Occipital Nerve Stimulation for Pain Modulation in Drug-Resistant Chronic Cluster Headache
Abstract
1. Introduction
2. Materials and Methods
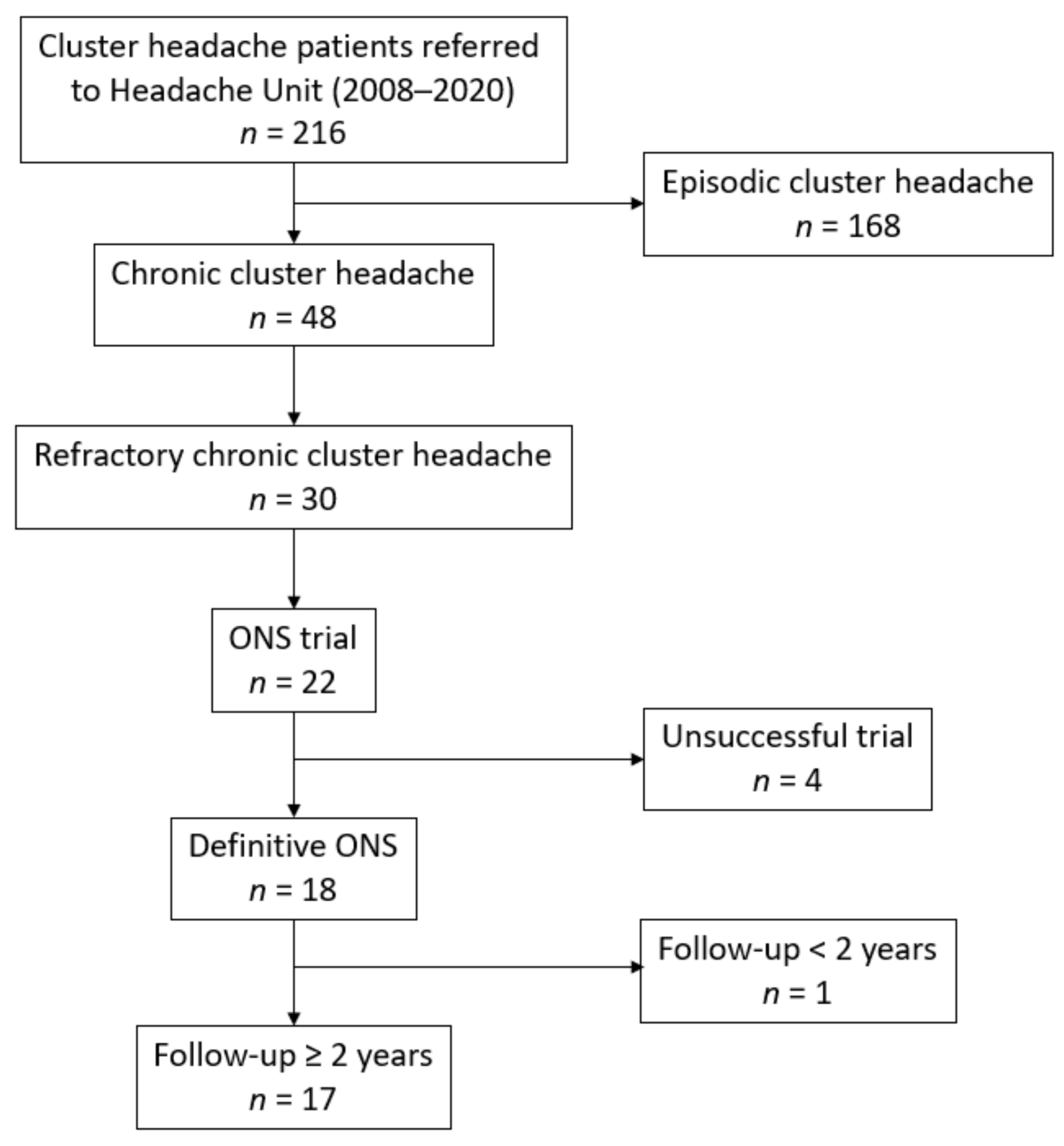
2.1. Study Population
2.2. Variables and Definitions
2.3. Data Collection
2.4. Surgical Procedures
2.5. Statistical Analysis
3. Results
3.1. Patients Characteristics
3.2. ONS Effect on Number of Weekly CH Attacks
3.3. ONS Effect on Pain Severity
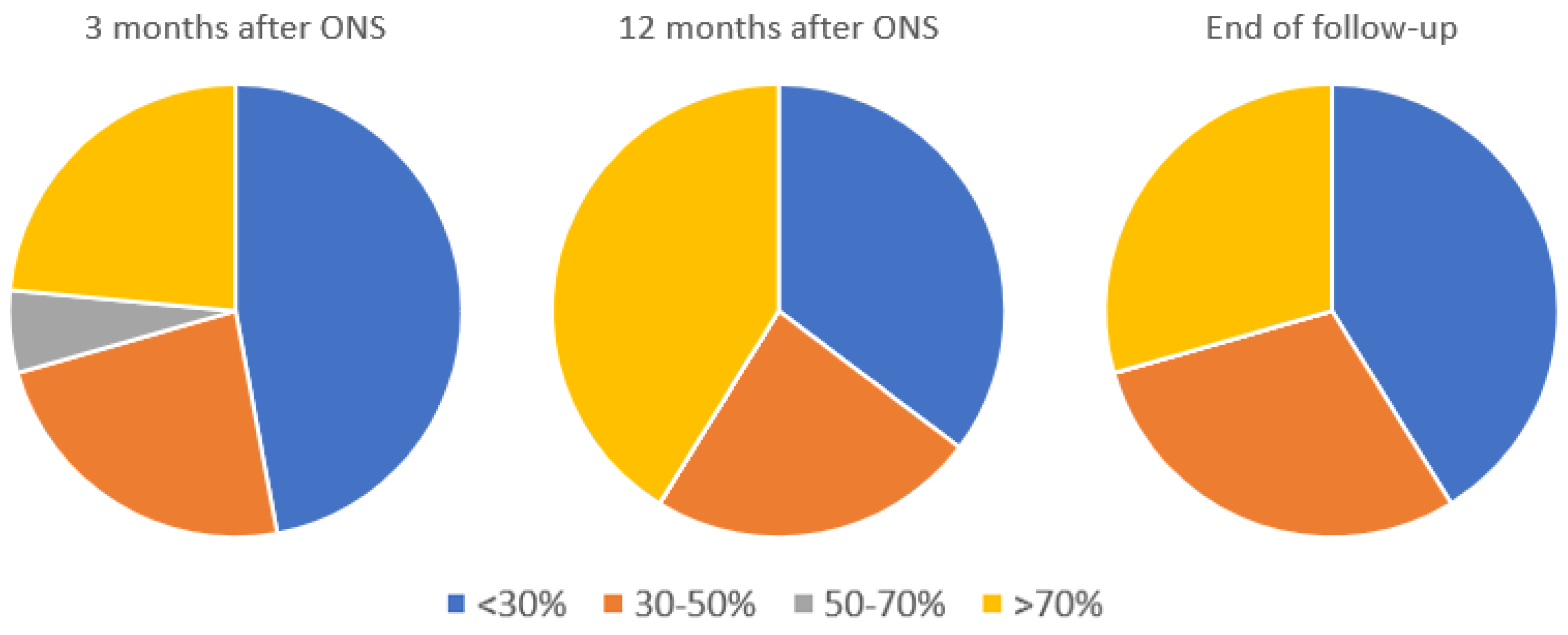
3.4. Overall Perceived Improvement
3.5. Preventive Medication Use
3.6. Triptan Use
3.7. Adverse Events
3.8. Non-Responders
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arnold, M. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef]
- May, A.; Schwedt, T.J.; Magis, D.; Pozo-Rosich, P.; Evers, S.; Wang, S.-J. Cluster headache. Nat. Rev. Dis. Primers 2018, 4, 18006. [Google Scholar] [CrossRef] [PubMed]
- Mitsikostas, D.D.; Edvinsson, L.; Jensen, R.H.; Katsarava, Z.; Lampl, C.; Negro, A.; Osipova, V.; Paemeleire, K.; Siva, A.; Valade, D. Refractory chronic cluster headache: A consensus statement on clinical definition from the European Headache Federation. J. Headache Pain 2014, 15, 79. [Google Scholar] [CrossRef] [PubMed]
- Vukovic Cvetkovic, V.; Jensen, R.H. Neurostimulation for the treatment of chronic migraine and cluster headache. Acta Neurol. Scand. 2019, 139, 4–17. [Google Scholar] [CrossRef]
- Martelletti, P.; Jensen, R.H.; Antal, A.; Arcioni, R.; Brighina, F.; de Tommaso, M.; Franzini, A.; Fontaine, D.; Heiland, M.; Jürgens, T.P.; et al. Neuromodulation of chronic headaches: Position statement from the European Headache Federation. J. Headache Pain 2013, 14, 86. [Google Scholar] [CrossRef]
- Belvís, R.; Irimia, P.; Seijo-Fernández, F.; Paz, J.; García-March, G.; Santos-Lasaosa, S.; Latorre, G.; González-Oria, C.; Rodríguez, R.; Pozo-Rosich, P.; et al. Neuromodulación En Cefaleas y Neuralgias Craneofaciales: Guía de La Sociedad Española de Neurología y de La Sociedad Española de Neurocirugía. Neurologia 2021, 36, 61–79. [Google Scholar] [CrossRef]
