Enhancing the Effects of Neurofeedback Training: The Motivational Value of the Reinforcers
Abstract
1. Introduction
The Role of Reinforcers in Operant Conditioning
2. Materials and Methods
2.1. Participants
2.2. Measures
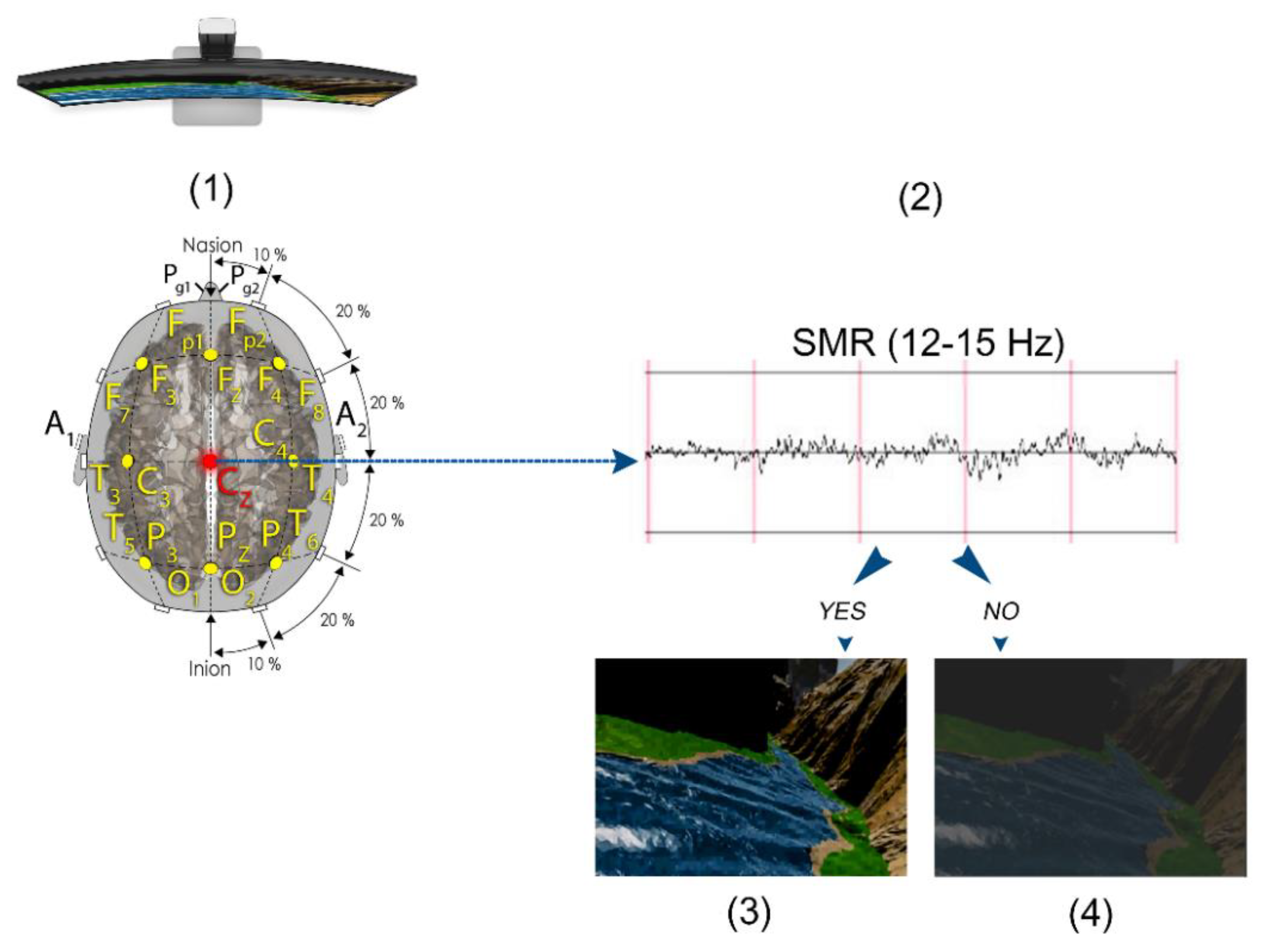
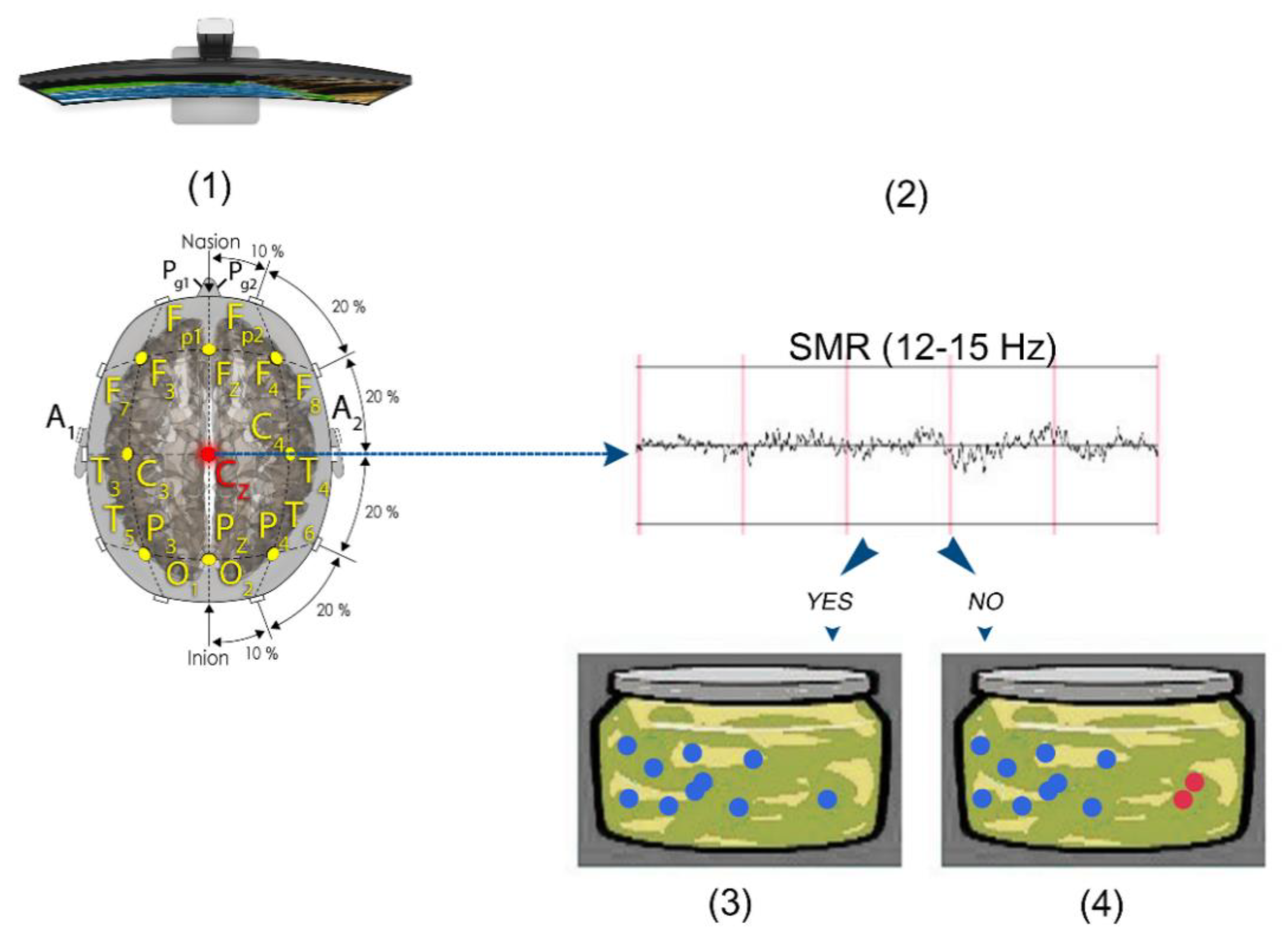
2.2.1. EEG Collection and QEEG Analysis
2.2.2. Montreal Cognitive Assessment (MoCA)
2.2.3. Visual Analogue Scale (VAS) for Reinforcer Rating
2.3. Procedure
2.4. SMR Training Neurofeedback
2.5. Statistical Analyses
3. Results
3.1. Differences between Groups
3.2. Effects of Reinforcers on NF
3.3. Reinforcer Rating
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Markram, H. Seven Challenges for Neuroscience. Funct. Neurol. 2013, 28, 145–151. [Google Scholar] [CrossRef]
- Battistin, L. Reflections of a Clinician on the Current Trends in Clinical Neuroscience—Molecular Neurobiology and/or Connectome? Eur. Neurol. Rev. 2018, 13, 16. [Google Scholar] [CrossRef]
- Watanabe, T.; Sasaki, Y.; Shibata, K.; Kawato, M. Advances in FMRI Real-Time Neurofeedback. Trends Cogn. Sci. 2017, 21, 997–1010. [Google Scholar] [CrossRef]
- Carrobles, J.A. Bio/neurofeedback. Clínica Salud 2016, 27, 125–131. [Google Scholar] [CrossRef]
- Lubar, J.F.; Swartwood, M.O.; Swartwood, J.N.; O’Donnell, P.H. Evaluation of the Effectiveness of EEG Neurofeedback Training for ADHD in a Clinical Setting as Measured by Changes in T.O.V.A. Scores, Behavioral Ratings, and WISC-R Performance. Biofeedback Self-Regul. 1995, 20, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Lubar, J.F.; Shouse, M.N. EEG and Behavioral Changes in a Hyperkinetic Child Concurrent with Training of the Sensorimotor Rhythm (SMR): A Preliminary Report. Biofeedback Self-Regul. 1976, 1, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Arns, M.; Drinkenburg, W.; Leon Kenemans, J. The Effects of QEEG-Informed Neurofeedback in ADHD: An Open-Label Pilot Study. Appl. Psychophysiol. Biofeedback 2012, 37, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Hammer, B.U.; Colbert, A.P.; Brown, K.A.; Ilioi, E.C. Neurofeedback for Insomnia: A Pilot Study of Z-Score SMR and Individualized Protocols. Appl. Psychophysiol. Biofeedback 2011, 36, 251–264. [Google Scholar] [CrossRef]
- Pérez-Elvira, R.; Carrobles, J.; López Bote, D.; Oltra-Cucarella, J. Efficacy of Live Z-Score Neurofeedback Training for Chronic Insomnia: A Single-Case Study. NeuroRegulation 2019, 6, 93–101. [Google Scholar] [CrossRef]
