Magnetoencephalography and High-Density Electroencephalography Study of Acoustic Event Related Potentials in Early Stage of Multiple Sclerosis: A Pilot Study on Cognitive Impairment and Fatigue
Abstract
1. Introduction
- Advanced topographic analysis of ERP and ERF and related ERSPs related to an acoustic oddball paradigm in a cohort of early and treatment-naïve pwMS;
- Correlations between P300 features with demographic, clinical, and neuropsychological characteristics, including fatigue, depression, and cognitive impairment.
2. Materials and Methods
2.1. Study Design and Population
- -
- A complete neurological examination with disability assessment by EDSS [46]
- -
- A 45-min neuropsychological evaluation
- -
- A simultaneous high density (hd) MEG and EEG recordings (50–55 min preceded by 20–25 min setup time) of event related fields/potentials (ERF/ERP) and resting state data.
2.2. Neuropsychological Evaluation
2.3. P300 Procedure
2.4. Simultaneous MEG and EEG Recording
2.5. Artifact Correction and Data Analysis
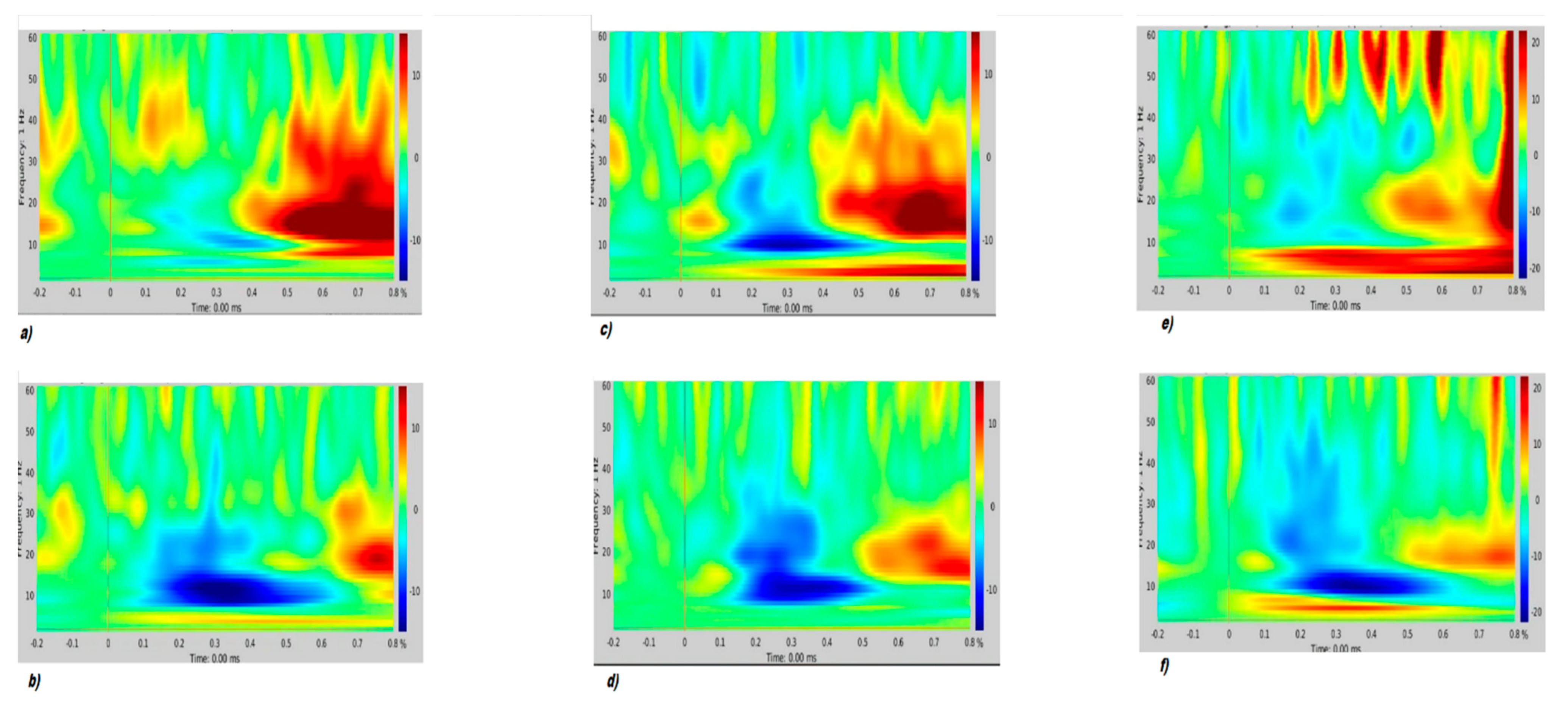
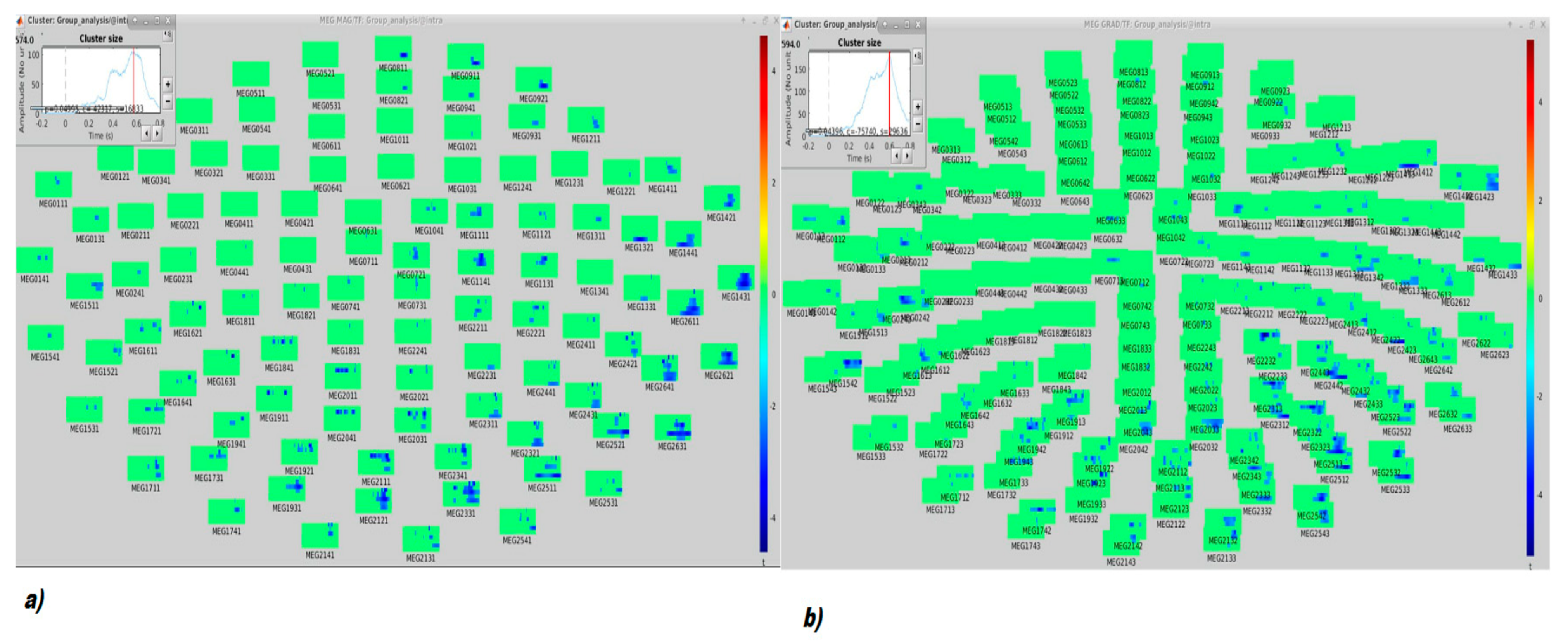
Time-Frequency (TF) Analysis
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chiaravalloti, N.D.; DeLuca, J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008, 7, 1139–1151. [Google Scholar] [CrossRef]
- Brochet, B.; Ruet, A. Cognitive Impairment in Multiple Sclerosis With Regards to Disease Duration and Clinical Phenotypes. Front. Neurol. 2019, 10, 261. [Google Scholar] [CrossRef] [PubMed]
- Ruano, L.; Portaccio, E.; Goretti, B.; Niccolai, C.; Severo, M.; Patti, F.; Cilia, S.; Gallo, P.; Grossi, P.; Ghezzi, A.; et al. Age and disability drive cognitive impairment in multiple sclerosis across disease subtypes. Mult. Scler. 2017, 23, 1258–1267. [Google Scholar] [CrossRef] [PubMed]
- Ruano, L.; Portaccio, E.; Goretti, B.; Niccolai, C.; Severo, M.; Patti, F.; Cilia, S.; Gallo, P.; Grossi, P.; Ghezzi, A. Early cognitive impairment in patients with clinically isolated syndrome suggestive of multiple sclerosis. Mult. Scler. 2007, 13, 124–174. [Google Scholar]
- Schulz, D.; Kopp, B.; Kunkel, A.; Faiss, J.H. Cognition in the early stage of multiple sclerosis. J. Neurol. 2006, 253, 1002–1010. [Google Scholar] [CrossRef]
- Rao, S.M.; Leo, G.J.; Bernardin, L.; Unverzagt, F. Cognitive dysfunction in multiple sclerosis. Frequency patterns and prediction. Neurology 1991, 41, 685–691. [Google Scholar] [CrossRef]
