Reversed Polarity bi-tDCS over M1 during a Five Days Motor Task Training Did Not Influence Motor Learning. A Triple-Blind Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
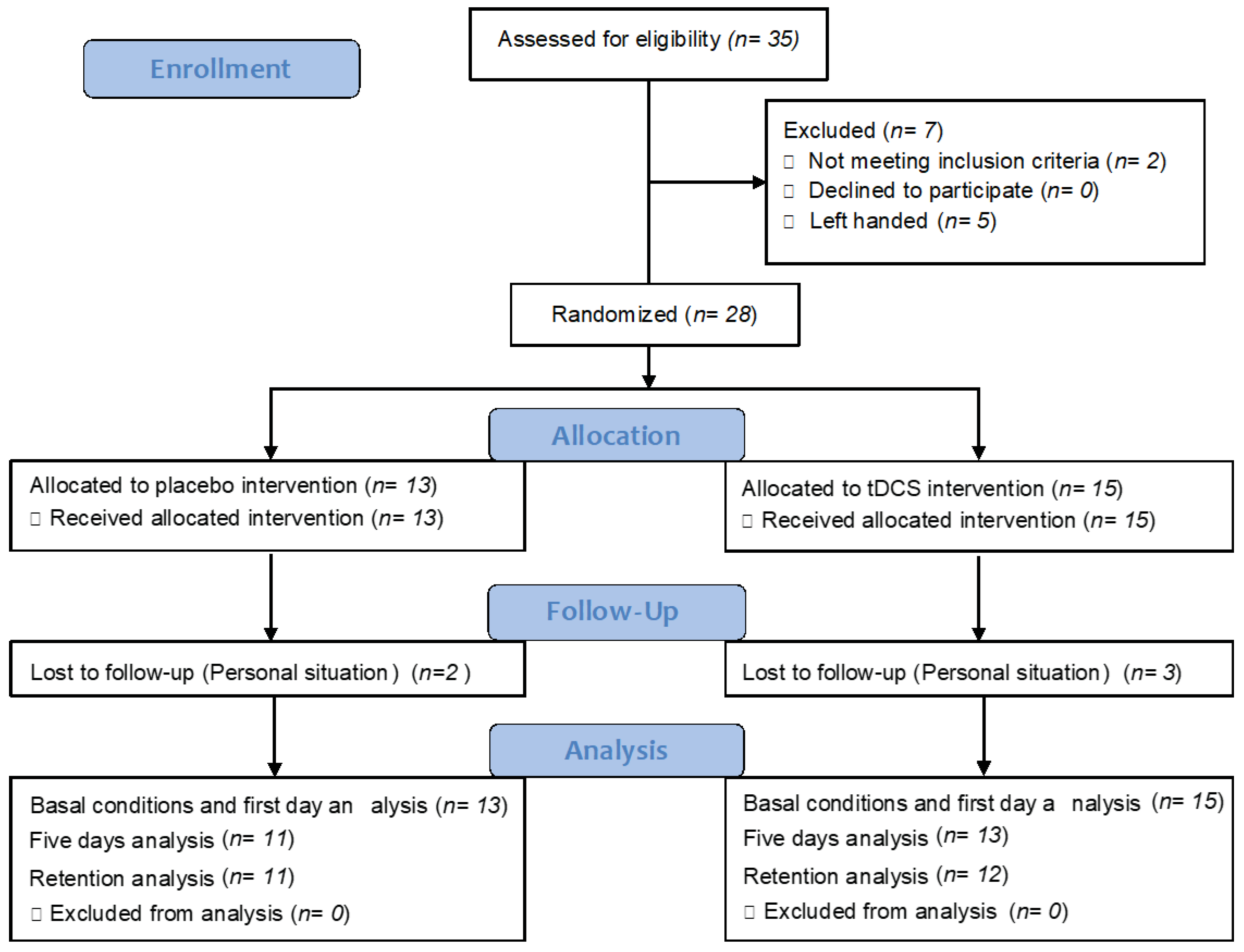
2.2. Participants
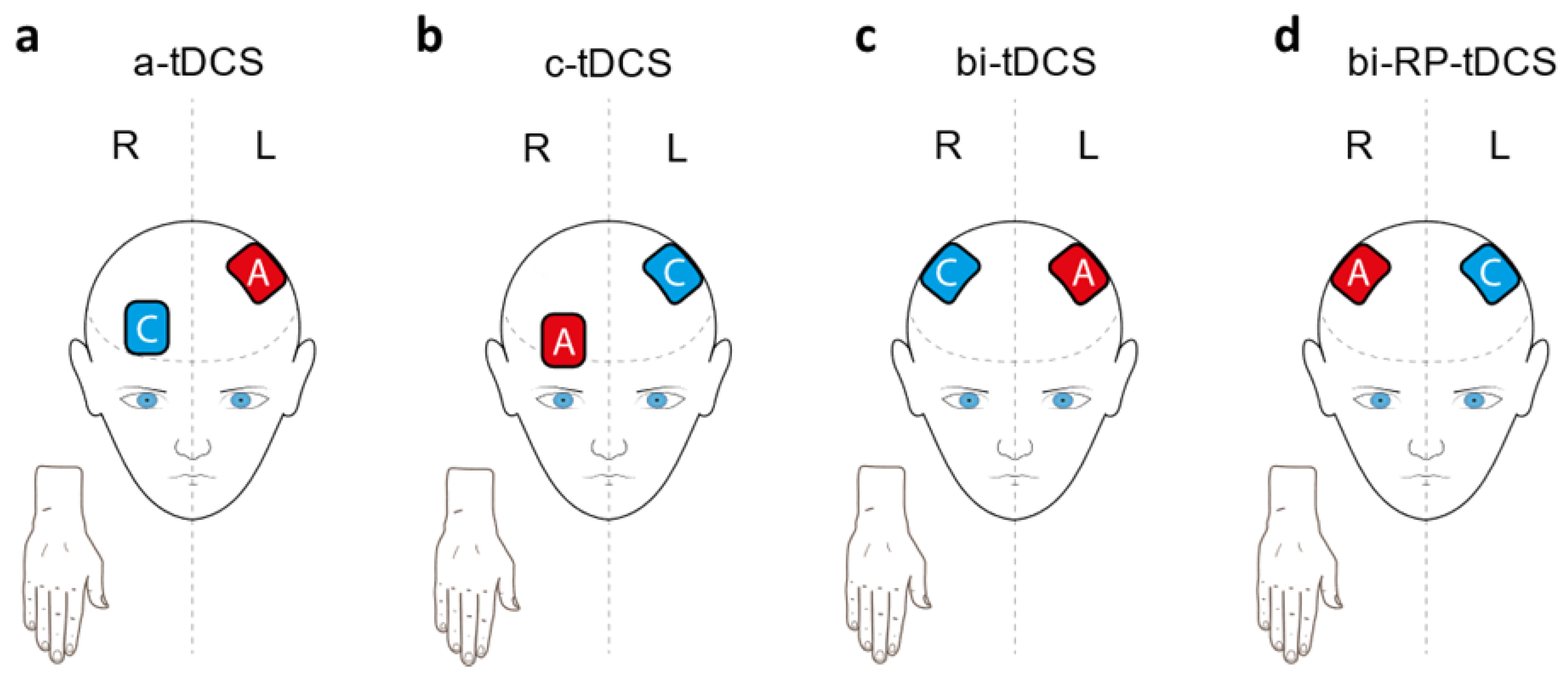
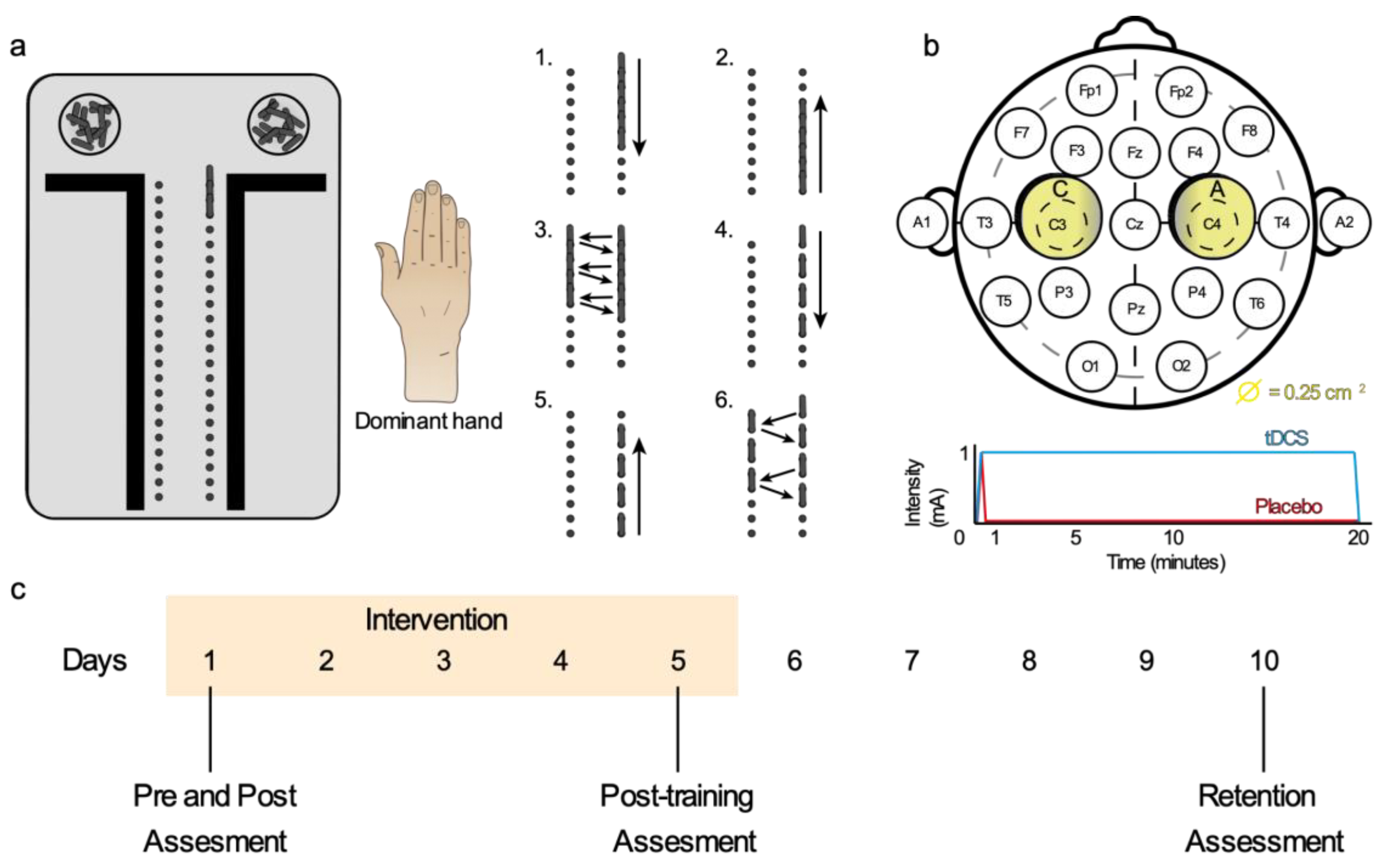
2.3. Intervention
2.3.1. Motor Dexterity Training
2.3.2. Transcranial Direct Current Stimulation
2.3.3. Placebo and Blinding Procedure
2.4. Measurement Protocol
2.4.1. Motor Dexterity Variables
2.4.2. Somatosensory Variables
2.4.3. Grip Strength
2.4.4. Physical Activity and Sleep Quality
2.5. Sample Size
2.6. Statistical Analysis
3. Results
3.1. Bi-RP-tDCS Effects on Motor Learning Training
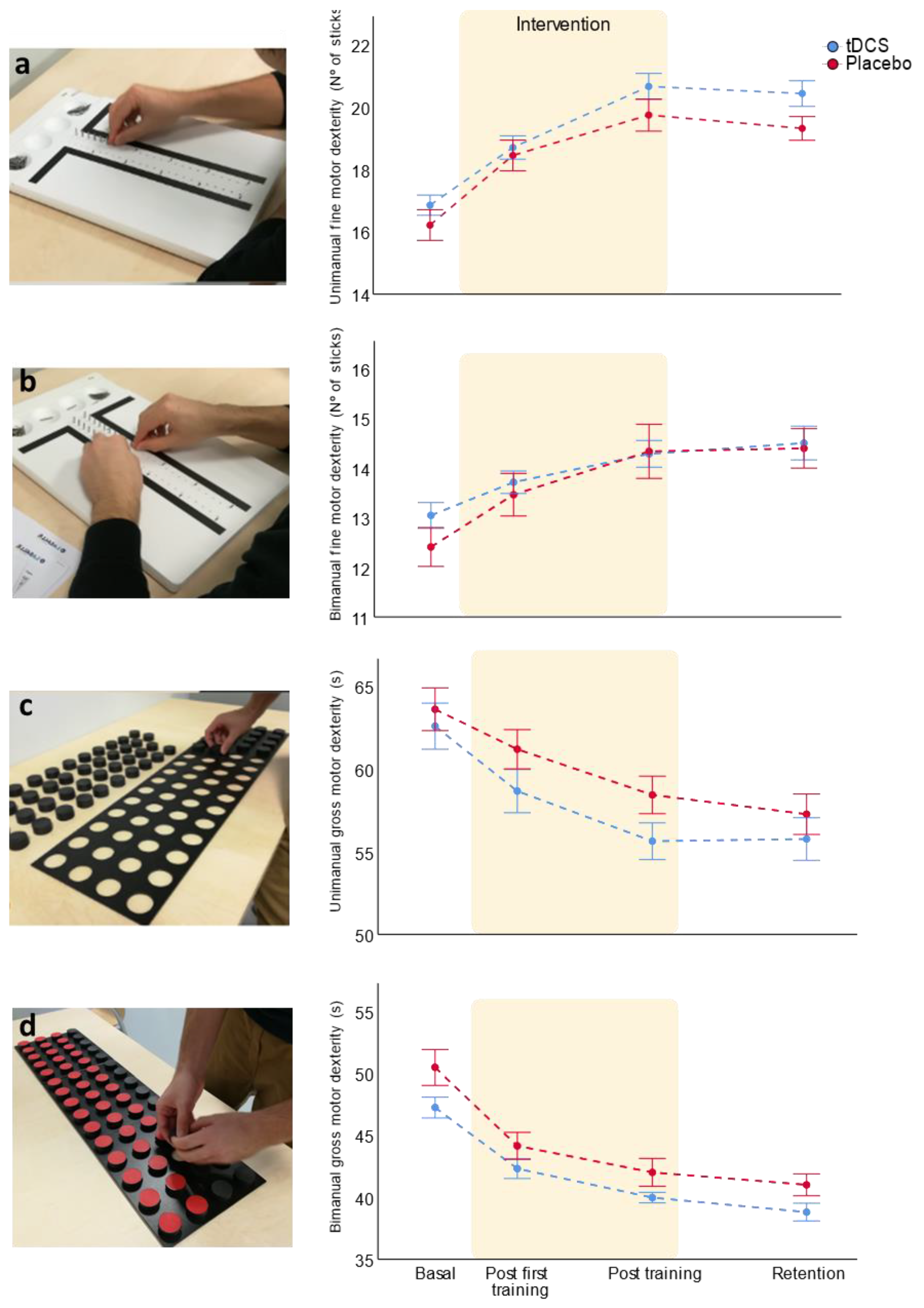
3.1.1. Unimanual Fine Motor Dexterity
3.1.2. Bimanual Fine Motor Dexterity
3.1.3. Uni- and Bimanual Gross Motor Dexterity
3.1.4. Sleep Quality Did Not Influence Motor Learning
3.2. Bi-RP-tDCS Effects on Grip Strength
3.3. Bi-RP-tDCS Effects on Somatosensory Variables
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yancosek, K.E.; Howell, D. A Narrative Review of Dexterity Assessments. J. Hand Ther. 2009, 22, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Tesio, L.; Simone, A.; Zebellin, G.; Rota, V.; Malfitano, C.; Perucca, L. Bimanual Dexterity Assessment: Validation of a Revised Form of the Turning Subtest from the Minnesota Dexterity Test. Int. J. Rehabil. Res. 2016, 39, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.; Zhang, X.; Wang, J. Hand Rehabilitation Robotics on Poststroke Motor Recovery. Behav. Neurol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; De Ferranti, S.; Després, J.P.; Fullerton, H.J.; Howard, V.J.; et al. Heart Disease and Stroke Statistics-2015 Update: A Report from the American Heart Association. Circulation 2015, 131, e29–e39. [Google Scholar] [CrossRef]
- Luengo-Fernandez, R.; Violato, M.; Candio, P.; Leal, J. Economic Burden of Stroke across Europe: A Population-Based Cost Analysis. Eur. Stroke J. 2020, 5, 17–25. [Google Scholar] [CrossRef]
- Duncan, P.W.; Zorowitz, R.; Bates, B.; Choi, J.Y.; Glasberg, J.J.; Graham, G.D.; Katz, R.C.; Lamberty, K.; Reker, D. Management of Adult Stroke Rehabilitation Care: A Clinical Practice Guideline. Stroke. 2005, 36, e100–e143. [Google Scholar] [CrossRef]
- Jolliffe, L.; Lannin, N.A.; Cadilhac, D.A.; Hoffmann, T. Systematic Review of Clinical Practice Guidelines to Identify Recommendations for Rehabilitation after Stroke and Other Acquired Brain Injuries. BMJ Open 2018, 8, e018791. [Google Scholar] [CrossRef]
- Hatem, S.M.; Saussez, G.; della Faille, M.; Prist, V.; Zhang, X.; Dispa, D.; Bleyenheuft, Y. Rehabilitation of Motor Function after Stroke: A Multiple Systematic Review Focused on Techniques to Stimulate Upper Extremity Recovery. Front. Hum. Neurosci. 2016, 10, 442. [Google Scholar] [CrossRef]