- Pérez-Elvira, R.; Oltra-Cucarella, J.; Carrobles, J.A.; Teodoru, M.; Bacila, C.; Neamtu, B. Individual Alpha Peak Frequency, an Important Biomarker for Live Z-Score Training Neurofeedback in Adolescents with Learning Disabilities. Brain Sci. 2021, 11, 167. [Google Scholar] [CrossRef]
- Fernández, T.; Bosch-Bayard, J.; Harmony, T.; Caballero, M.I.; Díaz-Comas, L.; Galán, L.; Ricardo-Garcell, J.; Aubert, E.; Otero-Ojeda, G. Neurofeedback in Learning Disabled Children: Visual versus Auditory Reinforcement. Appl. Psychophysiol. Biofeedback 2016, 41, 27–37. [Google Scholar] [CrossRef]
- Walker, J.E. QEEG-Guided Neurofeedback for Recurrent Migraine Headaches. Clin. Eeg Neurosci. 2011, 42, 59–61. [Google Scholar] [CrossRef]
- Cheon, E.-J.; Koo, B.-H.; Choi, J.-H. The Efficacy of Neurofeedback in Patients with Major Depressive Disorder: An Open Labeled Prospective Study. Appl. Psychophysiol. Biofeedback 2016, 41, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Peeters, F.; Oehlen, M.; Ronner, J.; van Os, J.; Lousberg, R. Neurofeedback As a Treatment for Major Depressive Disorder—A Pilot Study. PLoS ONE 2014, 9, e91837. [Google Scholar] [CrossRef]
- Hammond, D.C. Neurofeedback Treatment of Depression and Anxiety. J. Adult Develpment 2005, 12, 131–137. [Google Scholar] [CrossRef]
- Hammond, C. Neurofeedback with Anxiety and Affective Disorders. Child. Adolesc. Psychiatr. Clin. N. Am. 2005, 14, 105–123. [Google Scholar] [CrossRef]
- Weber, L.A.; Ethofer, T.; Ehlis, A.-C. Predictors of Neurofeedback Training Outcome: A Systematic Review. Neuroimage Clin. 2020, 27, 102301. [Google Scholar] [CrossRef]
- Bazanova, O.M.; Auer, T.; Sapina, E.A. On the Efficiency of Individualized Theta/Beta Ratio Neurofeedback Combined with Forehead EMG Training in ADHD Children. Front. Hum. Neurosci. 2018, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Reichert, J.L.; Kober, S.E.; Neuper, C.; Wood, G. Resting-State Sensorimotor Rhythm (SMR) Power Predicts the Ability to up-Regulate SMR in an EEG-Instrumental Conditioning Paradigm. Clin. Neurophysiol. 2015, 126, 2068–2077. [Google Scholar] [CrossRef]
- Nan, W.; Wan, F.; Vai, M.I.; Da Rosa, A.C. Resting and Initial Beta Amplitudes Predict Learning Ability in Beta/Theta Ratio Neurofeedback Training in Healthy Young Adults. Front. Hum. Neurosci. 2015, 9. [Google Scholar] [CrossRef]