- Zipoli, V.; Goretti, B.; Hakiki, B.; Siracusa, G.; Sorbi, S.; Portaccio, E.; Amato, M.P. Cognitive impairment predicts conversion to multiple sclerosis in clinically isolated syndromes. Mult. Scler. 2010, 16, 62–67. [Google Scholar] [CrossRef]
- Pitteri, M.; Romualdi, C.; Magliozzi, R.; Monaco, S.; Calabrese, M. Cognitive impairment predicts disability progression and cortical thinning in MS: An 8-year study. Mult. Scler. 2017, 23, 848–854. [Google Scholar] [CrossRef]
- Ghezzi, A.; Goretti, B.; Portaccio, E.; Roscio, M.; Amato, M.P. Cognitive impairment in pediatric multiple sclerosis. Neurol. Sci. 2010, 31 (Suppl. 2), S215–S218. [Google Scholar] [CrossRef]
- Rao, S.M.; Leo, G.J.; Ellington, L.; Nauertz, T.; Bernardin, L.; Unverzagt, F. Cognitive dysfunction in multiple sclerosis. II. Impact on employment and social functioning. Neurology 1991, 41, 692–696. [Google Scholar] [CrossRef]
- Ruet, A.; Deloire, M.; Hamel, D.; Ouallet, J.C.; Petry, K.; Brochet, B. Cognitive impairment, health-related quality of life and vocational status at early stages of multiple sclerosis: A 7-year longitudinal study. J. Neurol. 2013, 3, 776–784. [Google Scholar] [CrossRef]
- Sumowski, J.F.; Benedict, R.; Enzinger, C.; Filippi, M.; Geurts, J.J.; Hamalainen, P.; Hulst, H.; Inglese, M.; Leavitt, V.M.; Rocca, M.A.; et al. Cognition in multiple sclerosis: State of the field and priorities for the future. Neurology 2018, 90, 278–288. [Google Scholar] [CrossRef]
- Sumowski, J.F.; Benedict, R.; Enzinger, C.; Filippi, M.; Geurts, J.J.; Hamalainen, P.; Hulst, H.; Inglese, M.; Leavitt, V.M.; Rocca, M.A. Pathophysiological and cognitive mechanisms of fatigue in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2019, 90, 642–651. [Google Scholar]
- Bisecco, A.; Caiazzo, G.; d’Ambrosio, A.; Sacco, R.; Bonavita, S.; Docimo, R.; Cirillo, M.; Pagani, E.; Filippi, M.; Esposito, F.; et al. Fatigue in multiple sclerosis: The contribution of occult white matter damage. Mult. Scler. 2016, 22, 1676–1684. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, C.; Rocca, M.A.; Riccitelli, G.; Pagani, E.; Messina, R.; Preziosa, P.; Colombo, B.; Rodegher, M.; Falini, A.; Comi, G.; et al. Influence of the topography of brain damage on depression and fatigue in patients with multiple sclerosis. Mult. Scler. 2014, 20, 192–201. [Google Scholar] [CrossRef]