- Vyas, D.B.; Ho, A.L.; Dadey, D.Y.; Pendharkar, A.V.; Sussman, E.S.; Cowan, R.; Halpern, C.H. Deep brain stimulation for chronic cluster headache: A review. Neuromodulation Technol. Neural Interface 2019, 22, 388–397. [Google Scholar] [CrossRef]
- Magis, D.; Bruno, M.A.; Fumal, A.; Gérardy, P.Y.; Hustinx, R.; Laureys, S.; Schoenen, J. Central modulation in cluster headache patients treated with occipital nerve stimulation: An FDG-PET study. BMC Neurol. 2011, 11, 25. [Google Scholar] [CrossRef]
- Leone, M.; Proietti Cecchini, A.; Messina, G.; Franzini, A. Long-term occipital nerve stimulation for drug-resistant chronic cluster headache. Cephalalgia 2017, 37, 756–763. [Google Scholar] [CrossRef]
- Burns, L.; Watkins, L.; Goadsby, P.J. Treatment of intractable chronic cluster headache by occipital nerve stimulation in 14 patients. Neurology 2009, 72, 341–345. [Google Scholar] [CrossRef]
- Fontaine, D.; Sol, J.C.; Raoul, S.; Fabre, N.; Geraud, G.; Magne, C.; Sakarovitch, C.; Lanteri-Minet, M. Treatment of refractory chronic cluster headache by chronic occipital nerve stimulation. Cephalalgia 2011, 31, 1101–1105. [Google Scholar] [CrossRef] [PubMed]
- Robbins, M.S.; Starling, A.J.; Pringsheim, T.M.; Becker, W.J.; Schwedt, T.J. Treatment of cluster headache: The American headache society evidence-based guidelines: Headache. Headache 2016, 56, 1093–1106. [Google Scholar] [CrossRef]
- World medical association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191. [CrossRef]
- Olesen, J. The international classification of headache disorders. 2nd edition (ICHD-II). Revue Neurologique 2005, 161, 689–691. [Google Scholar] [CrossRef]
- Popeney, A.; Alo, K.M. Peripheral neurostimulation for the treatment of chronic, disabling transformed migraine. Headache 2003, 43, 369–375. [Google Scholar] [CrossRef]
- Slavin, K.V.; Nersesyan, H.; Wess, C. Peripheral neurostimulation for treatment of intractable occipital neuralgia. Neurosurgery 2006, 58, 112–119. [Google Scholar] [CrossRef]
- Weiner, R.L.; Reed, K.L. Peripheral neurostimulation for control of intractable occipital neuralgia. Neuromodulation Technol. Neural Interface 1999, 2, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Trentman, T.L.; Slavin, K.V.; Freeman, J.A.; Zimmerman, R.S. Occipital nerve stimulator placement via a retromastoid to infraclavicular approach: A technical report. Stereotact. Funct. Neurosurg. 2010, 88, 121–125. [Google Scholar] [CrossRef]