- Alkoby, O.; Abu-Rmileh, A.; Shriki, O.; Todder, D. Can We Predict Who Will Respond to Neurofeedback? A Review of the Inefficacy Problem and Existing Predictors for Successful EEG Neurofeedback Learning. Neuroscience 2018, 378, 155–164. [Google Scholar] [CrossRef]
- Diaz Hernandez, L.; Rieger, K.; Koenig, T. Low Motivational Incongruence Predicts Successful EEG Resting-State Neurofeedback Performance in Healthy Adults. Neuroscience 2018, 378, 146–154. [Google Scholar] [CrossRef]
- Wangler, S.; Gevensleben, H.; Albrecht, B.; Studer, P.; Rothenberger, A.; Moll, G.H.; Heinrich, H. Neurofeedback in Children with ADHD: Specific Event-Related Potential Findings of a Randomized Controlled Trial. Clin. Neurophysiol. 2011, 122, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Sherlin, L.H.; Arns, M.; Lubar, J.; Heinrich, H.; Kerson, C.; Strehl, U.; Sterman, M.B. Neurofeedback and Basic Learning Theory: Implications for Research and Practice. J. Neurother. 2011, 15, 292–304. [Google Scholar] [CrossRef]
- Domjan, M. The Essentials of Conditioning and Learning, 4th ed.; American Psychological Association: Washington, DC, USA, 2018; ISBN 978-1-4338-2778-5. [Google Scholar]
- Hutt, P.J. Rate of Bar Pressing as a Function of Quality and Quantity of Food Reward. J. Comp. Physiol. Psychol. 1954, 47, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Hulse, S.H. Reinforcement Contrast Effects in Rats Following Experimental Definition of a Dimension of Reinforcement Magnitude. J. Comp. Physiol. Psychol. 1973, 85, 160–170. [Google Scholar] [CrossRef]
- Hoedlmoser, K.; Pecherstorfer, T.; Gruber, G.; Anderer, P.; Doppelmayr, M.; Klimesch, W.; Schabus, M. Instrumental Conditioning of Human Sensorimotor Rhythm (12–15 Hz) and Its Impact on Sleep as Well as Declarative Learning. Sleep 2008, 31, 1401–1408. [Google Scholar]
- Schabus, M.; Griessenberger, H.; Gnjezda, M.-T.; Heib, D.P.J.; Wislowska, M.; Hoedlmoser, K. Better than Sham? A Double-Blind Placebo-Controlled Neurofeedback Study in Primary Insomnia. Brain 2017, 140, 1041–1052. [Google Scholar] [CrossRef]
- Gadea, M.; Aliño, M.; Hidalgo, V.; Espert, R.; Salvador, A. Effects of a Single Session of SMR Neurofeedback Training on Anxiety and Cortisol Levels. Neurophysiol. Clin. 2020, 50, 167–173. [Google Scholar] [CrossRef]
