Abstract
Dexmedetomidine, selective α2-adrenergic agonist dexmedetomidine, has been widely used clinically for sedation and anesthesia. The role of dexmedetomidine has been an interesting topic of neonatological and anesthetic research since a series of advantages of dexmedetomidine, such as enhancing recovery from surgery, reducing opioid prescription, decreasing sympathetic tone, inhibiting inflammatory reactions, and protecting organs, were reported. Particularly, an increasing number of animal studies have demonstrated that dexmedetomidine ameliorates the neurological outcomes associated with various brain and spinal cord injuries. In addition, a growing number of clinical trials have reported the efficacy of dexmedetomidine for decreasing the rates of postoperative neurological dysfunction, such as delirium and stroke, which strongly highlights the possibility of dexmedetomidine functioning as a neuroprotective agent for future clinical use. Mechanism studies have linked dexmedetomidine’s neuroprotective properties with its modulation of neuroinflammation, apoptosis, oxidative stress, and synaptic plasticity via the α2-adrenergic receptor, dependently or independently. By reviewing recent advances and preclinical and clinical evidence on the neuroprotective effects of dexmedetomidine, we hope to provide a complete understanding of the above mechanism and provide insights into the potential efficacy of this agent in clinical use for patients.
1. Introduction
Car accidents and falls are the main causes of nerve and brain injuries that can lead to permanent damage and dysfunction of a limb [1]. Furthermore, there are greater chances that this injury may develop anxiety in later stages [2]. Consequently, various kinds of the neuroprotective agent are tested preclinically and clinically on different steps of cascade in neuronal damage to ameliorate the aforementioned issues, thereby restoring their function by preventing further damage [3]. One of the most pertinent neuroprotective drugs is Dexmedetomidine (DEXM) which was approved in 1999 by the FDA as a short term (<24 h) sedative agent [4,5]. In 2008, the USA granted another indication of DEXM for use as a sedative agent in non-tracheal intubated patients before or during surgery [6,7,8].
Accordingly, the European Union approved DEXM in 2011 as an adult sedative agent for ICU [9]. A selective α2 agonist, DEXM has broad-spectrum effects, including being analgesic, sedative, and anxiolytic, with minimal depression of pulmonary function [9]. Previous works illustrated that DEXM has proved a crucial tool for critical patients by minimizing ventilation free hours [8,10]. On the other hand, it also attenuates delirium, improves postoperative cognitive dysfunction, and acts as a sympatholytic agent [11,12,13]. Recently, it caught the attention of researchers and clinicians due to its cardioprotective [14], renoprotective [15], pulmonoprotective [16], and neuroprotective effects [17]. However, there has been very limited research regarding DEXM as a neuroprotective agent against neuronal injury and only a few review articles have been reported. To the authors’ best knowledge, there is not a single review article on the neuroprotective effect of DEXM against neuronal injury. Therefore, to bridge this literature gap, an attempt has been made to provide the latest updates on the most recent contributions about DEXM, and which demonstrate the various growing research directions on its neuroprotective effects. The main contributions of this work are:
- It reviews recent studies in various animal models depicting DEXM’s anti-inflammatory role.
- Moreover, it elaborates the longitudinal studies of pre-and post-clinical trials on DEXM, which prove its ability to modulate apoptotic and necrotic events and also illustrate DEXM’s role in astroglia.
- Finally, the proposed study describes all the viable neuroprotective mechanisms of DEXM against nerve injury, which can support scientists and doctors in future studies.
The purpose of this article is to summarize and critically review the published data on the neuroprotective mechanisms of DEXM. This article also addresses the recent clinical applications of DEXM to have surfaced.
2. Pharmacology of Dexmedetomidine
2.1. Pharmacokinetics of Dexmedetomidine
DEXM is FDA approved IV drug that extensively undergoes a first-pass effect with a bioavailability of 16% [18]. It shows a better intranasal sedative and anxiolytic effect in comparison to clonidine [19]. DEXM exclusively binds with plasma protein (albumin) has the ability to cross the placenta and Blood-Brain Barriers (BBB) but its teratogenicity effect has not yet been fully explored [20].
DEXM is mainly metabolized in the liver via N-glucuronidation through uridine 5′-diphospho-glucuronosyltransferase (UGT2B10, UGT1A4) and cytochrome P450 (CYP2A6). The average half-life of DEXM in a healthy individual is 2.1–3.1 h, while in ICU it is 2.2–3.7 h.
2.2. Pharmacodynamics of Dexmedetomidine
The pharmacodynamic effect of DEXM is mainly dose-dependent. The biphasic hemodynamic responses, such as hypotension or hypertension, produce low or high plasma concentrations, respectively [21].
The concentration-dependent hypnotic and sedative action of DEXM is mediated through the activation of the α2 adrenergic receptor, which is anatomically located in the locus coeruleus [22]. The peculiar and unique pharmacodynamic properties of DEXM ensure “cooperative sedation”, in which even patients in the asleep stage can be easily aroused [23]. DEXM also can reduce pain via the α2 adrenergic receptor that alters perception, weakens the anxiolytic effect, and decreases the post-operative opioid need [24].
2.3. Adverse Effects of DEXM
DEXM’s main adverse effect is its hemodynamic instability because of α2 adrenoreceptor receptor activation that causes hypertension due to vasoconstriction, which leads to reflex bradycardia via carotid or aortic baroreceptor mediated autonomic activation. Moreover, DEXM is dose-dependent: a high dose of 1 or 2 μg/kg of DEXM over 2 min is required for rapid sedation. However, at the aforementioned dose concentration, an irregular and obstructive pattern of respiration has been reported [20].
The incidence of ventricular tachyarrhythmia, including ventricular fibrillation and ventricular tachycardia, is reduced after DEXM infusion during surgery. Conversely, DEXM shows no effects on atrial fibrillation [25], but overdose may cause I or II-degree atrioventricular blockage [20].
Newborn piglets experienced hypoxia and therapeutic hypothermia at a loading dose of 1 μg/kg with a maintenance infusion of 1.0 to 0.6 μg/kg/h, due to underdevelopment of the neonate cytochrome P450 enzyme and glucuronidation system. A few of the more adverse effects of DEXM include nausea, vomiting, and xerostomia [26].