- Aibar-Durán, J.A.; Álvarez Holzapfel, M.J.; Rodríguez Rodríguez, R.; Belvis Nieto, R.; Roig Arnall, C.; Molet Teixido, J. Occipital nerve stimulation and deep brain stimulation for refractory cluster headache: A prospective analysis of efficacy over time. J. Neurosurg. 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kinfe, T.M.; Schuss, P.; Vatter, H. Occipital nerve block prior to occipital nerve stimulation for refractory chronic migraine and chronic cluster headache: Myth or prediction? Cephalalgia 2015, 35, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Cadalso, R.T.; Daugherty, J.; Holmes, C.; Ram, S.; Enciso, R. Efficacy of electrical stimulation of the occipital nerve in intractable primary headache disorders: A systematic review with meta-analyses. J. Oral. Facial. Pain Headache 2018, 32, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Leplus, A.; Fontaine, D.; Donnet, A.; Regis, J.; Lucas, C.; Buisset, N.; Blond, S.; Raoul, S.; Guegan-Massardier, E.; Derrey, S.; et al. Long-term efficacy of occipital nerve stimulation for medically intractable cluster headache. Neurosurgery 2020, 373. [Google Scholar] [CrossRef]
- Pohl, H.; Gantenbein, A.R.; Sandor, P.S.; Schoenen, J.; Andrée, C. Interictal burden of cluster headache: Results of the EUROLIGHT cluster headache project, an internet-based, cross-sectional study of people with cluster headache. Headache 2020, 60, 360–369. [Google Scholar] [CrossRef]
- Fontaine, D.; Blond, S.; Lucas, C.; Regis, J.; Donnet, A.; Derrey, S.; Guegan-Massardier, E.; Jarraya, B.; Dang-Vu, B.; Bourdain, F.; et al. Occipital nerve stimulation improves the quality of life in medically-intractable chronic cluster headache: Results of an observational prospective study. Cephalalgia 2017, 37, 1173–1179. [Google Scholar] [CrossRef]