- Koski, L.; Xie, H.; Konsztowicz, S. Improving Precision in the Quantification of Cognition Using the Montreal Cognitive Assessment and the Mini-Mental State Examination. Int. Psychogeriatr. 2011, 23, 1107–1115. [Google Scholar] [CrossRef]
- Koski, L.; Xie, H.; Finch, L. Measuring Cognition in a Geriatric Outpatient Clinic: Rasch Analysis of the Montreal Cognitive Assessment. J. Geriatr. Psychiatry Neurol. 2009, 22, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool For Mild Cognitive Impairment: MOCA: A BRIEF SCREENING TOOL FOR MCI. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Ojeda del Pozo, N.; del Pino Sáez, R.; Ibarretxe Bilbao, N.; Schretlen, D.J.; Peña Lasa, J. Test de evaluación cognitiva de Montreal: Normalización y estandarización de la prueba en población española. Rev. Neurol. 2016, 63, 488. [Google Scholar] [CrossRef]
- Soutar, R.G.; Longo, R.E. Doing Neurofeedback: An. Introduction; ISNR Research Foundation: San Rafael, CA, USA, 2011; ISBN 978-0-9846085-4-6. [Google Scholar]
- Demos, J.N. Getting Started with Neurofeedback, 1st ed.; W.W. Norton: New York, NY, USA, 2005; ISBN 978-0-393-70450-1. [Google Scholar]
- Richardson, J.T.E. Eta Squared and Partial Eta Squared as Measures of Effect Size in Educational Research. Educ. Res. Rev. 2011, 6, 135–147. [Google Scholar] [CrossRef]
- Fritz, C.O.; Morris, P.E.; Richler, J.J. Effect Size Estimates: Current Use, Calculations, and Interpretation. J. Exp. Psychol. Gen. 2012, 141, 2–18. [Google Scholar] [CrossRef]
- Fisher, W.; Piazza, C.C.; Bowman, L.G.; Hagopian, L.P.; Owens, J.C.; Slevin, I. A Comparison of Two Approaches for Identifying Reinforcers for Persons with Severe and Profound Disabilities. J. Appl. Behav. Anal. 1992, 25, 491–498. [Google Scholar] [CrossRef]
- Burde, W.; Blankertz, B. Is the Locus of Control of Reinforcement a Predictor of Brain-Computer Interface Performance? In Proceedings of the 3th International Brain-Computer Interface Workshop and Training Course, Graz University of Technology, Graz, Austria, 21–24 September 2006; pp. 76–77. [Google Scholar]
- Flaherty, C.F. Incentive Contrast: A Review of Behavioral Changes Following Shifts in Reward. Anim. Learn. Behav. 1982, 10, 409–440. [Google Scholar] [CrossRef]
- Flaherty, C.F. Incentive Relativity; Cambridge University Press: Cambridge, UK, 1999; ISBN 978-0-521-65863-8. [Google Scholar]
- Hamilton, A.; Stellar, J.; Hart, E. Reward, Performance, and the Response Strength Method in Self-Stimulating Rats: Validation and Neuroleptics. Physiol. Behav. 1985, 35, 897–904. [Google Scholar] [CrossRef]