- Papale, A.E.; Hooks, B.M. Circuit Changes in Motor Cortex during Motor Skill Learning. Neuroscience 2018, 368, 283–297. [Google Scholar] [CrossRef]
- Makino, H.; Hwang, E.J.; Hedrick, N.G.; Komiyama, T. Circuit Mechanisms of Sensorimotor Learning. Neuron 2016, 92, 705–721. [Google Scholar] [CrossRef]
- Costa, R.M.; Cohen, D.; Nicolelis, M.A.L. Differential Corticostriatal Plasticity during Fast and Slow Motor Skill Learning in Mice. Curr. Biol. 2004, 14, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Mattar, A.A.; Nasir, S.M.; Darainy, M.; Ostry, D.J. Sensory change following motor learning. Prog. Brain Res. 2011, 191, 31–44. [Google Scholar]
- Bastani, A.; Jaberzadeh, S. Does Anodal Transcranial Direct Current Stimulation Enhance Excitability of the Motor Cortex and Motor Function in Healthy Individuals and Subjects with Stroke: A Systematic Review and Meta-Analysis. Clin. Neurophysiol. 2012, 123, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Vines, B.W.; Cerruti, C.; Schlaug, G. Dual-Hemisphere TDCS Facilitates Greater Improvements for Healthy Subjects’ Non-Dominant Hand Compared to Uni-Hemisphere Stimulation. BMC Neurosci. 2008, 9, 103. [Google Scholar] [CrossRef] [PubMed]
- Yavari, F.; Nitsche, M.A.; Ekhtiari, H. Transcranial Electric Stimulation for Precision Medicine: A Spatiomechanistic Framework. Front. Hum. Neurosci. 2017, 11, 159. [Google Scholar] [CrossRef]
- Woods, A.J.; Antal, A.; Bikson, M.; Boggio, P.S.; Brunoni, A.R.; Celnik, P.; Cohen, L.G.; Fregni, F.; Herrmann, C.S.; Kappenman, E.S.; et al. A Technical Guide to TDCS, and Related Non-Invasive Brain Stimulation Tools. Clin. Neurophysiol. 2016, 127, 1031–1048. [Google Scholar] [CrossRef]
- Reis, J.; Schambra, H.M.; Cohen, L.G.; Buch, E.R.; Fritsch, B.; Zarahn, E.; Celnik, P.A.; Krakauer, J.W. Noninvasive Cortical Stimulation Enhances Motor Skill Acquisition over Multiple Days through an Effect on Consolidation. Proc. Natl. Acad. Sci. USA. 2009, 106, 1590–1595. [Google Scholar] [CrossRef]
- Orban de Xivry, J.-J.; Shadmehr, R. Electrifying the Motor Engram: Effects of TDCS on Motor Learning and Control. Exp. Brain Res. 2014, 232, 3379–3395. [Google Scholar] [CrossRef]
- Hashemirad, F.; Zoghi, M.; Fitzgerald, P.B.; Jaberzadeh, S. The Effect of Anodal Transcranial Direct Current Stimulation on Motor Sequence Learning in Healthy Individuals: A Systematic Review and Meta-Analysis. Brain Cogn. 2016, 102, 1–12. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Excitability Changes Induced in the Human Motor Cortex by Weak Transcranial Direct Current Stimulation. J. Physiol. 2000, 527, 633–639. [Google Scholar] [CrossRef]