- Calabrese, M.; Rinaldi, F.; Grossi, P.; Mattisi, I.; Bernardi, V.; Favaretto, A.; Perini, P.; Gallo, P. Basal ganglia and frontal/parietal cortical atrophy is associated with fatigue in relapsing-remitting multiple sclerosis. Mult. Scler. 2010, 16, 1220–1228. [Google Scholar] [CrossRef]
- Cruz Gómez, Á.J.; Ventura Campos, N.; Belenguer, A.; Ávila, C.; Forn, C. Regional brain atrophy and functional connectivity changes related to fatigue in multiple sclerosis. PLoS ONE 2013, 8, e77914. [Google Scholar] [CrossRef]
- Pittion-Vouyovitch, S.; Debouverie, M.; Guillemin, F.; Vandenberghe, N.; Anxionnat, R.; Vespignani, H. Fatigue in multiple sclerosis is related to disability, depression and quality of life. J. Neurol. Sci. 2006, 15, 39–45. [Google Scholar] [CrossRef]
- Kos, D.; Kerckhofs, E.; Nagels, G.; D’hooghe, M.B.; Ilsbroukx, S. Origin of fatigue in multiple sclerosis: Review of the literature. Neurorehabil. Neural Repair 2008, 22, 91–100. [Google Scholar] [CrossRef]
- Strober, L.B.; Arnett, P.A. An examination of four models predicting fatigue in multiple sclerosis. Arch. Clin. Neuropychol. 2005, 20, 631–646. [Google Scholar] [CrossRef] [PubMed]
- Biberacher, V.; Schmidt, P.; Selter, R.C.; Pernpeinter, V.; Kowarik, M.C.; Knier, B.; Buck, D.; Hoshi, M.M.; Korn, T.; Berthele, A.; et al. Fatigue in multiple sclerosis: Associations with clinical, MRI and CSF parameters. Mult. Scler. 2018, 24, 1115–1125. [Google Scholar] [CrossRef]
- Iancheva, D.; Trenova, A.; Mantarova, S.; Terziyski, K. Functional Magnetic Resonance Imaging Correlations Between Fatigue and Cognitive Performance in Patients With Relapsing Remitting Multiple Sclerosis. Front. Psychiatry 2019, 10, 754. [Google Scholar] [CrossRef]
- Krupp, L.B.; LaRocca, N.G.; Muir-Nash, J.; Steinberg, A.D. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch. Neurol. 1989, 46, 1121–1123. [Google Scholar] [CrossRef] [PubMed]
- Penner, I.K.; Raselli, C.; Stöcklin, M.; Opwis, K.; Kappos, L.; Calabrese, P. The Fatigue Scale for Motor and Cognitive Functions (FSMC): Validation of a new instrument to assess multiple sclerosis-related fatigue. Mult. Scler. 2009, 15, 1509–1517. [Google Scholar] [CrossRef]
- Gross, J. Magnetoencephalography in Cognitive Neuroscience: A Primer. Neuron 2019, 104, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Tewarie, P.; Schoonheim, M.M.; Schouten, D.I.; Polman, C.H.; Balk, L.J.; Uitdehaag, B.M.; Geurts, J.J.; Hillebrand, A.; Barkhof, F.; Stam, C.J. Functional brain networks: Linking thalamic atrophy to clinical disability in multiple sclerosis, a multimodal fMRI and MEG study. Hum. Brain Mapp. 2015, 36, 603–618. [Google Scholar] [CrossRef]