2.4. Dexmedetomidine a Selective Alpha 2-Adrenoceptor Agonist
In 1948, Ahlquist challenged the opinion that adrenergic receptors were excitatory or inhibitory by differentiating the adrenergic receptors into an alpha and beta subtype [27]. The first α2-adrenoceptor agonist was manufactured in the early 1960s as a nasal decongestant (clonidine) [28]. Paton and his co-workers in 1996 found that a subclass of alpha adrenoceptors, located presynaptically, regulate the release of neurotransmitters [29]. This led to the subdivision of alpha adrenoceptors into postsynaptic alpha-1 and presynaptic alpha-2 adrenoreceptors. Other members of the α2 agonist receptors with α2/α1 selectivity include DEXM (1620:1), xylazine (160:1), Detomidine (260:1), Medotomidine (1620:1), clonidine (220:1), and Romifidine (affinity has not been confirmed yet) [30]. Alpha 2 adrenergic receptor is a transmembrane receptor that was differentiated into three iso-receptors, α2a, α2b, and α2c by Bylund. In contrast, its fourth type is only found in humans and is known as the α2d receptor, which is shown in Table 1. α2 receptor mediates effects through the potentiation of guanine-nucleotide regulatory binding proteins (G inhibitory protein), which ultimately attenuate the cAMP level. α2-adrenoceptor is widely distributed in the body, including the kidneys, pancreas, blood vessels, platelets, and eyes. Nonetheless, DEXM exerts its effects on α2-adrenoceptor distributed areas [23]. All subtypes of α-2 adrenoceptor perform different functions that include sedation, hypnosis, analgesia, sympatholysis, and neuroprotection via α-2A subtype [31,32]. Contrarily, the α-2B subtype is responsible for the suppression of central shivering, which causes peripheral vasoconstriction and analgesic effects on different sides of the spinal cord [33]. On the other hand, the stimulant induces locomotor activity, adrenaline outflow from the adrenal medulla, and mediation of cognitive sensory processing and emotional behavior, such as mood being controlled by α-2C [32]. In addition, DEXM performs all functions by binding with α-2 adrenoceptor [34,35], which links with the heterotrimeric transmembrane Gi protein that inhibits adenylyl cyclase. Inhibition of adenylyl cyclase decreases the formation of 3′5′cyclic adenosine monophosphate (cAMP), which acts as a second messenger. Gi protein activation opens inward potassium K+ rectifying channels that result in neuronal cell membrane hyperpolarization, which ultimately decreases the excitability of neuronal cells. On the other hand, the α-2 receptor also functions on the Go protein that inhibits Ca2+ entry into the cell and inhibits phospholipase C activity. On the other hand, phospholipase C inhibition attenuates Protein Kinase C (PKC), which has many roles in oxidative stress and apoptosis. Additional randomized clinical data also reported the binding of α-2 adrenoceptor with another undetermined G protein that works on sodium hydrogen (Na+/H+) exchanger [34,35], which is summarized in Figure 1.

Table 1.
Alpha 2 receptor subtypes, anatomical location, and functions.
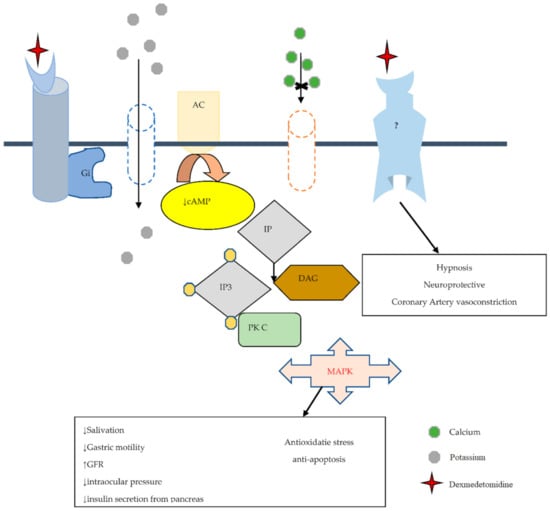
Figure 1.
The possible DEXM neuroprotective mechanism is mediated by α2 adrenoreceptor, which links with heterotrimeric transmembrane G inhibitory protein (Gi), which inactivates Adenyl cyclase (AC). Inactivation of AC attenuates the cAMP level (act as the second messenger). Gi opens inward, rectifying the K+ channel and leading to neuronal membrane hyperpolarization. Normally phosphoinositide (PI)hydrolysis to produce hydrolysis produces inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG) and activates PKC, and has a role in oxidative stress and apoptosis. Agonist agent of α2 receptor (DEXM) via G0 blocks Ca2+ translocation and inhibits phospholipase C activity, reducing PKC activity. “↑” increase and “↓” decrease.
2.5. Dexmedetomidine and Imidazoline Receptor
In 1987, Ernsberger et al. were the first to discover imidazoline binding sites in the ventrolateral medulla [41], belonging to a non-adrenergic family identified by compounds with an imidazoline moiety [42]. There are three imidazoline receptor subtypes found (I1, I2, and I3), among them, I2 is found in the medulla and brain, mainly the frontal cortex [36]. The neuroprotective effect of DEXM is mainly modulated by I2 noradrenergic receptors, not by I1 or I3 noradrenergic receptors [42]. I2 receptors associate with several distinct proteins but the identities of these proteins remain elusive. Its heterogeneous structure includes I2A and I2B subtypes, a division based on binding ability to the amiloride drug. Some data reported the evidence of DEXM binding with I2, causing Ca2+ influx into chromaffin cells and protecting neurons from damage. Nonetheless, DEXM’s neuroprotective effect via I2 requires further investigation, as few of its molecular mechanisms do not provide implicit information [43].
Manami Ingaki et al. [44] reported that Thapsigargin (non-competitive inhibitor of the Sarco/endoplasmic reticulum Ca2+ ATPase) can induce apoptosis and increase the influx of Ca2+. DEXM suppresses the Ca2+ level via the I2 receptor present on the outer membrane of mitochondria. The long-term potential (LTP) (a process of signal transmission in excitatory neurons of the hippocampus) is believed to be involved in the construction of neuronal circuits during learning and memory. DEXM can reduce LTP [45], and Yohimbine (α2 adrenoreceptor antagonist) is unable to fully abolish the DEXM effect on LTP, but combining with BU 224 hydrochloride (an imidazoline type 2 receptor antagonist) can reverse its effect. This information proves that DEXM also can bind with the imidazoline type 2 (I2) receptor [43,45].
3. Evidence of Dexmedetomidine as a Neuroprotective Agent
The role of DEXM as a neuroprotectant agent has been confined to animal experimental models. Even in laboratory animal models, the underlined neuroprotective mechanisms are not explicitly understood. Various DEXM neuroprotective properties are briefly discussed below.
3.1. Role of Dexmedetomidine in Overview Pathogenesis of Neuronal Damage and Death
Neural damage pathogenesis is a complex process, as it involves multiple molecular pathways. Numerous neuroprotective agents are applied to different types of neuronal injury such as necrosis, apoptosis, oxidative stress, and so on. DEXM exhibits a neuroprotective ability by modulating inflammatory markers and molecular pathways, which are summarized below [43].
3.2. The Influence of Dexmedetomidine against Necrosis
Necrosis is a type of irreversible cell death that usually occurs in acute phase injury. The pathophysiology of irreversible cell death consists of many components including the pre-necrotic event (pyroptosis) and the release of inflammatory markers, excitotoxic neurotransmitters, reactive oxygen species, and so on. All these components play a vital role in the pathogenies of cell death [46]. The signal transmission begins with a wave of depolarization and then repolarization that causes action potential (AP). The AP process exchanges cations of sodium (Na+) with potassium (K+), and during this process, the neurotransmitter acts as a chemical messenger. It is released from pre-synapses and binds to the corresponding receptor on a postsynaptic membrane, which causes depolarization or hyperpolarization [47,48]. Neurotransmitters are released in response to many processes, such as signal transmission, the release of hormones and peptides, and cell injury. Previous data have demonstrated that excitatory neurotransmitters such as glutamate and aspartate are released from the nerve terminal in response to nerve damage. These excitatory neurotransmitters also precipitate further nerve damage [49,50]. Additionally, this causes dysfunction of Na+/K+ ATPase, which leads to membrane hyperpolarization. Imbalance in levels of neurotransmitters such as catecholamine (CAT) increases nerve sensitivity to glutamate. The glutamate release by chelation of extracellular Ca2+ ions is associated with vesicular transportation. Glutamate binds to the NMDA (N-methyl-D-aspartate) receptor, which is a subtype of the glutamatergic receptor [49]. Furthermore, it activates the nitric oxide signaling pathway and enhances the intracellular Ca2+ ions that subsequently activate intracellular catabolic and proteolytic enzymes, for instance, protease and lipase [51,52]. These enzymes generate oxygen-free radicals that damage the membrane and results in cell death. Glutamate secretion is also regulated by mitogen-activated/extracellular signal-regulated kinase (MEK) [53].