- Mueller, O.; Diener, H.C.; Dammann, P.; Rabe, K.; Hagel, V.; Sure, U.; Gaul, C. Occipital nerve stimulation for intractable chronic cluster headache or migraine: A critical analysis of direct treatment costs and complications. Cephalalgia 2013, 33, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- Ford, J.H.; Nero, D.; Kim, G.; Chu, B.C.; Fowler, R.; Ahl, J.; Martinez, J.M. Societal burden of cluster headache in the United States: A descriptive economic analysis. J. Med. Econ. 2018, 21, 107–111. [Google Scholar] [CrossRef]
- Belvis, R.; Rodríguez, R.; Guasch, M.; Álvarez, M.J.; Molet, J.; Roig, C. Eficacia y seguridad del tratamiento quirúrgico en la cefalea en racimos. Med. Clín. 2020, 154, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Wilbrink, L.A.; Teernstra, O.P.M.; Haan, J.; van Zwet, E.W.; Evers, S.M.A.A.; Spincemaille, G.H.; Veltink, P.H.; Mulleners, W.; Brand, R.; Huygen, F.J.P.M.; et al. Occipital nerve stimulation in medically intractable, chronic cluster headache. The ICON study: Rationale and protocol of a randomised trial. Cephalalgia 2013, 33, 1238–1247. [Google Scholar] [CrossRef]

| Age | Gender | Duration of CH (Years) | Duration of Chronic CH (Years) | Preventive Oral Treatment Trials Prior to ONS | Therapeutic Techniques Prior to ONS | Prior to ONS Trial (Baseline) | Time of ONS Trial (Weeks) | ||
|---|---|---|---|---|---|---|---|---|---|
| Days of Pain per Month | Attacks per Day | Attack VAS Score | |||||||
| 37 | Female | 3 | 1 | 3 | None | 20 | 1 | 8 | 3 |
| 34 | Male | 23 | 9 | 3 | None | 30 | 10 | 10 | 7 |
| 38 | Female | 14 | 11 | 3 | OnabotA | 30 | 8 | 10 | 3 |
| 36 | Male | 8 | 5 | 4 | None | 30 | 2 | 10 | 2 |
| Patient No. | Age | Gender | Duration of CH (Years) | Duration of Chronic CH (Years) | Preventive Oral Treatment Trials Prior to ONS | Therapeutic Techniques Prior to ONS | Prior to ONS (Baseline) | ||
|---|---|---|---|---|---|---|---|---|---|
| Days of Pain per Month | Attacks per Day | Attack VAS Score | |||||||
| 1 | 54 | Female | 10 | 7 | 4 | OnabotA, GON TENS, SPG RFT, GON block | 30 | 4 | 9 |
| 2 | 31 | Female | 4 | 2 | 3 | GON block | 30 | 1 | 10 |
| 3 | 36 | Female | 17 | 11 | 6 | GON block | 30 | 10 ¶ | 10 |
| 4 | 49 | Female | 31 | 22 | 6 | GON block | 30 | 1 | 10 |
| 5 | 45 | Male | 33 | 25 | 5 | GON block | 30 | 5 | 10 |