- Pokryszko-Dragan, A.; Zagrajek, M.; Slotwinski, K.; Bilinska, M.; Gruszka, E.; Podemski, R. Event-related potentials and cognitive performance in multiple sclerosis patients with fatigue. Neurol. Sci. 2016, 37, 1545–1556. [Google Scholar] [CrossRef] [PubMed]
- Chinnadurai, S.A.; Venkatesan, S.A.; Shankar, G.; Samivel, B.; Ranganathan, L.N. A study of cognitive fatigue in multiple sclerosis with novel clinical and electrophysiological parameters utilizing the event related potential P300. Mult. Scler. Relat. Disord. 2016, 10, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lazarevic, S.; Azanjac Arsic, A.; Aleksic, D.; Toncev, G.; Miletic-Drakulic, S. Depression and Fatigue in Patients with Multiple Sclerosis Have No Influence on the Parameters of Cognitive Evoked Potentials. J. Clin. Neurophysiol. 2021, 38, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Aminoff, J.C.; Goodin, D.S. Long-latency cerebral event-related potentials in multiple sclerosis. J. Clin. Neurophysiol. 2001, 18, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Gil, R.; Zai, L.; Neau, J.P.; Jonveaux, T.; Agbo, C.; Rosolacci, T.; Burbaud, P.; Ingrand, P. Event-related auditory evoked potentials and multiple sclerosis. Electroencephalogr. Clin. Neurophysiol. 1993, 88, 182–187. [Google Scholar] [CrossRef]
- Piras, M.R.; Magnano, I.; Canu, E.D.; Paulus, K.S.; Satta, W.M.; Soddu, A.; Conti, M.; Achene, A.; Solinas, G.; Aiello, I. Longitudinal study of cognitive dysfunction in multiple sclerosis: Neuropsychological, neuroradiological, and neurophysiological findings. J. Neurol. Neurosurg. Psychiatry 2003, 74, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Ivica, N.; Titlic, M.; Pavelin, S. P300 wave changes in patients with multiple sclerosis. Acta Inform. Med. 2013, 21, 205–207. [Google Scholar] [CrossRef]
- Giesser, B.S.; Schroeder, M.M.; LaRocca, N.G.; Kurtzberg, D.; Ritter, W.; Vaughan, H.G.; Scheinberg, L.C. Endogenous event-related potentials as indices of dementia in multiple sclerosis patients. Electroencephalogr. Clin. Neurophysiol. 1992, 82, 320–329. [Google Scholar] [CrossRef]
- Ellger, T.; Bethke, F.; Frese, A.; Luettmann, R.J.; Buchheister, A.; Ringelstein, E.B.; Evers, S. Event-related potentials in different subtypes of multiple sclerosis—A cross-sectional study. J. Neurol. Sci. 2002, 205, 35–40. [Google Scholar] [CrossRef]