Experimental models corroborate DEXM’s ability to modulate glutamate, and its inhibitory effect releases glutamate by reducing the release of CAT. DEXM inhibits glutamate release by binding with the α2 receptors that are expressed on neurons. This reduces the CAT release along with the CAT-associated nerve sensitivity for glutamate [53,54]. Additionally, DEXM directly blocks the MEK pathway and voltage-dependent calcium channel. It improves the expression of excitatory amino acid transporter 1 by increasing the N-methyl-D-aspartate receptor (NMDA) that decreases glutamate [55,56]. Excitatory Amino Acid Transporter 3 (EAAT3) is a member of the glutamate transporter family that removes glutamate from the synaptic cleft and extrasynaptic sites via glutamate re-uptake into glial cells and neurons. DEXM enhances EAAT3 expression [54,55]. The aforementioned discussion concludes that DEXM could prevent neuronal damage by inhibiting neurotransmitter release, as depicted in Figure 2.
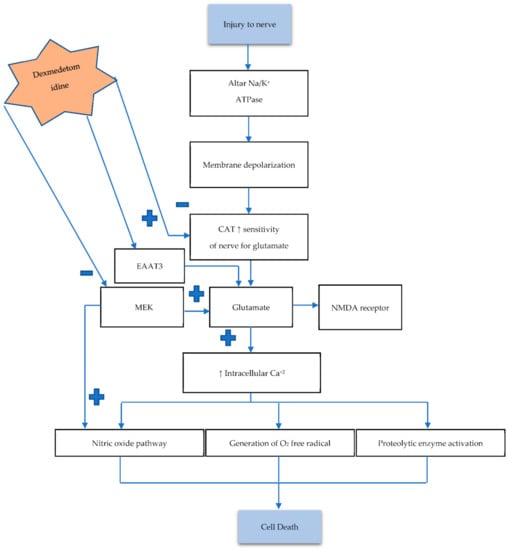
Figure 2.
Nerve injury causes dysfunction of NA/K ATPase, leading to an imbalance between neurotransmitters. Increasing CAT level enhances the sensitivity of nerves to glutamate (Glu). Excessive Glu increases intracellular Ca2+, which in return activates the aforementioned pathways, resulting in cell death. Activation of the mitogen-activated/extracellular signal-regulated kinase (MAK) pathway also increases Glu concentration. The α2 adrenoreceptor exerts its possible neuroprotective mechanism by hyperpolarization, which blocks the Ca2+ entry into presynase. Low Ca2+ diminishes neurotransmitter release (CAT) and Glu and also decreases nerve sensitivity for Glu. DEXM also enhances Excitatory amino acid transporter 3 (EAAT3) activity, which removes Glu from the synaptic cleft. Hyperpolarization also reduces NMDA receptors, which also decreases firing and reduces intracellular Ca2+. DEXM also blocks MEK, resulting in decreased excitotoxic neuronal injury.
Pyroptosis is a proinflammatory form of cell death that regulates necrosis. The clinical features of pyroptosis include rapid cell membrane rapture, cellular swelling, DNA fragmentation, and extravagation of cellular components resulting in the death of a neighboring healthy or normal cell, and inducing necrosis. The inflammasome is a protein that works as a sensor to detect cellular damage and invading pathogens. The LPS is released in response to pyroptosis. The LPS upregulates leucine rich repeated kinase (LRRK), nucleotide-binding domain (NLRP3), and apoptosis-associated speck like protein, Gasdermin D expression. The assembly of NLRP3 leads to the release of caspase 1 depending on cytokines IL-1β, IL-18 as well as Gasdermin D which mediate pyroptosis [57,58]. Previous data reports that cells treated with DEXM were found to protect against LPS inducing pyroptosis. Histone is a nuclear protein released from a dying cell into the extracellular space. LPS challenge the cell increase concentration of histone; it has been found that DEXM markedly inhibits histone release after LPS challenge [53,58,59].
3.3. The Response of Dexmedetomidine against Apoptosis
Apoptosis (programmed cell death) usually occurs in a late phase of injury and has an integral role in the normal development of a tissue or organ. The process of apoptosis involves B cell lymphoma/leukemia-2 (Bcl-2), a family of pro and anti-apoptotic regulatory proteins. The process of cellular death is delicately balanced by these proteins, which assess the integrity of the mitochondrial membrane, the release of cytochrome c, and potentiators of other apoptotic factors. BCL Associated Death protein (BAD) is a pro-apoptotic protein that is provoked by protooncogene protein C-akt (AKT) through phosphorylation of its serine residue. After AKT activation, BAD attaches to a cytosolic protein called 14-3-3 to reduce BCL-XL (anti-apoptotic protein), which delicately inhibits programmed cell death by binding with Bax (pro-apoptotic protein) [59].
It can be inferred that BCL-2 and BCL-XL proteins perform their anti-apoptotic effects by preventing the release of cytochrome C from mitochondria. Moreover, this also prevents Bax translocation and maintains mitochondrial membrane potential. DEXM potentially exhibits an anti-apoptotic property by regulating many pro and anti-apoptotic proteins, as it increases BCL2 [59], pERK concentration, and Murine Double Minute 2 (MDM-2). An increase in MDM-2 results in a reduction of pro-apoptotic mediator p53 [60]. Subsequently, it also increases Focal Adhesion Kinase (FAK), as well as the concentration of phosphorylated extracellular signal regulator kinase 1/2 (ERK1/2) [61]. FAK regulates its anti-apoptotic property by activating Phospohoinositide 3′-OH Kinase (PI3K) and the AKT pathway [62]. FAK simultaneously activates Nuclear Factor-kB (NF-kB), Inhibitor of Apoptosis Proteins (IAP), and IAP subtypes such as cIAP-1, cIAp-2, and XIAP, which alleviates apoptosis by inhibiting the caspase 3 activation [43].