| 6 | 39 | Female | 23 | 16 | 3 | None | 30 | 9 ¶ | 8 |
| 7 | 61 | Male | 44 | 10 | 4 | None | 30 | 2 | 10 |
| 8 | 34 | Female | 2 | 1 | 5 | None | 30 | 4 | 9 |
| 9 | 37 | Female | 3 | 2 | 3 | OnabotA | 30 | 16 ¶ | 10 |
| 10 | 31 | Male | 4 | 3 | 4 | None | 10 | 1 | 10 |
| 11 | 41 | Male | 4 | 3 | 3 | None | 30 | 14 ¶ | 10 |
| 12 | 43 | Male | 4 | 2 | 8 | OnabotA | 30 | 3 | 10 |
| 13 | 42 | Male | 6 | 4 | 3 | None | 30 | 2 | 9 |
| 14 | 33 | Male | 4 | 1 | 3 | GON block | 30 | 7 | 10 |
| 15 | 31 | Male | 9 | 6 | 4 | GON block, OnabotA | 30 | 3 | 10 |
| 16 | 43 | Male | 7 | 5 | 8 | Gon block, OnabotA | 30 | 3 | 10 |
| 17 | 51 | Male | 11 | 6 | 5 | GON block, OnabotA | 30 | 5 | 10 |
| Patient No. | Follow-Up * | Adverse Effects | 3 Months after ONS | 1 Year after ONS | At the End of Follow-Up | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Days of Pain per Month | Attacks per Day | Attack VAS Score | Overall Perceived Improvement | Days of Pain per Month | Attacks per Day | Attack VAS Score | Overall Perceived Improvement | Days of Pain per Month | Attacks per Day | Attack VAS Score | Overall Perceived Improvement | |||
| 1 | 4 years (deactivated) | None | 3 | 1 | 3 | >70% | 3 | 1 | 3 | >70% | 9 | 30 µ | 4 | <30% |
| 2 | 5 years (deactivated) | Electrode migration | 1 | 1 | 10 | >70% | 1 | 1 | 10 | >70% | 30 | 1 | 10 | <30% |
| 3 | 5 years (removed) | None | 30 | 10 | 10 | <30% | 30 | 10 | 10 | <30% | 30 | 10 | 10 | <30% |
| 4 | 12 years (ongoing) | Posttraumatic disconnection (reintervention needed) | 0 | 0 | NA | >70% | 0 | 0 | NA | >70% | Once every 3 months | Once every 3 months | 3 | >70% |
| 5 | 9 years (ongoing) | Mild pain on surgery site | 30 | 5 | 10 | <30% | 15 | 5 | 6 | 30–50% | Once every 3 months | Once every 3 months | 6 | >70% |
| 6 | 8 years (removed) | None | 30 | 9 | 7 | <30% | 30 | 9 | 7 | <30% | 30 | 9 | 7 | <30% |
| 7 | 10 years (ongoing) | None | 30 | 2 | 7 | 30–50% | 30 | 2 | 7 | 30–50% | 30 | 2 | 7 | 30–50% |
| 8 | 3 years (removed) | None | 30 | 4 | 7 | <30% | 30 | 6 | 9 | <30% | 30 | 6 | 9 | <30% |
| 9 | 7 years (ongoing) | Electrode migration | 30 | 5 | 6 | 30–50% | 0 | 0 | NA | >70% | 0 | 0 | NA | >70% |