- Triantafyllou, N.I.; Voumvourakis, K.; Zalonis, I.; Sfagos, K.; Mantouvalos, V.; Malliara, S.; Papageorgiou, C. Cognition in relapsing-remitting multiple sclerosis: A multichannel event-related potential (P300) study. Acta Neurol. Scand. 1992, 85, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Honig, L.S.; Ramsay, R.E.; Sheremata, W.A. Event-related potential P300 in multiple sclerosis. Relation to magnetic resonance imaging and cognitive impairment. Arch. Neurol. 1992, 49, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Magnano, I.; Aiello, I.; Piras, M.R. Cognitive impairment and neurophysiological correlates in MS. J. Neurol. Sci. 2006, 245, 117–122. [Google Scholar] [CrossRef]
- Zwecker, M.; Sarova, I.; Lavie, M.; Zeilig, G.; Achiron, A. Detection of Cognitive Impairment in Multiple Sclerosis Based on P300 Event-Related Potential. Int. J. Phys. Med. Rehabil. 2018, 6, 4. [Google Scholar] [CrossRef]
- Magnié, M.N.; Bensa, C.; Laloux, L.; Bertogliati, C.; Faure, S.; Lebrun, C. Contribution of cognitive evoked potentials for detecting early cognitive disorders in multiple sclerosis. Rev. Neurol. 2007, 163, 1065–1074. [Google Scholar] [CrossRef]
- Kocer, B.; Unal, T.; Nazliel, B.; Biyikli, Z.; Yesilbudak, Z.; Karakas, S.; Irkec, C. Evaluating sub-clinical cognitive dysfunction and event-related potentials (P300) in clinically isolated syndrome. Neurol. Sci. 2008, 29, 435–444. [Google Scholar] [CrossRef]
- Pokryszko-Dragan, A.; Dziadkowiak, E.; Zagrajek, M.; Slotwinski, K.; Gruszka, E.; Bilinska, M.; Podemski, R. Cognitive performance, fatigue and event-related potentials in patients with clinically isolated syndrome. Clin. Neurol. Neurosurg. 2016, 149, 68–74. [Google Scholar] [CrossRef]
- Kiiski, H.; Reilly, R.B.; Lonergan, R.; Kelly, S.; O’Brien, M.C.; Kinsella, K.; Bramham, J.; Burke, T.; ODonnchadha, S.; Nolan, H.; et al. Only low frequency event-related EEG activity is compromised in multiple sclerosis: Insights from an independent component clustering analysis. PLoS ONE 2012, 7, e45536. [Google Scholar] [CrossRef]
- Sundgren, M.; Nikulin, V.V.; Maurex, L.; Wahlin, L.; Piehl, F.; Brismar, T. P300 amplitude and response speed relate to preserved cognitive function in relapsing-remitting multiple sclerosis. Clin. Neurophysiol. 