The global cerebral ischemic insult may activate adaptive pathways and alter gene expression within the area of injury. Hypoxia Inducible Factor (HIF-1α) is expressed in response to hypoxia and it exponentially escalates several other genes, such as Vascular Endothelial Growth Factor (VEGF), erythropoietin, and DNA damage response 1 (REDD1/RTP801). The role of REDD1 is controversial in injury, but the reported data give evidence that it suppresses lactate dehydrogenase LHD release from neurons [58]. Data also indicate that DEXM upregulates HIF-1α, VEGF [63], and REDD1 [62] expression to protect the cell from oxygen-glucose deprivation induced injury. Oxygen glucose deprivation (OGD) insult is commonly used in vitro for hypoxia/ischemic modeling [64]. OGD exposure to cells causes deterioration of mitochondrial function and cell viability loss, and this death is usually by apoptosis (55%) and not by necrosis (only 7%) [63]. Furthermore, 1 µM of DEXM through the I2 imidazole receptor reduces the OGD induced cell death apoptosis to 22% and necrosis to 2% [62]. Moreover, a cell pretreated with BU 224 or idazoxan (I2 imidazoline antagonist) inhibits the DEXM protection by 82.3% and 92.4%, respectively. DEXM binds the I2 imidazole receptor, which activates the PI3K/AKT pathway and increases the HIF1α, VEGF, and REDD1 gene expression [62]. The imbalance between two pathways (phosphoinositide 3′-OH kinase (PI3K) and AKT protein kinase) plays a significant role in apoptosis [62], as these signaling pathways (PI3K/AKT) act with anti-apoptosis to enhance cell survival and proliferation [65]. DEXM phosphorylates AKT and increases expression of PI3K [65,66]. Furthermore, DEXM counteracts the OGD influenced inhibition of the p38 Mitogen Activated Protein Kinase (MAPK)/ERK1/2 pathway by elevating the phosphorylated level of p38 MAPK and ERK1/2 [67]. Caspase-3 expression was further investigated to observe the DEXM anti-apoptosis effect that protects against OGD-induced injury. Caspase-3 activation was increased by 8.7-fold due to OGD insult, while DEXM mitigated OGD induce caspase-3 expression to 1.8-fold [62]. The role of DEXM in apoptotic events is demonstrated in Figure 3.
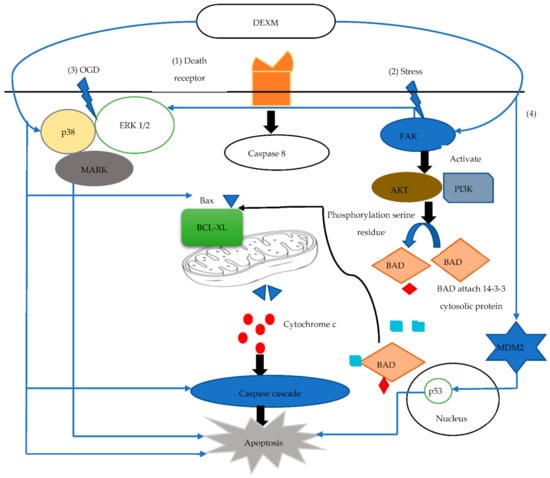
Figure 3.
(1) Activation of death receptor release Caspase 8 that helps Ba bind with BCL-XL and releases cytochrome C (Cyt C) from mitochondria. Cyt C activates caspase cascade induced apoptosis. (2) Stress activates FAK, which activates AKT/PI3 K and ERK ½. AKT helps BAD serine residue phosphorylation. Phosphorylated BAD attaches cytosolic 14-3-3 protein that unbinds Ba with BCL-XL, attenuating apoptosis. (3) OGD induces necrosis by decreasing the p38 Mitogen-activated protein kinase (MAPK)/ERK1/2 pathways that attenuate apoptosis. (4) MDM2 reduces p53 activity, which induces apoptosis. DEXM enhances FAK activity and also phosphorylates AKT and increases expression of PI3K, increasing detachment of Ba-BCL-XL. DEXM counteracts OGD-influenced inhibition by elevating the phosphorylated level of p38 MAPK and ERK1/2. DEXM modulates p53 activity via Mdm2.
3.4. The Response of Dexmedetomidine against Immunologic Response
The significance of immunological or inflammatory response is not entirely clear because, on the one hand, it exacerbates oxidative stress, and on the other hand its phagocytes kill or damage nerve cells and help maintain neurogenesis. Inflammation or neuroinflammation is a complex process that involves many molecules, including pro-inflammatory cytokines (interleukins, TNF), chemokine, and inflammatory cells (monocytes, macrophages, Natural Killer Cell (NKC), astrocytes, and lymphocytes). Interleukin 1β or simple IL-1β has many names, such as a leukocytic endogenous mediator, mononuclear cell factor, leukocytic pyrogen, lymphocyte activating factor, and so on. It is a pro-inflammatory cytokine that is secreted by phagocytes (macrophage and monocytes). During acute insult, IL-1β is first released by microglia and then by astrocytes. Moreover, IL-1β has the ability to induce the release of other cytokines such as Tumor Necrotic Factor- α (TNF-α) and IL-6, which are released from astrocytes and microglial cells. IL-6 acts as a pro and anti-inflammatory cytokine, which affects metabolism, organ development, and hematopoiesis. In addition, IL-6 plays an essential role in infection, inflammation, neurodegenerative disease, and other ischemic injuries.
IL-1β induces mRNA expression of IL-6 in glioma cells through many pathways like phosphorylation of p38 Mitogen-Activated Protein Kinases (MAPK), Stress Activated Protein Kinase (SAPK)/c-Jun-N Terminal Kinase (JNK), and nuclear factor kappa-B kinase IκB/NF-κB [68,69]. IL-1β activities and synthesis IL-6 by binding within SMase/Src kinase/NMDA receptor through Calmodulin-Dependent Protein Kinase II and cAMP Response Element-Binding Protein (CamKII/CREB) activation pathway. DEXM exerts neuroprotective effects directly and indirectly by astrocytes as well as reduces the number of activated macrophages that involves in inflammation. Cell pretreated with DEXM decreases GFAP level (marker for astrocytes) [70] and CD116B level (marker for macrophage and monocytes) [70,71]. Previous work reported that DEXM alleviates IL-1 β-inducing-IL-6 release but it exerts very little effect on IL-6 suppression [72,73].
It has been experimentally proven in [72] that DEXM decreases IL-6 through suppression of IL-β but independently of the adenylyl cyclase-cAMP pathway, as 8-Bromo cAMP (analog of cAMP) increases the IL-6 level and Forskolin (direct activator of adenylyl cyclase) significantly magnifies the IL-1β-induced-release of IL-6. However, DEXM is unable to affect the forskolin-induced cAMP accumulation [72]. Yohimbine (α2-adrenoceptor antagonist) did not invert the suppressive effects of DEXM on the IL-1β-induced IL-6 release. It seems that DEXM, by employing α2-adrenoceptors, has a marginal chance of alleviating the IL-1β-evoked IL-6 release [72]. The above discussion signifies that more work needs to be done to accurately identify the molecular mechanisms.
DEXM not only reduces IL-β and IL-6 but also other pro-inflammatory cytokines such as TNF-α and NF-κB [74,75]. Meanwhile, the α2 receptor on the medulla oblongata is stimulated by DEXM, which alleviates the Sympathetic Nervous System (SNS) and dominant Parasympathetic Nervous System (PNS). Moreover, DEXM significantly decreases systemic cytokine levels in association with an enhancement in the discharge frequency of the cervical vagus nerve in response to endotoxin [70,76]. The inflammatory mediators are not only released by damaged or injured cells but also from supportive cells of the nervous system. Glial cells are the supportive cells of the nervous system that include oligodendrocytes, astrocytes, ependymal cells, and microglia. Glial cells not only help in neuronal repair but also support regeneration and maintain migration hemostasis and BBB. Astrocytes perform a complex function in both healthy and diseased cells of the nervous system.
