 422–432. [Google Scholar] [CrossRef]
- Xu, X.-Y.; Deng, C.-Q.; Wang, J.; Deng, X.-J.; Xiao, Q.; Li, Y.; He, Q.; Fan, W.-H.; Quan, F.-Y.; Zhu, Y.-P.; et al. Plasma levels of soluble receptor for advanced glycation end products in Alzheimer’s disease. Int. J. Neurosci. 2017, 127, 454–458. [Google Scholar] [CrossRef]
- Zhang, D.-Q.; Wang, R.; Li, T.; Zhou, J.-P.; Chang, G.-Q.; Zhao, N.; Yang, L.-N.; Zhai, H.; Li, Y. Reduced soluble RAGE is associated with disease severity of axonal Guillain-Barré syndrome. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Vistoli, G.; De Maddis, D.; Cipak, A.; Zarkovic, N.; Carini, M.; Aldini, G. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): An overview of their mechanisms of formation. Free. Radic. Res. 2013, 47 (Suppl. 1), 3–27. [Google Scholar] [CrossRef] [Green Version]
- Sobów, T.; Flirski, M.; Liberski, P.P. Amyloid-beta and tau proteins as biochemical markers of Alzheimer’s disease. Acta Neu-robiol. Exp. 2004, 64, 53–70. [Google Scholar] [PubMed]
- Leszek, J.; Małyszczak, K.; Bartyś, A.; Staniszewska, M.; Gamian, A. Analysis of Serum of Patients With Alzheimer’s Disease for the Level of Advanced Glycation End Products. Am. J. Alzheimer’s Dis. Other Dement. 2006, 21, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Staniszewska, M.; Leszek, J.; Małyszczak, K.; Gamian, A. Are advanced glycation end-products specific biomarkers for Alzheimer’s disease? Int. J. Geriatr. Psychiatry 2005, 20, 896–897. [Google Scholar] [CrossRef] [PubMed]
- Lyons, T.J.; Basu, A. Biomarkers in diabetes: Hemoglobin A1c, vascular and tissue markers. Transl. Res. 2012, 159, 303–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Ames, J.M.; Smith, R.D.; Baynes, J.W.; Metz, T.O. A Perspective on the Maillard Reaction and the Analysis of Protein Glycation by Mass Spectrometry: Probing the Pathogenesis of Chronic Disease. J. Proteome Res. 2009, 8, 754–769. [Google Scholar] [CrossRef] [Green Version]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef] [Green Version]
- Yonekura, H.; Yamamoto, Y.; Sakurai, S.; Petrova, G.R.; Abedin Md, J.; Li, H.; Yasui, K.; Takeuchi, M.; Makita, Z.; Takasawa, S.; et al. Expression and function of novel splice variants of RAGE in human vascular endo-thelial cells and pericytes. Biochem. J. 2003, 370, 1097–1109. [Google Scholar] [CrossRef] [PubMed]
- Wetzels, S.; Wouters, K.; Schalkwijk, C.G.; Vanmierlo, T.; Hendriks, J.J.A. Methylglyoxal-Derived Advanced Glycation Endproducts in Multiple Sclerosis. Int. J. Mol. Sci. 2017, 18, 421. [Google Scholar] [CrossRef] [Green Version]
- Wetzels, S.; Wouters, K.; Miyata, T.; Scheijen, J.L.J.M.; Hendriks, J.J.A.; Schalkwijk, C.G.; Vanmierlo, T. Advanced Glycation Endproducts Are Increased in the Animal Model of Multiple Sclerosis but Cannot Be Reduced by Pyridoxamine Treatment or Glyoxalase 1 Overexpression. Int. J. Mol. Sci. 2018, 19, 1311. [Google Scholar] [CrossRef] [Green Version]
- Selmaj, K. Stwardnienie Rozsiane; Wydawnictwo Medyczne Termedia: Poznań, Poland, 2013; pp. 11–12. ISBN 978-83-63622-99-2. (In Polish) [Google Scholar]
- Edwards, S.; Zvartau, M.; Clarke, H.; Irving, W.; Blumhardt, L.D. Clinical relapses and disease activity on magnetic resonance imaging associated with viral upper respiratory tract infections in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 1998, 64, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Kalousová, M.; Havrdova, E.K.; Mrazova, K.; Spacek, P.; Braun, M.; Uhrova, J.; Germanová, A.; Zima, T. Advanced glycoxidation end products in patients with multiple sclerosis. Prague Med. Rep. 2005, 106, 167–174. [Google Scholar]