- Stagg, C.J.; Jayaram, G.; Pastor, D.; Kincses, Z.T.; Matthews, P.M.; Johansen-Berg, H. Polarity and Timing-Dependent Effects of Transcranial Direct Current Stimulation in Explicit Motor Learning. Neuropsychologia 2011, 49, 800–804. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Nitsche, M.S.; Klein, C.C.; Tergau, F.; Rothwell, J.C.; Paulus, W. Level of Action of Cathodal DC Polarisation Induced Inhibition of the Human Motor Cortex. Clin. Neurophysiol. 2003, 114, 600–604. [Google Scholar] [CrossRef]
- Boggio, P.S.; Castro, L.O.; Savagim, E.A.; Braite, R.; Cruz, V.C.; Rocha, R.R.; Rigonatti, S.P.; Silva, M.T.A.; Fregni, F. Enhancement of Non-Dominant Hand Motor Function by Anodal Transcranial Direct Current Stimulation. Neurosci. Lett. 2006, 404, 232–236. [Google Scholar] [CrossRef]
- Jacobson, L.; Koslowsky, M.; Lavidor, M. TDCS Polarity Effects in Motor and Cognitive Domains: A Meta-Analytical Review. Exp. Brain Res. 2012, 216, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Schauenburg, A.; Lang, N.; Liebetanz, D.; Exner, C.; Paulus, W.; Tergau, F. Facilitation of Implicit Motor Learning by Weak Transcranial Direct Current Stimulation of the Primary Motor Cortex in the Human. J. Cogn. Neurosci. 2003, 15, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Vines, B.W.; Nair, D.G.; Schlaug, G. Contralateral and Ipsilateral Motor Effects after Transcranial Direct Current Stimulation. Neuroreport 2006, 17, 671–674. [Google Scholar] [CrossRef]
- Ferbert, A.; Priori, A.; Rothwell, J.C.; Day, B.L.; Colebatch, J.G.; Marsden, C.D. Interhemispheric Inhibition of the Human Motor Cortex. J. Physiol. 1992, 453, 525–546. [Google Scholar] [CrossRef]
- Halakoo, S.; Ehsani, F.; Hosnian, M.; Zoghi, M.; Jaberzadeh, S. The Comparative Effects of Unilateral and Bilateral Transcranial Direct Current Stimulation on Motor Learning and Motor Performance: A Systematic Review of Literature and Meta-Analysis. J. Clin. Neurosci. 2020, 72, 8–14. [Google Scholar] [CrossRef]
- Buch, E.R.; Santarnecchi, E.; Antal, A.; Born, J.; Celnik, P.A.; Classen, J.; Gerloff, C.; Hallett, M.; Hummel, F.C.; Nitsche, M.A.; et al. Effects of TDCS on Motor Learning and Memory Formation: A Consensus and Critical Position Paper. Clin. Neurophysiol. 2017, 128, 589–603. [Google Scholar] [CrossRef]
- Naros, G.; Geyer, M.; Koch, S.; Mayr, L.; Ellinger, T.; Grimm, F.; Gharabaghi, A. Enhanced Motor Learning with Bilateral Transcranial Direct Current Stimulation: Impact of Polarity or Current Flow Direction? Clin. Neurophysiol. 2016, 127, 2119–2126. [Google Scholar] [CrossRef]
- Tsubota, K.; Nakamori, K. Effects of Ocular Surface Area and Blink Rate on Tear Dynamics. Arch. Ophthalmol. 1995, 113, 155–158. [Google Scholar] [CrossRef]
- Waters, S.; Wiestler, T.; Diedrichsen, J. Cooperation Not Competition: Bihemispheric TDCS and FMRI Show Role for Ipsilateral Hemisphere in Motor Learning. J. Neurosci. 2017, 37, 7500–7512. [Google Scholar] [CrossRef] [PubMed]