On this basis, they are divided into good and bad astrocytes. Data studies reported that DEXM influences inhibitory effects by reducing astrocyte overactivation in response to nerve injury, or ischemic or reperfusion damage. Longitudinal studies also suggest that DEXM inhibits attenuate inflammatory mediator TNF-α and glial fibrillary acidic protein, which suggests DEXM has a neuroprotective effect [43]. DEXM and BDNF (Brain-Derived Neurotrophic Factor) provide a significant neuroprotective effect. DEXM enhances Bdnf4 and Bdnf5 transcription and also increases the BDNF in astrocytes through the extracellular signal-regulated kinase-dependent pathway, subsequently providing neuroprotection. As BDNF is a neurotrophin found in the healthy and diseased brain and body, evidence suggests that BNDF is associated with neurotransmitter regulation, neuronal plasticity, maintenance, and survival [77].
To summarize, DEXM treatment has been observed to eliminate or alleviate neuroinflammatory mediators (IL-6, TNF-α, NF-κB) and also increase Bdnf4 and Bdnf5 transcription and BDNF in astrocytes, which ultimately produces its neuroprotective effect, as summarized in Table 2.

Table 2.
Summary of DEXM-associated neuroprotective effects.
3.5. Clinical Evidence of Dexmedetomidine as Neuroprotective Agent
Some randomized clinical trials that suggested DEXM as a neuroprotective agent are shown in Table 3. Some randomized clinical trials that suggested DEXM is a neuroprotective agent are also displayed in Table 2. Xiahong Luo et al. conducted a randomized clinical trial to explore the neuroprotective efficacy of DEXM. Sixty glioma patients underwent craniotomy resection, among them thirty patients received IV 1 μg/kg 10 min of DEXM with a maintenance dose of 0.4 μg/(kg/h). It was found that the DEXM could significantly reduce serum expression of an inflammatory mediator (TNF α, IL-6), inhibit free radical generation (superoxide dismutase), and stabilize hemodynamic parameters (Mean arterial pressure and heart rate) [19]. Xiahong Luo et al. [19] and Ashish Bindra et al. [78] ruled out the brain injury biomarkers: Neuron-Specific enolase of Enzyme (NSE) (enzyme release during neuronal injury) and S100b (express by type 2 astrocytes). These biomarkers cause cerebral insult in chronic temporal lobe epilepsy. Intraoperative DEXM treatment attenuates S100b and NSE, which suggests its neuroprotective property during epilepsy surgery [78].

Table 3.
Randomized clinical trials of Dexmedetomidine as a neuroprotective agent.
Brain-Derived Neurotrophic Factor (BDNF) is a member of the neurotrophin family, which has an important role in axonal growth, synaptic plasticity, and neuronal survival. An elevated level of BDNF in the CNS can protect neurons from ischemic injury [79,80]. A randomized clinical trial suggested that IV DEXM infusion during surgery improves cognition after carotid endarterectomy by increasing BDNF concentration [71]. It helped by attenuating the duration of postoperative delirium [79]. The above studies provide a vital theoretical aspect for the application of DEXM in neuroprotection.
4. Conclusions
This work demonstrates the role of DEXM as a neuroprotective agent, which has been tested in various experimental models. It has been corroborated from the literature survey that DEXM abolishes its neuroprotective effects through the upregulation of α2 adrenoreceptor. DEXM imidazoline moiety binds with the I2 receptor, which is present on the outer surface of mitochondria that reduces Ca2+ modulated apoptosis. Moreover, preclinical data inferred that DEXM considerably decreases neuroinflammation and neurodegeneration following neurological insult. After a rigorous literature survey on the DEXM drug, the following decisive concluding remarks can be made:
- (i)
- Clinical investigations of DEXM should be designed to verify the efficacy of different steps of the cascade of neuronal damage.
- (ii)
- DEXM improves neuroinflammatory behavior by suppressing inflammatory mediators. It not only controls apoptotic signaling pathways but also decreases oxygen-free radical generation.
- (iii)
- The randomized clinical trials on DEXM suggest its ability to enhance BDNF to protect the brain from ischemic insult.
- (iv)
- DEXM not only improves cognition but also attenuates the duration of postoperative delirium.
The aforementioned conclusions regarding the neuroprotective agent (DEXM) can serve as a benchmark for researchers and doctors to substantiate DEXM’s efficacy in exerting anti-inflammatory properties that ameliorate and prevent nerve injuries. However, many areas are yet to be explored, for instance, certain extended applications of DEXM require further evaluation to ensure the safe use of DEXM before it is designed in a clinical trial. It is imperative to carefully select patients and to determine the appropriate dosage, as DEXM usage is still limited in the pediatric population.
Author Contributions
Conceptualization, funding acquisition, resources, and supervision, L.Z. and R.F., Z.L., P.L.M.Z., and X.X. wrote the manuscript. L.Z. and R.F. reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work is made possible by the National Natural Science Foundation of China, grant number 82071496 to R.F., the Young Teacher Foundation of Sun Yat-sen University grant number 59000-18841219 to R.F., and Natural Science Foundation of Guangdong Province grant number 2021A1515010463 to R.F.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Park, H.R.; Lee, G.S.; Kim, I.S.; Chang, J.-C. Brachial plexus injury in adults. Nerve 2017, 3, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Yannascoli, S.M.; Stwalley, D.; Saeed, M.J.; Olsen, M.A.; Dy, C.J. A population-based assessment of depression and anxiety in patients with brachial plexus injuries. J. Hand Surg. 2018, 43, 1136.e1–1136.e9. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; Brimble, M. A review of neuroprotective agents. Curr. Med. Chem. 2004, 11, 2383–2397. [Google Scholar] [CrossRef] [PubMed]