- Sternberg, Z.; Hennies, C.; Sternberg, D.; Wang, P.; Kinkel, P.; Hojnacki, D.; Weinstock-Guttmann, B.; Munschauer, F. Diagnostic potential of plasma carboxymethyllysine and carboxyethyllysine in multiple sclerosis. J. Neuroinflammation 2010, 7, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagai, R.; Murray, D.B.; Metz, T.O.; Baynes, J.W. Chelation: A Fundamental Mechanism of Action of AGE Inhibitors, AGE Breakers, and Other Inhibitors of Diabetes Complications. Diabetes 2012, 61, 549–559. [Google Scholar] [CrossRef] [Green Version]
- Forbes, J.M.; Cooper, M.E.; Thallas, V.; Burns, W.C.; Thomas, M.; Brammar, G.C.; Lee, F.; Grant, S.L.; Burrell, L.M.; Jerums, G.; et al. Reduction of the Accumulation of Advanced Glycation End Products by ACE Inhibition in Experimental Diabetic Nephropathy. Diabetes 2002, 51, 3274–3282. [Google Scholar] [CrossRef]
- Vazzana, N.; Santilli, F.; Cuccurullo, C.; Davì, G. Soluble forms of RAGE in internal medicine. Intern. Emerg. Med. 2009, 4, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Glasnović, A.; Cvija, H.; Stojić, M.; Tudorić-Đeno, I.; Ivčević, S.; Romić, D.; Tičinović, N.; Vuletić, V.; Lazibat, I.; Grčević, D. Decreased level of sRAGE in the cerebrospinal fluid of multiple sclerosis patients at clinical onset. Neuroimmunomodulation 2014, 2, 226–233. [Google Scholar] [CrossRef]
- Sternberg, Z.; Weinstock-Guttman, B.; Hojnacki, D.; Zamboni, P.; Zivadinov, R.; Chadha, K.; Lieberman, A.; Kazim, L.; Drake, A.; Rocco, P.; et al. Soluble receptor for advanced glycation end products in multiple sclerosis: A potential marker of disease severity. Mult. Scler. J. 2008, 14, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Yonekura, H.; Yamagishi, S.-I.; Fujimori, H.; Yamamoto, Y.; Yamamoto, H. The Receptor for Advanced Glycation End Products Is Induced by the Glycation Products Themselves and Tumor Necrosis Factor-α through Nuclear Factor-κB, and by 17β-Estradiol through Sp-1 in Human Vascular Endothelial Cells. J. Biol. Chem. 2000, 275, 25781–25790. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.T.; Ramanathan, M.; Weinstock-Guttman, B.; Baier, M.; Brownscheidle, C.; Jacobs, L.D. Sex differences in in vitro pro-inflammatory cytokine production from peripheral blood of multiple sclerosis patients. J. Neurol. Sci. 2003, 209, 93–99. [Google Scholar] [CrossRef]
- Sternberg, Z.; Sternberg, D.; Drake, A.; Chichelli, T.; Yu, J.; Hojnacki, D. Disease modifying drugs modulate endogenous secretory receptor for advanced glycation end-products, a new biomarker of clinical relapse in multiple sclerosis. J. Neuroimmunol. 2014, 27, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Cierny, D.; Michalik, J.; Hanysova, S.; Kantorova, E.; Škereňová, M.; Kurca, E.; Dobrota, D.; Lehotsky, J. The increased serum level of sRAGE is associated with multiple sclerosis but not with disability progression. Neurol. Sci. Neurophysiol. 2018, 35, 70–76. [Google Scholar] [CrossRef]
- Rahimi, M.; Afjeh, S.S.A.; Omrani, M.D.; Arsang-Jang, S.; Ganji, M.; Noroozi, R.; Taheri, M.; Ghafouri-Fard, S. Soluble Receptor for Advanced Glycation End Products (sRAGE) is Up-Regulated in Multiple Sclerosis Patients Treated with Interferon β-1a. Cell. Physiol. Biochem. 2018, 46, 561–567. [Google Scholar] [CrossRef]
- Asadikaram, G.; Noroozi, S.; Meimand, H.A.E.; Sanjari, M.; Zainodini, N.; Khoramdelazad, H.; Shahrokhi, N.; Arababadi, M.K. Interferon-beta 1a modulates expression of RAGE but not S100A12 and nuclear factor-kappa B in multiple sclerosipatients. Neuroimmunomodulation 2016, 23, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, Z.; Kolb, C.; Chadha, K.; Nir, A.; Nir, R.; George, R.; Johnson, J.; Yu, J.; Hojnacki, D. Fingolimod anti-inflammatory and neuroprotective effects modulation of RAGE axis in multiple sclerosis patients. Neuropharmacology 2018, 130, 71–76. [Google Scholar] [CrossRef]