- Di Pino, G.; Pellegrino, G.; Assenza, G.; Capone, F.; Ferreri, F.; Formica, D.; Ranieri, F.; Tombini, M.; Ziemann, U.; Rothwell, J.C.; et al. Modulation of Brain Plasticity in Stroke: A Novel Model for Neurorehabilitation. Nat. Rev. Neurol. 2014, 10, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Waters-Metenier, S.; Husain, M.; Wiestler, T.; Diedrichsen, J. Bihemispheric Transcranial Direct Current Stimulation Enhances Effector-Independent Representations of Motor Synergy and Sequence Learning. J. Neurosci. 2014, 34, 1037–1050. [Google Scholar] [CrossRef] [PubMed]
- Jamil, A.; Batsikadze, G.; Kuo, H.I.; Labruna, L.; Hasan, A.; Paulus, W.; Nitsche, M.A. Systematic Evaluation of the Impact of Stimulation Intensity on Neuroplastic After-Effects Induced by Transcranial Direct Current Stimulation. J. Physiol. 2017, 595, 1273–1288. [Google Scholar] [CrossRef] [PubMed]
- Monte-Silva, K.; Kuo, M.-F.; Hessenthaler, S.; Fresnoza, S.; Liebetanz, D.; Paulus, W.; Nitsche, M.A. Induction of Late LTP-Like Plasticity in the Human Motor Cortex by Repeated Non-Invasive Brain Stimulation. Brain Stimul. 2013, 6, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.; Gual, A.; Colon, J. Alcohol y Atención Primaria de La Salud: Informaciones Clínicas Básicas Para La Identificación y El Manejo de Riesgos y Problemas; Monteiro, M., Ed.; Pan American Health Organization: Washington, DC, USA, 2008. [Google Scholar]
- Wulf, G.; Shea, C.; Lewthwaite, R. Motor Skill Learning and Performance: A Review of Influential Factors. Med. Educ. 2010, 44, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.; Fischer, J.T.; Prichard, G.; Weiller, C.; Cohen, L.G.; Fritsch, B. Time- but Not Sleep-Dependent Consolidation of TDCS-Enhanced Visuomotor Skills. Cereb. Cortex 2015, 25, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, B.; Reis, J.; Martinowich, K.; Schambra, H.M.; Ji, Y.; Cohen, L.G.; Lu, B. Direct Current Stimulation Promotes BDNF-Dependent Synaptic Plasticity: Potential Implications for Motor Learning. Neuron 2010, 66, 198–204. [Google Scholar] [CrossRef]
- Saucedo Marquez, C.M.; Zhang, X.; Swinnen, S.P.; Meesen, R.; Wenderoth, N. Task-Specific Effect of Transcranial Direct Current Stimulation on Motor Learning. Front. Hum. Neurosci. 2013, 7, 333. [Google Scholar] [CrossRef]
- Gandiga, P.C.; Hummel, F.C.; Cohen, L.G. Transcranial DC Stimulation (TDCS): A Tool for Double-Blind Sham-Controlled Clinical Studies in Brain Stimulation. Clin. Neurophysiol. 2006, 117, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Alonzo, A.; Aaronson, S.; Bikson, M.; Husain, M.; Lisanby, S.; Martin, D.; McClintock, S.M.; McDonald, W.M.; O’Reardon, J.; Esmailpoor, Z.; et al. Study Design and Methodology for a Multicentre, Randomised Controlled Trial of Transcranial Direct Current Stimulation as a Treatment for Unipolar and Bipolar Depression. Contemp. Clin. Trials 2016, 51, 65–71. [Google Scholar] [CrossRef]