2015, 126, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Whelan, R.; Lonergan, R.; Kiiski, H.; Nolan, H.; Kinsella, K.; Bramham, J.; O’Brien, M.; Reilly, R.B.; Hutchinson, M.; Tubridy, N. A high-density ERP study reveals latency, amplitude and topo graphical differences in multiple sclerosis patients versus controls. Clin. Neurophysiol. 2010, 121, 1420–1426. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Sanders, E.A.; Reulen, J.P.; Van der Velde, E.A.; Hogenhuis, L.A. The diagnosis of multiple sclerosis. Contribution of non clinical tests. J. Neurol. Sci. 1986, 72, 273–285. [Google Scholar] [CrossRef]
- Hase, Y.; Horsburgh, K.; Ihara, M.; Kalaria, R.N. White matter degeneration in vascular and other ageing-related dementias. J. Neurochem. 2018, 144, 617–633. [Google Scholar] [CrossRef]
- Lublin, F.D. New multiple sclerosis phenotypic classification. Eur. Neurol. 2014, 72 (Suppl. 1), 1–5. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Olsson, T.; Barcellos, L.F.; Alfredsson, L. Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat. Rev. Neurol. 2017, 13, 25–36. [Google Scholar] [CrossRef]
- Boringa, J.B.; Lazeron, R.H.; Reuling, I.E.; Adèr, H.J.; Pfennings, L.; Lindeboom, J.; de Sonneville, L.M.; Kalkers, N.F.; Polman, C.H. The brief repeatable battery of neuropsychological tests: Normative values allow application in multiple sclerosis clinical practice. Mult. Scler. 2001, 7, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Tombaugh, T.N. Trail Making Test A and B: Normative data stratified by age and education. Arch. Clin. Neuropsychol. 2004, 19, 203–214. [Google Scholar] [CrossRef]
- Amato, M.P.; Portaccio, E.; Goretti, B.; Zipoli, V.; Ricchiuti, L.; De Caro, M.F.; Patti, F.; Vecchio, R.; Sorbi, S.; Trojano, M. The Rao’s Brief Repeatable Battery and Stroop Test: Normative values with age, education and gender corrections in an Italian population. Mult. Scler. 2006, 12, 787–793. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Brown, G.K. Manual for Beck Depression Inventory-II; Psychological Corporation: San Antonio, TX, USA, 1996. [Google Scholar]
- Maris, E.; Oostenveld, R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods 2007, 164, 177–190. [Google Scholar] [CrossRef]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef]
- Waliszewska-Prosół, M.; Nowakowska-Kotas, M.; Kotas, R.; Bańkowski, T.; Pokryszko-Dragan, A.; Podemski, R. The relationship between event-related potentials, stress perception and personality type in patients with multiple sclerosis without cognitive impairment: A pilot study. Adv. Clin. Exp. Med. 2018, 27, 787–794. [Google Scholar] [CrossRef]