- Chrysostomou, C.; Schmitt, C.G. Dexmedetomidine: Sedation, analgesia and beyond. Expert Opin. Drug Metab. Toxicol. 2008, 4, 619–627. [Google Scholar] [CrossRef]
- Grewal, A. Dexmedetomidine: New avenues. J. Anaesthesiol. Clin. Pharmacol. 2011, 27, 297–302. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, Y.-Q. Advances in the Clinical Use of Dexmedetomidine. Med Recapitul. 2011, 3, 434–437. (In Chinese) [Google Scholar]
- Marwaha, A. Anesthesia Considerations for Tracheal Reconstruction. In Clinical Thoracic Anesthesia; Springer: Singapore, 2020; pp. 181–201. [Google Scholar]
- Keating, G.M. Dexmedetomidine: A review of its use for sedation in the intensive care setting. Drugs 2015, 75, 1119–1130. [Google Scholar] [CrossRef]
- Weerink, M.A.; Struys, M.M.; Hannivoort, L.N.; Barends, C.R.; Absalom, A.R.; Colin, P. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin. Pharmacokinet. 2017, 56, 893–913. [Google Scholar] [CrossRef] [Green Version]
- Elgebaly, A.S.; Sabry, M. Sedation effects by dexmedetomidine versus propofol in decreasing duration of mechanical ventilation after open heart surgery. Ann. Card. Anaesth. 2018, 21, 235–242. [Google Scholar] [CrossRef]
- Deiner, S.; Luo, X.; Lin, H.-M.; Sessler, D.I.; Saager, L.; Sieber, F.E.; Lee, H.B.; Sano, M.; Jankowski, C.; Bergese, S.D. Intraoperative infusion of dexmedetomidine for prevention of postoperative delirium and cognitive dysfunction in elderly patients undergoing major elective noncardiac surgery: A randomized clinical trial. JAMA Surg. 2017, 152, e171505. [Google Scholar] [CrossRef]
- Zhao, W.; Hu, Y.; Chen, H.; Wang, X.; Wang, L.; Wang, Y.; Wu, X.; Han, F. The Effect and Optimal Dosage of Dexmedetomidine Plus Sufentanil for Postoperative Analgesia in Elderly Patients with Postoperative Delirium and Early Postoperative Cognitive Dysfunction: A Single-Center, Prospective, Randomized, Double-Blind, Controlled Trial. Front. Neurosci. 2020, 14, 549516. [Google Scholar]
- Lee, S. Dexmedetomidine: Present and future directions. Korean J. Anesthesiol. 2019, 72, 323–330. [Google Scholar] [CrossRef]
- Yoshikawa, Y.; Hirata, N.; Kawaguchi, R.; Tokinaga, Y.; Yamakage, M. Dexmedetomidine maintains its direct cardioprotective effect against ischemia/reperfusion injury in hypertensive hypertrophied myocardium. Anesth. Analg. 2018, 126, 443–452. [Google Scholar] [CrossRef]
- Ma, J.; Chen, Q.; Li, J.; Zhao, H.; Mi, E.; Chen, Y.; Yi, B.; Ning, J.; Ma, D.; Lu, K. Dexmedetomidine-mediated prevention of renal ischemia-reperfusion injury depends in part on cholinergic anti-inflammatory mechanisms. Anesth. Analg. 2020, 130, 1054–1062. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, Y.; Zhang, Z.; Ding, X.; Gong, C.; Qian, Y. Activation of PI3K/Akt/HIF-1α signaling is involved in lung protection of dexmedetomidine in patients undergoing video-assisted thoracoscopic surgery: A pilot study. Drug Des. Dev. Ther. 2020, 14, 5155–5166. [Google Scholar] [CrossRef]
- Lv, H.; Li, Y.; Cheng, Q.; Chen, J.; Chen, W. Neuroprotective Effects Against Cerebral Ischemic Injury Exerted by Dexmedetomidine via the HDAC5/NPAS4/MDM2/PSD-95 Axis. Mol. Neurobiol. 2021, 58, 1990–2004. [Google Scholar] [CrossRef]
- Anttila, M.; Penttilä, J.; Helminen, A.; Vuorilehto, L.; Scheinin, H. Bioavailability of dexmedetomidine after extravascular doses in healthy subjects. Br. J. Clin. Pharmacol. 2003, 56, 691–693. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Zheng, X.; Huang, H. Protective effects of dexmedetomidine on brain function of glioma patients undergoing craniotomy resection and its underlying mechanism. Clin. Neurol. Neurosurg. 2016, 146, 105–108. [Google Scholar] [CrossRef]
- Gertler, R.; Brown, H.C.; Mitchell, D.H.; Silvius, E.N. Dexmedetomidine: A novel sedative-analgesic agent. Bayl. Univ. Med Cent. Proc. 2001, 14, 13–21. [Google Scholar] [CrossRef]
- Colin, P.; Hannivoort, L.N.; Eleveld, D.J.; Reyntjens, K.M.; Absalom, A.R.; Vereecke, H.E.; Struys, M.M. Dexmedetomidine pharmacodynamics in healthy volunteers: 2. Haemodynamic profile. BJA Br. J. Anaesth. 2017, 119, 211–220. [Google Scholar] [CrossRef] [Green Version]
- Mantz, J. Dexmedetomidine. Drugs Today 1999, 35, 151–157. [Google Scholar] [CrossRef]
- Yu, S.-B. Dexmedetomidine sedation in ICU. Korean J. Anesthesiol. 2012, 62, 405–411. [Google Scholar] [CrossRef] [Green Version]
- Naik, B.I.; Nemergut, E.C.; Kazemi, A.; Fernández, L.; Cederholm, S.K.; McMurry, T.L.; Durieux, M.E. The effect of dexmedetomidine on postoperative opioid consumption and pain after major spine surgery. Anesth. Analg. 2016, 122, 1646–1653. [Google Scholar] [CrossRef]
- Ling, X.; Zhou, H.; Ni, Y.; Wu, C.; Zhang, C.; Zhu, Z. Does dexmedetomidine have an antiarrhythmic effect on cardiac patients? A meta-analysis of randomized controlled trials. PLoS ONE 2018, 13, e0193303. [Google Scholar] [CrossRef] [PubMed]
- Botero Posada, L.F.; Arias Jiménez, J.M.; Vasseur Arboleda, J.P. The use of dexmedetomidine (DXM) for implanting a cardiac resynchronization device: Is it really safe? Colomb. J. Anestesiol. 2011, 39, 425–431. [Google Scholar] [CrossRef] [Green Version]
- Seyrek, M.; Halici, Z.; Yildiz, O.; Ulusoy, H.B. Interaction between dexmedetomidine and α-adrenergic receptors: Emphasis on vascular actions. J. Cardiothorac. Vasc. Anesth. 2011, 25, 856–862. [Google Scholar] [CrossRef]
- Afonso, J.; Reis, F. Dexmedetomidine: Current role in anesthesia and intensive care. Braz. J. Anesthesiol. 2012, 62, 118–133. [Google Scholar] [CrossRef] [Green Version]
- Murrell, J.C.; Hellebrekers, L.J. Medetomidine and dexmedetomidine: A review of cardiovascular effects and antinociceptive properties in the dog. Vet. Anaesth. Analg. 2005, 32, 117–127. [Google Scholar] [CrossRef]
- Arcangeli, A.; D’alo, C.; Gaspari, R. Dexmedetomidine use in general anaesthesia. Curr. Drug Targets 2009, 10, 687–695. [Google Scholar] [CrossRef]