- Hahn, C.; Rice, J.; Macuff, S.; Minhas, P.; Rahman, A.; Bikson, M. Methods for Extra-Low Voltage Transcranial Direct Current Stimulation: Current and Time Dependent Impedance Decreases. Clin. Neurophysiol. 2013, 124, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Tiffin, J.; Asher, E.J. The Purdue Pegboard: Norms and Studies of Reliability and Validity. J. Appl. Psychol. 1948, 32, 234–247. [Google Scholar] [CrossRef] [PubMed]
- López, L.A.; López, L.A.A.; Gómez, M.I.C.; Lozano, E.C.; Durán, M.A. Estándares Del Test de Minnesota de Destreza Manual En Una Muestra de Adultos Residentes En Cuatro Localidades de Bogotá. Morfolia 2014, 6, 3. [Google Scholar]
- Wang, Y.-C.; Wickstrom, R.; Yen, S.-C.; Kapellusch, J.; Grogan, K.A. Assessing Manual Dexterity: Comparing the WorkAbility Rate of Manipulation Test with the Minnesota Manual Dexterity Test. J. Hand Ther. 2017, 85, 909–917. [Google Scholar] [CrossRef]
- Rozand, V.; Pageaux, B.; Marcora, S.M.; Papaxanthis, C.; Lepers, R. Does Mental Exertion Alter Maximal Muscle Activation? Front. Hum. Neurosci. 2014, 8, 755. [Google Scholar] [CrossRef]
- Slater, H.; Arendt-Nielsen, L.; Wright, A.; Graven-Nielsen, T. Experimental Deep Tissue Pain in Wrist Extensors-a Model of Lateral Epicondylalgia. Eur. J. Pain 2003, 7, 277–288. [Google Scholar] [CrossRef]
- Torgén, M.; Swerup, C. Individual Factors and Physical Work Load in Relation to Sensory Thresholds in a Middle-Aged General Population Sample. Eur. J. Appl. Physiol. 2002, 86, 418–427. [Google Scholar] [CrossRef]
- Kregel, J.; van Wilgen, C.P.; Zwerver, J. Pain Assessment in Patellar Tendinopathy Using Pain Pressure Threshold Algometry: An Observational Study. Pain Med. 2013, 14, 1769–1775. [Google Scholar] [CrossRef]
- Hamilton, G.F.; McDonald, C.; Chenier, T.C. Measurement of Grip Strength: Validity and Reliability of the Sphygmomanometer and Jamar Grip Dynamometer. J. Orthop. Sports Phys. Ther. 1992, 16, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International Physical Activity Questionnaire: 12-Country Reliability and Validity. Med. Sci. Sport. Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A New Instrument for Psychiatric Practice and Research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Mollayeva, T.; Thurairajah, P.; Burton, K.; Mollayeva, S.; Shapiro, C.M.; Colantonio, A. The Pittsburgh Sleep Quality Index as a Screening Tool for Sleep Dysfunction in Clinical and Non-Clinical Samples: A Systematic Review and Meta-Analysis. Sleep Med. Rev. 2016, 25, 52–73. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Pixa, N.H.; Pollok, B. Effects of TDCS on Bimanual Motor Skills: A Brief Review. Front. Behav. Neurosci. 2018, 12, 1–9. [Google Scholar] [CrossRef]
- Pixa, N.H.; Steinberg, F.; Doppelmayr, M. High-Definition Transcranial Direct Current Stimulation to Both Primary Motor Cortices Improves Unimanual and Bimanual Dexterity. Neurosci. Lett. 2017, 643, 84–88. [Google Scholar] [CrossRef]