- Münch, G.; Deuther-Conrad, W.; Gasic-Milenkovic, J. Glycoxidative stress creates a vicious cycle of neurodegeneration in Alzheimer’s disease—A target for neuroprotective treatment strategies? Ageing Dement. Curr. Future Concepts 2002, 62, 303–307. [Google Scholar] [CrossRef]
- Nedeljkovic, U.; Dackovic, J.; Tepavcevic, D.K.; Basuroski, I.D.; Mesaros, S.; Pekmezovic, T.; Drulovic, J. Multidisciplinary rehabilitation and steroids in the management of multiple sclerosis relapses: A randomized controlled trial. Arch. Med. Sci. 2016, 2, 380–389. [Google Scholar] [CrossRef]

| Characteristics | MS Patients |
|---|---|
| N | 52 |
| Age (years) mean ± SD | 37.9 ± 9.4 |
| Gender female (%) male (%) | 35 (67%) 17 (33%) |
| Employment full-time employment/student part-time employment pension/sickness pension | 28 (54%) 5 (10%) 19 (36%) |
| Smoking active smokers passive smokers non-smokers | 8 (15%) 12 (23%) 32 (62%) |
| Environmental exposure chemical pollution/industrial plants heat plants/power plants increased car traffic | 25 (48%) 5 (10%) 25 (48%) |
| Moving no problem with the help of elbow crutches on wheelchair | 38 (73%) 10 (19%) 4 (8%) |
| Forms of physical activity walking/nordic walking biking none | 41 (79%) 4 (8%) 5 (10%) |
| Active forms of rehabilitation every day several times a year once a year every few years never | 2 (4%) 25 (48%) 11 (19%) 4 (8%) 10 (19%) |
| Disease duration (years) 0–5 6–10 11–15 16 and more | 15 (29%) 17 (33%) 13 (24%) 7 (14%) |
| Form of the disease relapsing-remitting secondary progressive | 38 (73%) 14 (27%) |
| EDSS score (points) 0–1.5 2–4.5 5 and more | 21 (40%) 17 (33%) 14 (27%) |
| The most common symptoms weakness in at least one limb balance disorders mood disorders sensory disturbances visual impairment bladder problems | 40 (76%) 26 (50%) 22 (42%) 22 (42%) 18 (34%) 11 (21%) |
| DMD treatment interferon beta glatiramer acetate natalizumab fingolimod | 13 (25%) 2 (4%) 2 (4%) 3 (6%) |
| Parameter (Mean ± SD) | MS Patients (n = 52) | Control Group (n = 40) | ||||
|---|---|---|---|---|---|---|
| Men (n = 17) | Women (n = 35) | p | Men (n = 15) | Women (n = 25) | p | |
| AGE (µg/mL) | 47.75 ± 9.12 | 46.30 ± 9.96 | 0.438 | 43.16 ± 11.39 | 45.47 ± 13.13 | 0.555 |
| sRAGE (pg/mL) | 303.66 ± 122.03 | 345.56 ± 132.37 | 0.322 | 373.98 ± 167.80 | 346.00 ± 176.56 | 0.472 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damasiewicz-Bodzek, A.; Łabuz-Roszak, B.; Kumaszka, B.; Tadeusiak, B.; Tyrpień-Golder, K. The Assessment of Serum Concentrations of AGEs and Their Soluble Receptor (sRAGE) in Multiple Sclerosis Patients. Brain Sci. 2021, 11, 1021. https://doi.org/10.3390/brainsci11081021
Damasiewicz-Bodzek A, Łabuz-Roszak B, Kumaszka B, Tadeusiak B, Tyrpień-Golder K. The Assessment of Serum Concentrations of AGEs and Their Soluble Receptor (sRAGE) in Multiple Sclerosis Patients. Brain Sciences. 2021; 11(8):1021. https://doi.org/10.3390/brainsci11081021
Chicago/Turabian StyleDamasiewicz-Bodzek, Aleksandra, Beata Łabuz-Roszak, Bartłomiej Kumaszka, Bartosz Tadeusiak, and Krystyna Tyrpień-Golder. 2021. "The Assessment of Serum Concentrations of AGEs and Their Soluble Receptor (sRAGE) in Multiple Sclerosis Patients" Brain Sciences 11, no. 8: 1021. https://doi.org/10.3390/brainsci11081021
APA StyleDamasiewicz-Bodzek, A., Łabuz-Roszak, B., Kumaszka, B., Tadeusiak, B., & Tyrpień-Golder, K. (2021). The Assessment of Serum Concentrations of AGEs and Their Soluble Receptor (sRAGE) in Multiple Sclerosis Patients. Brain Sciences, 11(8), 1021. https://doi.org/10.3390/brainsci11081021