- Bylund, D.B. Subtypes of α1-and α2-adrenergic receptors. FASEB J. 1992, 6, 832–839. [Google Scholar] [CrossRef]
- Ciccarelli, M.; Sorriento, D.; Coscioni, E.; Iaccarino, G.; Santulli, G. Adrenergic receptors. Endocrinol. Heart Health Dis. 2017, 285–315. [Google Scholar]
- Kim, H.; Choi, S.-H.; Ha, S.-H.; Chang, W.-S.; Heo, G.-A.; Jeong, J.; Min, K.T. Haemodynamic changes and incisional bleeding after scalp infiltration of dexmedetomidine with lidocaine in neurosurgical patients. Anaesth. Crit. Care Pain Med. 2019, 38, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Liu, Z.; Kaindl, J.; Maeda, S.; Zhao, J.; Sun, X.; Xu, J.; Gmeiner, P.; Wang, H.-W.; Kobilka, B.K. Activation of the α 2B adrenoceptor by the sedative sympatholytic dexmedetomidine. Nat. Chem. Biol. 2020, 16, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Hossain, M.; Rajakumaraswamy, N.; Arshad, M.; Sanders, R.D.; Franks, N.P.; Maze, M. Dexmedetomidine produces its neuroprotective effect via the α2A-adrenoceptor subtype. Eur. J. Pharmacol. 2004, 502, 87–97. [Google Scholar] [CrossRef]
- Zhang, Y.; Kimelberg, H.K. Neuroprotection by alpha 2-adrenergic agonists in cerebral ischemia. Curr. Neuropharmacol. 2005, 3, 317–323. [Google Scholar] [CrossRef] [Green Version]
- De Vos, H.; Vauquelin, G.; De Keyser, J.; De Backer, J.P.; Van Liefde, I. Regional Distribution of α2A-and α2B-Adrenoceptor Subtypes in Postmortem Human Brain. J. Neurochem. 1992, 58, 1555–1560. [Google Scholar] [CrossRef]
- Scheinin, M.; Lomasney, J.W.; Hayden-Hixson, D.M.; Schambra, U.B.; Caron, M.G.; Lefkowitz, R.J.; Fremeau Jr, R.T. Distribution of α2-adrenergic receptor subtype gene expression in rat brain. Mol. Brain Res. 1994, 21, 133–149. [Google Scholar] [CrossRef]
- Sinclair, M.D. A review of the physiological effects of α2-agonists related to the clinical use of medetomidine in small animal practice. Can. Vet. J. 2003, 44, 885–897. [Google Scholar]
- Ruffolo Jr, R.R.; Stadel, J.M.; Hieble, J.P. α-adrenoceptors: Recent developments. Med. Res. Rev. 1994, 14, 229–270. [Google Scholar] [CrossRef]
- Khan, Z.; Ferguson, C.; Jones, R. Alpha-2 and imidazoline receptor agonistsTheir pharmacology and therapeutic role. Anaesthesia 1999, 54, 146–165. [Google Scholar] [CrossRef]
- Bousquet, P.; Hudson, A.; García-Sevilla, J.A.; Li, J.-X. Imidazoline receptor system: The past, the present, and the future. Pharmacol. Rev. 2020, 72, 50–79. [Google Scholar] [CrossRef]
- Janke, E.L.; Samra, S. Dexmedetomidine and neuroprotection. Semin. Anesth. Perioper. Med. Pain 2006, 25, 71–76. [Google Scholar] [CrossRef]
- Inagaki, M.; Somei, M.; Oguchi, T.; Ono, R.; Fukutaka, S.; Matsuoka, I.; Tsuji, M.; Oguchi, K. Neuroprotective Effects of Dexmedetomidine against-induced ER-stress via Activity of α2-adrenoceptors and Imidazoline Receptors. AIMS Neurosci. 2016, 3, 237–252. [Google Scholar] [CrossRef]
- Takamatsu, I.; Iwase, A.; Ozaki, M.; Kazama, T.; Wada, K.; Sekiguchi, M. Dexmedetomidine reduces long-term potentiation in mouse hippocampus. J. Am. Soc. Anesthesiol. 2008, 108, 94–102. [Google Scholar] [CrossRef] [Green Version]
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef]
- Süudhof, T.C. Neurotransmitter release. In Pharmacology of Neurotransmitter Release; Springer: Berlin, Germany, 2008; pp. 1–21. [Google Scholar]
- Südhof, T.C. Calcium control of neurotransmitter release. Cold Spring Harb. Perspect. Biol. 2012, 4, a011353. [Google Scholar] [CrossRef]
- Degos, V.; Charpentier, T.L.; Chhor, V.; Brissaud, O.; Lebon, S.; Schwendimann, L.; Bednareck, N.; Passemard, S.; Mantz, J.; Gressens, P. Neuroprotective effects of dexmedetomidine against glutamate agonist-induced neuronal cell death are related to increased astrocyte brain-derived neurotrophic factor expression. Anesthesiology 2013, 118, 1123–1132. [Google Scholar] [CrossRef]
- Zhao, Y.; He, J.; Yu, N.; Jia, C.; Wang, S. Mechanisms of dexmedetomidine in neuropathic pain. Front. Neurosci. 2020, 14, 330. [Google Scholar] [CrossRef]
- Noch, E.; Khalili, K. Molecular mechanisms of necrosis in glioblastoma: The role of glutamate excitotoxicity. Cancer Biol. Ther. 2009, 8, 1791–1797. [Google Scholar] [CrossRef]
- Ji, X.; Guo, Y.; Zhou, G.; Wang, Y.; Zhang, J.; Wang, Z.; Wang, Q. Dexmedetomidine protects against high mobility group box 1-induced cellular injury by inhibiting pyroptosis. Cell Biol. Int. 2019, 43, 651–657. [Google Scholar] [CrossRef]
- Chiu, K.-M.; Lin, T.-Y.; Lu, C.-W.; Wang, S.-J. Inhibitory effect of glutamate release from rat cerebrocortical nerve terminals by α2 adrenoceptor agonist dexmedetomidine. Eur. J. Pharmacol. 2011, 670, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Do, S.-H.; Park, S.-J.; Shin, H.-J.; Paik, H.-S.; Zuo, Z.; Yoon, H.-J.; Ryu, J.-H. Dexmedetomidine increases the activity of excitatory amino acid transporter type 3 expressed in Xenopus oocytes: The involvement of protein kinase C and phosphatidylinositol 3-kinase. Eur. J. Pharmacol. 2014, 738, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shan, Y.; Tang, Z.; Gao, L.; Liu, H. Neuroprotective effects of dexmedetomidine against isoflurane-induced neuronal injury via glutamate regulation in neonatal rats. Drug Des. Dev. Ther. 2019, 13, 153–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, M.; Ling, X.; Song, R.; Gao, X.; Liang, Z.; Fang, F.; Cang, J. Upregulation of GLT-1 via PI3K/Akt pathway contributes to neuroprotection induced by dexmedetomidine. Front. Neurol. 2019, 10, 1041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Zhao, H.; Wang, D.; Ma, D. Dexmedetomidine alleviates LPS-induced pyroptosis in astrocytes in vitro. Br. J. Anaesth. 2018, 120, e8–e9. [Google Scholar] [CrossRef]
- Sun, Y.-B.; Zhao, H.; Mu, D.-L.; Zhang, W.; Cui, J.; Wu, L.; Alam, A.; Wang, D.-X.; Ma, D. Dexmedetomidine inhibits astrocyte pyroptosis and subsequently protects the brain in in vitro and in vivo models of sepsis. Cell Death Dis. 2019, 10, 167. [Google Scholar] [CrossRef] [Green Version]