- Cohen, M.X.; Elger, C.E.; Ranganath, C. Reward Expectation Modulates Feedback-Related Negativity and EEG Spectra. NeuroImage 2007, 35, 968–978. [Google Scholar] [CrossRef]
- Vachon-Presseau, E.; Achim, A.; Benoit-Lajoie, A. Direction of SMR and Beta Change with Attention in Adults. J. Neurother. 2009, 13, 22–29. [Google Scholar] [CrossRef]
- Estes, W.K. Human learning and memory. In Stevens’ Handbook of Experimental Psychology: Perception and Motivation; Learning and Cognition, 2nd ed.; John Wiley & Sons: Oxford, UK, 1988; Volume 1–2, pp. 351–415. ISBN 0-471-04203-X. [Google Scholar]
- Wearden, J.H. Some neglected problems in the analysis of human operant behavior. In Human Operant Conditioning and Behavior Modification; John Wiley & Sons: Oxford, UK, 1988; pp. 197–224. ISBN 0-471-91637-4. [Google Scholar]
- Mangum, A.; Fredrick, L.; Pabico, R.; Roane, H. The Role of Context in the Evaluation of Reinforcer Efficacy: Implications for the Preference Assessment Outcomes. Res. Autism Spectr. Disord. 2012, 6, 158–167. [Google Scholar] [CrossRef]
- Piazza, C.C.; Fisher, W.W.; Hagopian, L.P.; Bowman, L.G.; Toole, L. USING A CHOICE ASSESSMENT TO PREDICT REINFORCER EFFECTIVENESS. J. Appl. Behav. Anal. 1996, 29, 1–9. [Google Scholar] [CrossRef]
- Svartdal, F.; Mortensen, T. Effects of Reinforcer Value on Sensitivity to Non-Verbal Operant Contingencies in Humans. Q. J. Exp. Psychol. Sect. A 1993, 46, 347–364. [Google Scholar] [CrossRef]
- Killeen, P.R.; Jacobs, K.W. Coal Is Not Black, Snow Is Not White, Food Is Not a Reinforcer: The Roles of Affordances and Dispositions in the Analysis of Behavior. Behav. Anal. 2017, 40, 17–38. [Google Scholar] [CrossRef]
- Strehl, U. What Learning Theories Can Teach Us in Designing Neurofeedback Treatments. Front. Hum. Neurosci. 2014, 8. [Google Scholar] [CrossRef]
- Dworkin, B. Operant Mechanisms in Physiological Regulation. In Self Regulation of the Brain and Behavior; Elbert, T., Rockstroh, B., Lutzenberger, W., Birbaumer, N., Eds.; Springer: Berlin/Heidelberg, Germany, 1984; pp. 296–309. ISBN 978-3-642-69381-6. [Google Scholar]
- Dragoi, V.; Staddon, J.E.R. The Dynamics of Operant Conditioning. Psychol. Rev. 1999, 106, 20–61. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.B. Learning: Principles and Applications, 7th ed.; SAGE: Los Angeles, CA, USA, 2015; ISBN 978-1-4522-7194-1. [Google Scholar]