- Bastani, A.; Jaberzadeh, S. Within-Session Repeated a-TDCS: The Effects of Repetition Rate and Inter-Stimulus Interval on Corticospinal Excitability and Motor Performance. Clin. Neurophysiol. 2014, 125, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Antal, A.; Nitsche, M.A.; Kincses, T.Z.; Kruse, W.; Hoffmann, K.P.; Paulus, W. Facilitation of Visuo-Motor Learning by Transcranial Direct Current Stimulation of the Motor and Extrastriate Visual Areas in Humans. Eur. J. Neurosci. 2004, 19, 2888–2892. [Google Scholar] [CrossRef]
- Arias, P.; Corral-Bergantiños, Y.; Robles-García, V.; Madrid, A.; Oliviero, A.; Cudeiro, J. Bilateral TDCS on Primary Motor Cortex: Effects on Fast Arm Reaching Tasks. PLoS ONE 2016, 11, e0160063. [Google Scholar] [CrossRef]
- Karok, S.; Fletcher, D.; Witney, A.G. Task-Specificity of Unilateral Anodal and Dual-M1 TDCS Effects on Motor Learning. Neuropsychologia 2017, 94, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.; Swayne, O.B.; Vandermeeren, Y.; Camus, M.; Dimyan, M.A.; Harris-Love, M.; Perez, M.A.; Ragert, P.; Rothwell, J.C.; Cohen, L.G. Contribution of Transcranial Magnetic Stimulation to the Understanding of Cortical Mechanisms Involved in Motor Control. J. Physiol. 2008, 586, 325–351. [Google Scholar] [CrossRef] [PubMed]
- Kidgell, D.J.; Goodwill, A.M.; Frazer, A.K.; Daly, R.M. Induction of Cortical Plasticity and Improved Motor Performance Following Unilateral and Bilateral Transcranial Direct Current Stimulation of the Primary Motor Cortex. BMC Neurosci. 2013, 14, 64. [Google Scholar] [CrossRef]
- Nelson, J.T.; McKinley, R.A.; Golob, E.J.; Warm, J.S.; Parasuraman, R. Enhancing Vigilance in Operators with Prefrontal Cortex Transcranial Direct Current Stimulation (TDCS). NeuroImage 2014, 85, 909–917. [Google Scholar] [CrossRef]
- Mattar, A.A.G.; Darainy, M.; Ostry, D.J. Motor Learning and Its Sensory Effects: Time Course of Perceptual Change and Its Presence with Gradual Introduction of Load. J. Neurophysiol. 2013, 109, 782–791. [Google Scholar] [CrossRef]
- Montenegro, R.; Okano, A.; Gurgel, J.; Porto, F.; Cunha, F.; Massaferri, R.; Farinatti, P. Motor Cortex TDCS Does Not Improve Strength Performance in Healthy Subjects. Motriz. Rev. Educ. Fis. 2015, 21, 185–193. [Google Scholar] [CrossRef]
- Cho, H.S.; Cha, H.G. Effect of Mirror Therapy with TDCS on Functional Recovery of the Upper Extremity of Stroke Patients. J. Phys. Ther. Sci. 2015, 27, 1045–1047. [Google Scholar] [CrossRef]
- Hordacre, B.; Goldsworthy, M.R. Commentary: Cooperation Not Competition: Bihemispheric TDCS and FMRI Show Role for Ipsilateral Hemisphere in Motor Learning. Front. Hum. Neurosci. 2018, 12, 97. [Google Scholar] [CrossRef]
- Bernardi, N.F.; Darainy, M.; Bricolo, E.; Ostry, D.J. Observing Motor Learning Produces Somatosensory Change. J. Neurophysiol. 2013, 110, 1804–1810. [Google Scholar] [CrossRef]