- Han, J.-H.; Kim, D.-O.; Yi, J.-W.; Park, S.-W.; Kang, W.-J.; Choi, Y.-K.; Kim, S.-H.; Ko, I.-G.; Jin, J.-J.; Kim, S.-E. Dexmedetomidine, α2-adrenoceptor agonist, does not induce apoptosis in the brachial plexus of rats. Anim. Cells Syst. 2014, 18, 407–415. [Google Scholar] [CrossRef] [Green Version]
- Thomasova, D.; Bruns, H.A.; Kretschmer, V.; Ebrahim, M.; Romoli, S.; Liapis, H.; Kotb, A.M.; Endlich, N.; Anders, H.-J. Murine double minute-2 prevents p53-overactivation-related cell death (podoptosis) of podocytes. J. Am. Soc. Nephrol. 2015, 26, 1513–1523. [Google Scholar] [CrossRef] [Green Version]
- Dahmani, S.; Rouelle, D.; Gressens, P.; Mantz, J. Effects of dexmedetomidine on hippocampal focal adhesion kinase tyrosine phosphorylation in physiologic and ischemic conditions. J. Am. Soc. Anesthesiol. 2005, 103, 969–977. [Google Scholar] [CrossRef]
- Zhang, F.; Ding, T.; Yu, L.; Zhong, Y.; Dai, H.; Yan, M. Dexmedetomidine protects against oxygen–glucose deprivation-induced injury through the I2 imidazoline receptor-PI3K/AKT pathway in rat C6 glioma cells. J. Pharm. Pharmacol. 2012, 64, 120–127. [Google Scholar] [CrossRef]
- Ding, X.-D.; Zheng, N.-N.; Cao, Y.-Y.; Zhao, G.-Y.; Zhao, P. Dexmedetomidine preconditioning attenuates global cerebral ischemic injury following asphyxial cardiac arrest. Int. J. Neurosci. 2016, 126, 249–256. [Google Scholar] [CrossRef]
- Pugliese, A.M.; Latini, S.; Corradetti, R.; Pedata, F. Brief, repeated, oxygen-glucose deprivation episodes protect neurotransmission from a longer ischemic episode in the in vitro hippocampus: Role of adenosine receptors. Br. J. Pharmacol. 2003, 140, 305–314. [Google Scholar] [CrossRef] [Green Version]
- Shen, M.; Wang, S.; Wen, X.; Han, X.-R.; Wang, Y.-J.; Zhou, X.-M.; Zhang, M.-H.; Wu, D.-M.; Lu, J.; Zheng, Y.-L. Dexmedetomidine exerts neuroprotective effect via the activation of the PI3K/Akt/mTOR signaling pathway in rats with traumatic brain injury. Biomed. Pharmacother. 2017, 95, 885–893. [Google Scholar] [CrossRef]
- Zhu, Y.-M.; Wang, C.-C.; Chen, L.; Qian, L.-B.; Ma, L.-L.; Yu, J.; Zhu, M.-H.; Wen, C.-Y.; Yu, L.-N.; Yan, M. Both PI3K/Akt and ERK1/2 pathways participate in the protection by dexmedetomidine against transient focal cerebral ischemia/reperfusion injury in rats. Brain Res. 2013, 1494, 1–8. [Google Scholar] [CrossRef]
- Wang, K.; Zhu, Y. Dexmedetomidine protects against oxygen-glucose deprivation/reoxygenation injury-induced apoptosis via the p38 MAPK/ERK signalling pathway. J. Int. Med Res. 2018, 46, 675–686. [Google Scholar] [CrossRef]
- Tanabe, K.; Kozawa, O.; Iida, H. Midazolam suppresses interleukin-1β-induced interleukin-6 release from rat glial cells. J. Neuroinflamm. 2011, 8, 68. [Google Scholar] [CrossRef] [Green Version]
- Tanabe, K.; Kozawa, O.; Iida, H. cAMP/PKA enhances interleukin-1β-induced interleukin-6 synthesis through STAT3 in glial cells. Cell. Signal. 2016, 28, 19–24. [Google Scholar] [CrossRef]
- Zhu, Y.-J.; Peng, K.; Meng, X.-W.; Ji, F.-H. Attenuation of neuroinflammation by dexmedetomidine is associated with activation of a cholinergic anti-inflammatory pathway in a rat tibial fracture model. Brain Res. 2016, 1644, 1–8. [Google Scholar] [CrossRef]
- Ge, Y.; Li, Q.; Nie, Y.; Gao, J.; Luo, K.; Fang, X.; Wang, C. Dexmedetomidine improves cognition after carotid endarterectomy by inhibiting cerebral inflammation and enhancing brain-derived neurotrophic factor expression. J. Int. Med Res. 2019, 47, 2471–2482. [Google Scholar] [CrossRef]
- Tanabe, K.; Matsushima-Nishiwaki, R.; Kozawa, O.; Iida, H. Dexmedetomidine suppresses interleukin-1β-induced interleukin-6 synthesis in rat glial cells. Int. J. Mol. Med. 2014, 34, 1032–1038. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wang, J.; Qian, W.; Zhao, J.; Sun, L.; Qian, Y.; Xiao, H. Dexmedetomidine inhibits tumor necrosis factor-alpha and interleukin 6 in lipopolysaccharide-stimulated astrocytes by suppression of c-Jun N-terminal kinases. Inflammation 2014, 37, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Kim, H.-C.; Lee, S.; Ryu, H.-G.; Park, Y.-H.; Kim, J.H.; Lim, Y.-J.; Park, H.-P. Dexmedetomidine confers neuroprotection against transient global cerebral ischemia/reperfusion injury in rats by inhibiting inflammation through inactivation of the TLR-4/NF-κB pathway. Neurosci. Lett. 2017, 649, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, H.; Zhang, L.; Wang, G.; Zhang, M.; Yu, Y. Neuroprotection of dexmedetomidine against cerebral ischemia-reperfusion injury in rats: Involved in inhibition of NF-κB and inflammation response. Biomol. Ther. 2017, 25, 383–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, H.; Hu, B.; Li, Z.; Li, J. Dexmedetomidine controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Inflammation 2014, 37, 1763–1770. [Google Scholar] [CrossRef]
- Giacobbo, B.L.; Doorduin, J.; Klein, H.C.; Dierckx, R.A.; Bromberg, E.; de Vries, E.F. Brain-derived neurotrophic factor in brain disorders: Focus on neuroinflammation. Mol. Neurobiol. 2019, 56, 3295–3312. [Google Scholar] [CrossRef] [Green Version]
- Bindra, A.; Kaushal, A.; Prabhakar, H.; Chaturvedi, A.; Chandra, P.S.; Tripathi, M.; Subbiah, V.; Sathianathan, S.; Banerjee, J.; Prakash, C. Neuroprotective role of dexmedetomidine in epilepsy surgery: A preliminary study. Neurol. India 2019, 67, 163–168. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, L.; Gao, M.; Guo, W.; Ma, Y. Dexmedetomidine reduces postoperative delirium after joint replacement in elderly patients with mild cognitive impairment. Aging Clin. Exp. Res. 2016, 28, 729–736. [Google Scholar] [CrossRef]
- Yang, L.; Xu, J.-M.; Jiang, X.; Ruan, W.; Cui, Y.; He, L. Effect of dexmedetomidine on plasma brain-derived neurotrophic factor: A double-blind, randomized and placebo-controlled study. Upsala J. Med Sci. 2013, 118, 235–239. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).