- Artemiadis, A.K.; Anagnostouli, M.C.; Zalonis, I.G.; Chairopoulos, K.G.; Triantafyllou, N.I. Structural MRI Correlates of Cognitive Event-Related Potentials in Multiple Sclerosis. J. Clin. Neurophysiol. 2018, 35, 399–407. [Google Scholar] [CrossRef]
- de Tommaso, M.; Betti, V.; Bocci, T.; Bolognini, N.; Di Russo, F.; Fattapposta, F.; Ferri, R.; Invitto, S.; Koch, G.; Miniussi, C.; et al. Pearls and pitfalls in brain functional analysis by event-related potentials: A narrative review by the Italian Psychophysiology and Cognitive Neuroscience Society on methodological limits and clinical reliability-part I. Neurol. Sci. 2020, 41, 2711–2735. [Google Scholar] [CrossRef]
- de Tommaso, M.; Betti, V.; Bocci, T.; Bolognini, N.; Di Russo, F.; Fattapposta, F.; Ferri, R.; Invitto, S.; Koch, G.; Miniussi, C.; et al. Pearl and pitfalls in brain functional analysis by event-related potentials: A narrative review by the Italian Psychophysiology and Cognitive Neuroscience Society on methodological limits and clinical reliability-part II. Neurol. Sci. 2020, 41, 3503–3515. [Google Scholar] [CrossRef]
- Linden, D.E.J. The P300: Where in the Brain Is It Produced and What Does It Tell Us? Neuroscientist 2005, 11, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Kiiski, H.; Whelan, R.; Lonergan, R.; Nolan, H.; Kinsella, K.; Hutchinson, M.; Tubridy, N.; Reilly, R.B. Preliminary evidence for correlation between PASAT performance and P3a and P3b amplitudes in progressive multiple sclerosis. Eur. J. Neurol. 2011, 18, 792–795. [Google Scholar] [CrossRef]


| Patient (n = 16) | Controls (n = 19) | p Value | |
|---|---|---|---|
| Sex, n° (%) | |||
| F | 14 (87.50%) | 13 (68.42%) | 0.19 × |
| M | 2 (12.50%) | 6 (31.58%) | |
| Age (years) * | 36.5 ± 7.5 | 36.0 ± 16.0 | 0.98 ° |
| Education (years) * | 13 ± 1 | 13 ± 4 | 0.49 ° |
| Duration of disease (months) * | 11.44 ± 19.96 | ||
| EDSS * | 2.0 ± 1 | ||
| Clinical phenotype | |||
| CIS, n° (%) | 1 (6.25%) | ||
| RRMS, n° (%) | 15 (93.75%) |
| Mean | Error DS | 95% CI | ANOVA | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| PASAT | |||||
| Control | 140.000 | 9.162 | 121.338 | 158.662 | F 5.21 |
| Patient | 109.500 | 9.717 | 89.706 | 129.294 | P 0.029 |
| SDMT | |||||
| Control | 52.611 | 3.303 | 45.884 | 59.339 | F 1.32 |
| Patient | 47.063 | 3.503 | 39.927 | 54.198 | P 0.25 |
| FSS | |||||
| Control | 1.139 | 0.408 | 0.308 | 1.970 | F 6.29 |