- Yoshida, N.; Suzuki, T.; Ogahara, K.; Higashi, T.; Sugawara, K. Somatosensory Temporal Discrimination Threshold Changes during Motor Learning. Somatosens. Mot. Res. 2020, 37, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Costa, T.L.; Lapenta, O.M.; Boggio, P.S.; Ventura, D.F. Transcranial Direct Current Stimulation as a Tool in the Study of Sensory-Perceptual Processing. Atten. Percept. Psychophys. 2015, 77, 1813–1840. [Google Scholar] [CrossRef]
- Matsunaga, K. Effect of Transcranial DC Sensorimotor Cortex Stimulation on Somatosensory Evoked Potentials in Humans. Clin. Neurophysiol. 2004, 115, 456–460. [Google Scholar] [CrossRef]
- Meeker, T.J.; Keaser, M.L.; Khan, S.A.; Gullapalli, R.P.; Seminowicz, D.A.; Greenspan, J.D. Non-Invasive Motor Cortex Neuromodulation Reduces Secondary Hyperalgesia and Enhances Activation of the Descending Pain Modulatory Network. Front. Neurosci. 2019, 13, 467. [Google Scholar] [CrossRef] [PubMed]
- Vaseghi, B.; Zoghi, M.; Jaberzadeh, S. Does Anodal Transcranial Direct Current Stimulation Modulate Sensory Perception and Pain? A Meta-Analysis Study. Clin. Neurophysiol. 2014, 125, 1847–1858. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, C.G.; Muschinsky, S.; Nitsche, M.A.; Rolke, R.; Magerl, W.; Treede, R.D.; Paulus, W.; Happe, S. Transcranial Direct Current Stimulation of the Motor Cortex Induces Distinct Changes in Thermal and Mechanical Sensory Percepts. Clin. Neurophysiol. 2010, 121, 2083–2089. [Google Scholar] [CrossRef]


| Variables | Bi-RP-tDCS Group (n = 15) | Sham Group (n = 13) | p-Value |
|---|---|---|---|
| Quantitative | |||
| Age | 26.1 ± 5.5 | 22.9 ± 1.3 | 0.006 |
| Sleep Quality | 4 ± 2.2 | 6.9 ± 3.1 | 0.015 |
| Physical Activity | 8962.5 ± 1698.7 | 7828 ± 1952.6 | 0.797 |
| Edinburgh Inventory | 20 ± 3.7 | 19.2 ± 3.4 | 0.570 |
| Qualitative | |||
| Gender | |||
| Women | 6 | 5 | |
| Men | 9 | 8 | 0.256 |
| Educational level | |||
| High school | 3 | 1 | |
| University | 12 | 12 | 0.353 |
| Motor Dexterity | Group | Basal Condition | Post First Training | Post Training | Post 5 Days without Training | RM ANOVA Factor Time | RM ANOVA Time * Group | |||
|---|---|---|---|---|---|---|---|---|---|---|
| F | p-Value | F | p-Value | |||||||
| Fine | n = 28 | n = 28 | n = 24 | n = 23 | n = 23 | |||||
| Unimanual | Bi-RP-tDCS Placebo | 16.8 ± 1.2 16.2 ± 3.1 | 18.7 ± 1.5 18.4 ± 1.8 | 20.6 ± 1.5 19.7 ± 1.7 | 20.4 ± 1.4 19.3 ± 1.2 | 84.8 | <0.001 | 2.3 | 0.085 | |
| Bimanual | Bi-RP-tDCS Placebo | 13 ± 1 12.4 ± 1.4 | 13.7 ± 0.9 13.5 ± 1.5 | 14.3 ± 1 14.3 ± 1.8 | 14.5 ± 1.2 14.4 ± 1.3 | 33.1 | <0.001 | 0.7 | 0.563 | |
| Gross | n = 25 | n = 25 | n = 21 | n = 20 | n = 20 | |||||
| Unimanual | Bi-RP-tDCS Placebo | 62.6 ± 5 63.6 ± 4.5 | 58.7 ± 4.7 61.2 ± 4.1 | 55.6 ± 3.6 58.4 ± 3.6 | 55.8 ± 4.1 57.3 ± 3.9 | 41.8 | <0.001 | 2.3 | 0.089 | |
| Bimanual | Bi-RP-tDCS Placebo | 47.2 ± 3 50.4 ± 5 | 42.3 ± 2.9 44.1 ± 3.8 | 40 ± 1.4 42 ± 3.5 | 38.8 ± 2.3 41 ± 2.8 | 116.8 | <0.001 | 0.1 | 0.955 | |