| 10 | 9 years (removed) | None | 30 | 5 | 10 | <30% | 30 | 1 | 10 | <30% | 30 | 1 | 7 | <30% |
| 11 | 6 years (ongoing) | None | 30 | 1 | 6 | 50–70% | 30 | 10 | 8 | 30–50% | 30 | 2 | 10 | 30–50% |
| 12 | 5 years (ongoing) | Surgical site infection | 30 | 1 | 6 | 30–50% | 30 | 1 | 7 | 30-50% | 30 | 2 | 8 | 30–50% |
| 13 | 2 years (removed) | Surgical site infection | 20 | 2 | 9 | 30–50% | 30 | 2 | 10 | <30% | 30 | 2 | 10 | <30% |
| 14 | 12 years (ongoing) | None | 15 | 1 | 5 | >70% | 5 | 3 | 5 | >70% | 3 | 1 | 5 | >70% § |
| 15 | 3 years (removed) # | None | 30 | 3 | 10 | <30% | 10 | 3 | 5 | >70% | Once every 6 months | 1 | 5 | >70% |
| 16 | 5 years (removed) | Electrode infection | 30 | 3 | 10 | <30% | 10 | 1 | 5 | >70% | 30 | 1 | 5 | 30–50% |
| 17 | 7 years (ongoing) | None | 30 | 5 | 10 | <30% | 30 | 5 | 10 | <30% | 30 | 3 | 3 | 30–50% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-de-Terán, J.; Membrilla, J.A.; Paz-Solís, J.; de Lorenzo, I.; Roa, J.; Lara-Lara, M.; Gil-Martínez, A.; Díez-Tejedor, E. Occipital Nerve Stimulation for Pain Modulation in Drug-Resistant Chronic Cluster Headache. Brain Sci. 2021, 11, 236. https://doi.org/10.3390/brainsci11020236
Díaz-de-Terán J, Membrilla JA, Paz-Solís J, de Lorenzo I, Roa J, Lara-Lara M, Gil-Martínez A, Díez-Tejedor E. Occipital Nerve Stimulation for Pain Modulation in Drug-Resistant Chronic Cluster Headache. Brain Sciences. 2021; 11(2):236. https://doi.org/10.3390/brainsci11020236
Chicago/Turabian StyleDíaz-de-Terán, Javier, Javier A. Membrilla, José Paz-Solís, Iñigo de Lorenzo, Javier Roa, Manuel Lara-Lara, Alfonso Gil-Martínez, and Exuperio Díez-Tejedor. 2021. "Occipital Nerve Stimulation for Pain Modulation in Drug-Resistant Chronic Cluster Headache" Brain Sciences 11, no. 2: 236. https://doi.org/10.3390/brainsci11020236
APA StyleDíaz-de-Terán, J., Membrilla, J. A., Paz-Solís, J., de Lorenzo, I., Roa, J., Lara-Lara, M., Gil-Martínez, A., & Díez-Tejedor, E. (2021). Occipital Nerve Stimulation for Pain Modulation in Drug-Resistant Chronic Cluster Headache. Brain Sciences, 11(2), 236. https://doi.org/10.3390/brainsci11020236