- Siniatchkin, M.; Kropp, P.; Gerber, W.-D. Neurofeedback—The Significance of Reinforcement and the Search for an Appropriate Strategy for the Success of Self Regulation. Appl. Psychophysiol. Biofeedback 2000, 25, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Schabus, M.; Heib, D.P.J.; Lechinger, J.; Griessenberger, H.; Klimesch, W.; Pawlizki, A.; Kunz, A.B.; Sterman, B.M.; Hoedlmoser, K. Enhancing Sleep Quality and Memory in Insomnia Using Instrumental Sensorimotor Rhythm Conditioning. Biol. Psychol. 2014, 95, 126–134. [Google Scholar] [CrossRef]
- Weber, E.; Köberl, A.; Frank, S.; Doppelmayr, M. Predicting Successful Learning of SMR Neurofeedback in Healthy Participants: Methodological Considerations. Appl. Psychophysiol. Biofeedback 2011, 36, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Escolano, C.; Navarro-Gil, M.; Garcia-Campayo, J.; Minguez, J. The Effects of a Single Session of Upper Alpha Neurofeedback for Cognitive Enhancement: A Sham-Controlled Study. Appl. Psychophysiol. Biofeedback 2014, 39, 227–236. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Kim, H.-G.; Cheon, E.-J.; Kim, K.; Choi, J.-H.; Kim, J.-Y.; Kim, J.-M.; Koo, B.-H. The Analysis of Electroencephalography Changes Before and After a Single Neurofeedback Alpha/Theta Training Session in University Students. Appl. Psychophysiol. Biofeedback 2019, 44, 173–184. [Google Scholar] [CrossRef]
- MacDuffie, K.E.; MacInnes, J.; Dickerson, K.C.; Eddington, K.M.; Strauman, T.J.; Adcock, R.A. Single Session Real-Time FMRI Neurofeedback Has a Lasting Impact on Cognitive Behavioral Therapy Strategies. Neuroimage Clin. 2018, 19, 868–875. [Google Scholar] [CrossRef]
- Krigbaum, G.; Wigton, N.L. When Discussing Neurofeedback, Does Modality Matter? NeuroRegulation 2014, 1, 48–60. [Google Scholar] [CrossRef]
- Autenrieth, M.; Kober, S.E.; Neuper, C.; Wood, G. How Much Do Strategy Reports Tell About the Outcomes of Neurofeedback Training? A Study on the Voluntary Up-Regulation of the Sensorimotor Rhythm. Front. Hum. Neurosci. 2020, 14, 218. [Google Scholar] [CrossRef]
| Imposed (n = 45) | Selected (n = 48) | Placebo (n = 20) | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | ||
| Sex | Male | 21 | 46.7 | 19 | 39.6 | 9 | 45 | 49 | 43.36 |
| Female | 24 | 53.3 | 29 | 60.4 | 11 | 55 | 64 | 56.63 | |
| Education | Low | 1 | 2.2 | 2 | 4.2 | 1 | 5 | 4 | 3.53 |