| Variable | Group | Basal Condition | Post First Training | Post Training | Post 5 Days without Training | RM ANOVA Factor Time | RM ANOVA Time * Group | ||
|---|---|---|---|---|---|---|---|---|---|
| F | p-Value | F | p-Value | ||||||
| n = 28 | n = 28 | n = 24 | n = 23 | n = 23 | |||||
| Grip strength | Bi-RP-tDCS Placebo | 32.6 ± 8.9 29 ± 9.6 | 33.8 ± 8.3 30.5 ± 10.4 | 35.1 ± 9.8 29.9 ± 11.8 | 35.2 ± 9.7 28.9 ± 9.2 | 19 | 0.007 | 2.3 | 0.472 |
| Sensory Thresholds | Group | Basal Condition | Post First Training | Post Training | Post 5 Days without Training | RM ANOVA Factor Time | RM ANOVA Time * Group | ||
|---|---|---|---|---|---|---|---|---|---|
| F | p-Value | F | p-Value | ||||||
| n = 28 | n = 28 | n = 24 | n = 23 | n = 0.23 | |||||
| Two-point discrimination | Bi-RP-tDCS Placebo | 13.5 ± 6 12.3 ± 5.2 | 12.1 ± 6.1 11.5 ± 5.6 | 11.9 ± 4.9 8.8 ± 4.5 | 10.7 ± 6.5 8.8 ± 5.7 | 2.6 | 0.057 | 0.5 | 0.681 |
| Mechanical detection | Bi-RP-tDCS Placebo | 0.031 ± 0.018 0.028 ± 0.014 | .028 ± 0.012 .027 ± 0.016 | 0.029 ± 0.013 0.028 ± 0.016 | 0.033 ± 0.013 0.023 ± 0.012 | 0.5 | 0.655 | 1.2 | 0.319 |
| Thenar pressure pain | Bi-RP-tDCS Placebo | 5.9 ± 1.7 5.5 ± 1.6 | 5.8 ± 1.8 5.5 ± 1.4 | 5.9 ± 1.7 5.3 ± 1.7 | 6.4 ± 1.7 5.6 ± 1.6 | 0.5 | 0.682 | 0.2 | 0.901 |
| Bone pressure pain | Bi-RP-tDCS Placebo | 7.5 ± 2.9 7.1 ± 2.7 | 6.9 ± 2.6 7.6 ± 2.9 | 7.6 ± 2.5 7.1 ± 2.8 | 7.8 ± 2.8 7.9 ± 3.4 | 5.8 | 0.001 | 1.7 | 0.175 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flix-Díez, L.; Delicado-Miralles, M.; Gurdiel-Álvarez, F.; Velasco, E.; Galán-Calle, M.; Lerma Lara, S. Reversed Polarity bi-tDCS over M1 during a Five Days Motor Task Training Did Not Influence Motor Learning. A Triple-Blind Clinical Trial. Brain Sci. 2021, 11, 691. https://doi.org/10.3390/brainsci11060691
Flix-Díez L, Delicado-Miralles M, Gurdiel-Álvarez F, Velasco E, Galán-Calle M, Lerma Lara S. Reversed Polarity bi-tDCS over M1 during a Five Days Motor Task Training Did Not Influence Motor Learning. A Triple-Blind Clinical Trial. Brain Sciences. 2021; 11(6):691. https://doi.org/10.3390/brainsci11060691
Chicago/Turabian StyleFlix-Díez, Laura, Miguel Delicado-Miralles, Francisco Gurdiel-Álvarez, Enrique Velasco, María Galán-Calle, and Sergio Lerma Lara. 2021. "Reversed Polarity bi-tDCS over M1 during a Five Days Motor Task Training Did Not Influence Motor Learning. A Triple-Blind Clinical Trial" Brain Sciences 11, no. 6: 691. https://doi.org/10.3390/brainsci11060691
APA StyleFlix-Díez, L., Delicado-Miralles, M., Gurdiel-Álvarez, F., Velasco, E., Galán-Calle, M., & Lerma Lara, S. (2021). Reversed Polarity bi-tDCS over M1 during a Five Days Motor Task Training Did Not Influence Motor Learning. A Triple-Blind Clinical Trial. Brain Sciences, 11(6), 691. https://doi.org/10.3390/brainsci11060691