| Patient | 2.631 | 0.433 | 1.750 | 3.513 | P 0.017 |
| BDI | |||||
| Control | 2.72 | 1.13 | 0.4 | 5.04 | F 4.51 |
| Patient | 6.25 | 1.21 | 3.79 | 8.71 | P 0.041 |
| pw (n = 16) | HCs (n = 15) | p-Value | |
|---|---|---|---|
| Attention and processing speed | 121 [122–142] | 157 [125.5–165] | p < 0.01 |
| Memory | 105 [84.5–110] | 122 [101–135] | p = 0.16 |
| Verbal Fluency | 19 [15–20.5] | 23 [19–25] | p = 0.08 |
| Executive Functions | 122 [75–131] | 83 [66.5–84] | p = 0.44 |
| Patients (n = 16) | Controls (n = 19) | ANOVA: F | p-Value | |
|---|---|---|---|---|
| P300 latency (msec) | 326.15 ± 28.85 | 311.37 ± 31.3 | 1.66 | 0.21 |
| P300 amplitude (uV) | 5.7 ± 4 | 6.7 ± 3.4 | 0.61 | 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paolicelli, D.; Manni, A.; Iaffaldano, A.; Tancredi, G.; Ricci, K.; Gentile, E.; Viterbo, R.G.; Quitadamo, S.G.; de Tommaso, M.; Trojano, M. Magnetoencephalography and High-Density Electroencephalography Study of Acoustic Event Related Potentials in Early Stage of Multiple Sclerosis: A Pilot Study on Cognitive Impairment and Fatigue. Brain Sci. 2021, 11, 481. https://doi.org/10.3390/brainsci11040481
Paolicelli D, Manni A, Iaffaldano A, Tancredi G, Ricci K, Gentile E, Viterbo RG, Quitadamo SG, de Tommaso M, Trojano M. Magnetoencephalography and High-Density Electroencephalography Study of Acoustic Event Related Potentials in Early Stage of Multiple Sclerosis: A Pilot Study on Cognitive Impairment and Fatigue. Brain Sciences. 2021; 11(4):481. https://doi.org/10.3390/brainsci11040481
Chicago/Turabian StylePaolicelli, Damiano, Alessia Manni, Antonio Iaffaldano, Giusy Tancredi, Katia Ricci, Eleonora Gentile, Rosa Gemma Viterbo, Silvia Giovanna Quitadamo, Marina de Tommaso, and Maria Trojano. 2021. "Magnetoencephalography and High-Density Electroencephalography Study of Acoustic Event Related Potentials in Early Stage of Multiple Sclerosis: A Pilot Study on Cognitive Impairment and Fatigue" Brain Sciences 11, no. 4: 481. https://doi.org/10.3390/brainsci11040481
APA StylePaolicelli, D., Manni, A., Iaffaldano, A., Tancredi, G., Ricci, K., Gentile, E., Viterbo, R. G., Quitadamo, S. G., de Tommaso, M., & Trojano, M. (2021). Magnetoencephalography and High-Density Electroencephalography Study of Acoustic Event Related Potentials in Early Stage of Multiple Sclerosis: A Pilot Study on Cognitive Impairment and Fatigue. Brain Sciences, 11(4), 481. https://doi.org/10.3390/brainsci11040481