| Middle | 10 | 22.2 | 9 | 18.8 | 8 | 40 | 27 | 23.89 | |
| High | 34 | 75.6 | 37 | 77.1 | 11 | 55 | 82 | 72.56 | |
| Imposed (n = 45) | Selected (n = 48) | Placebo (n = 20) | Total | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age | 38.42 | 12.17 | 35.90 | 12.02 | 27.05 | 3.25 | 35.38 | 11.61 |
| MoCA | 28.18 | 1.60 | 28.17 | 1.42 | 27.55 | 0.94 | 28.06 | 1.43 |
| SMR Pre-treatment | 4.54 | 1.64 | 4.53 | 1.40 | 4.51 | 0.83 | 4.53 | 1.41 |
| SMR Post-treatment | 4.60 | 1.77 | 4.79 | 1.44 | 4.41 | 0.89 | 4.64 | 1.50 |
| Reinforcer rating | 4.11 | 3.01 | 7.79 | 1.93 | 7.3 | 1.55 | 6.24 | 2.93 |
| Imposed | Selected | Placebo | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low Feedback | High Feedback | Low Feedback | High Feedback | Low Feedback | High Feedback | |||||||
| Variables | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| SMR Pre-treatment | 4.50 | 1.84 | 4.61 | 1.25 | 4.55 | 1.32 | 4.52 | 1.44 | 4.99 | 0.15 | 4.45 | 0.86 |
| SMR Post-treatment | 4.64 | 2.01 | 4.53 | 1.27 | 4.61 | 1.45 | 4.83 | 1.46 | 5.05 | 0.24 | 4.33 | 0.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Elvira, R.; Oltra-Cucarella, J.; Carrobles, J.A.; Moltó, J.; Flórez, M.; Parra, S.; Agudo, M.; Saez, C.; Guarino, S.; Costea, R.M.; et al. Enhancing the Effects of Neurofeedback Training: The Motivational Value of the Reinforcers. Brain Sci. 2021, 11, 457. https://doi.org/10.3390/brainsci11040457
Pérez-Elvira R, Oltra-Cucarella J, Carrobles JA, Moltó J, Flórez M, Parra S, Agudo M, Saez C, Guarino S, Costea RM, et al. Enhancing the Effects of Neurofeedback Training: The Motivational Value of the Reinforcers. Brain Sciences. 2021; 11(4):457. https://doi.org/10.3390/brainsci11040457
Chicago/Turabian StylePérez-Elvira, Rubén, Javier Oltra-Cucarella, José Antonio Carrobles, Jorge Moltó, Mercedes Flórez, Salvador Parra, María Agudo, Clara Saez, Sergio Guarino, Raluca Maria Costea, and et al. 2021. "Enhancing the Effects of Neurofeedback Training: The Motivational Value of the Reinforcers" Brain Sciences 11, no. 4: 457. https://doi.org/10.3390/brainsci11040457
APA StylePérez-Elvira, R., Oltra-Cucarella, J., Carrobles, J. A., Moltó, J., Flórez, M., Parra, S., Agudo, M., Saez, C., Guarino, S., Costea, R. M., & Neamtu, B. (2021). Enhancing the Effects of Neurofeedback Training: The Motivational Value of the Reinforcers. Brain Sciences, 11(4), 457. https://doi.org/10.3390/brainsci11040457





