Distinctive Frontal and Occipitotemporal Surface Features in Neglectful Parenting
Abstract
1. Introduction
1.1. Neuroanatomical Correlates of Neglectful Caregiving
1.2. Relations between Brain Features, Personality Traits and Quality of Mother-Child Interaction
2. Materials and Methods
2.1. Participants
2.2. MRI Data Acquisition
2.3. Cortical Thickness and Surface Area
2.4. Behavioral and Personality Measures
2.5. Statistical Analyses for Brain Measures
2.6. Statistical Analyses for Cortical-EA Associations
3. Results
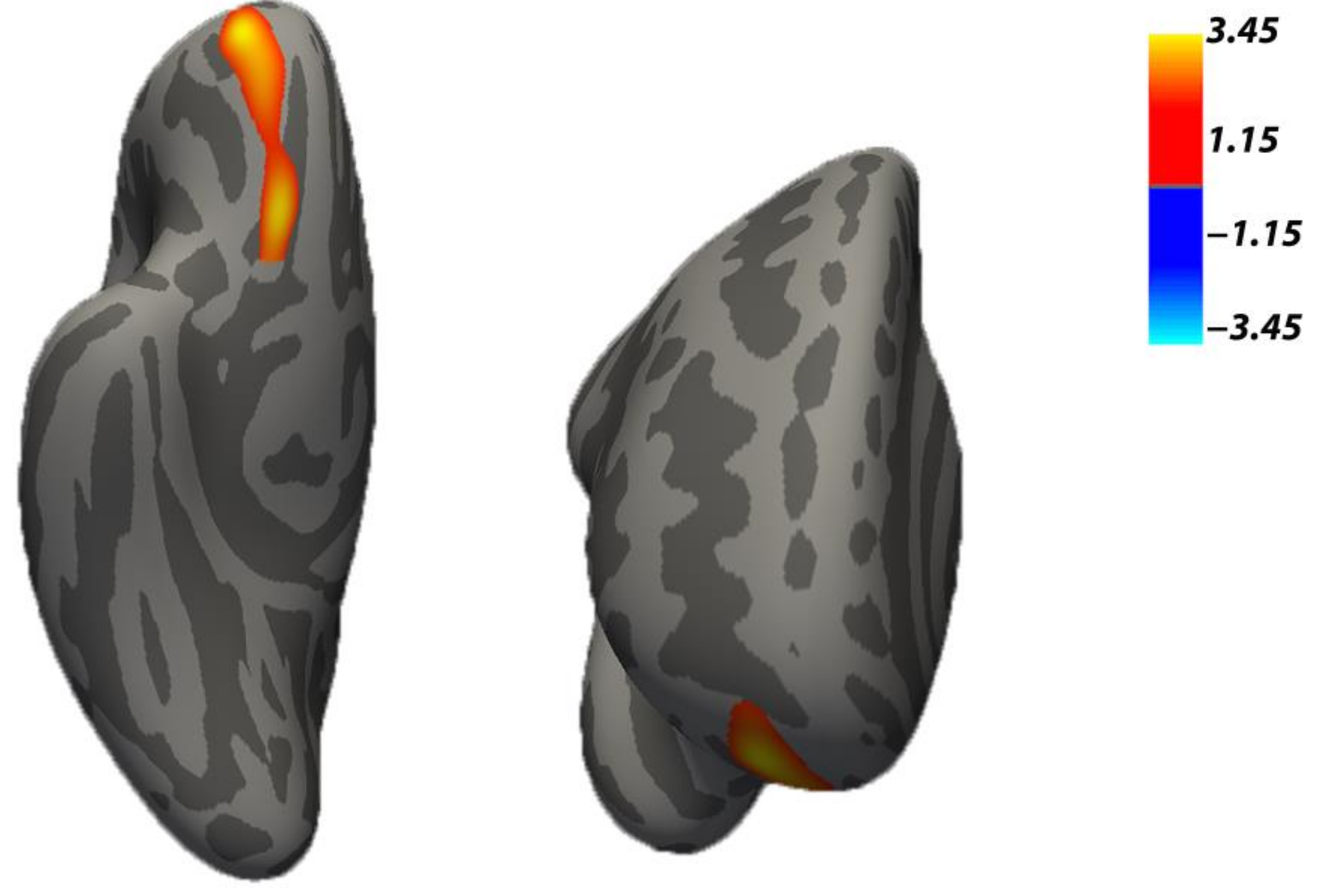
3.1. Differential Cortical Features in Neglect and Control Groups
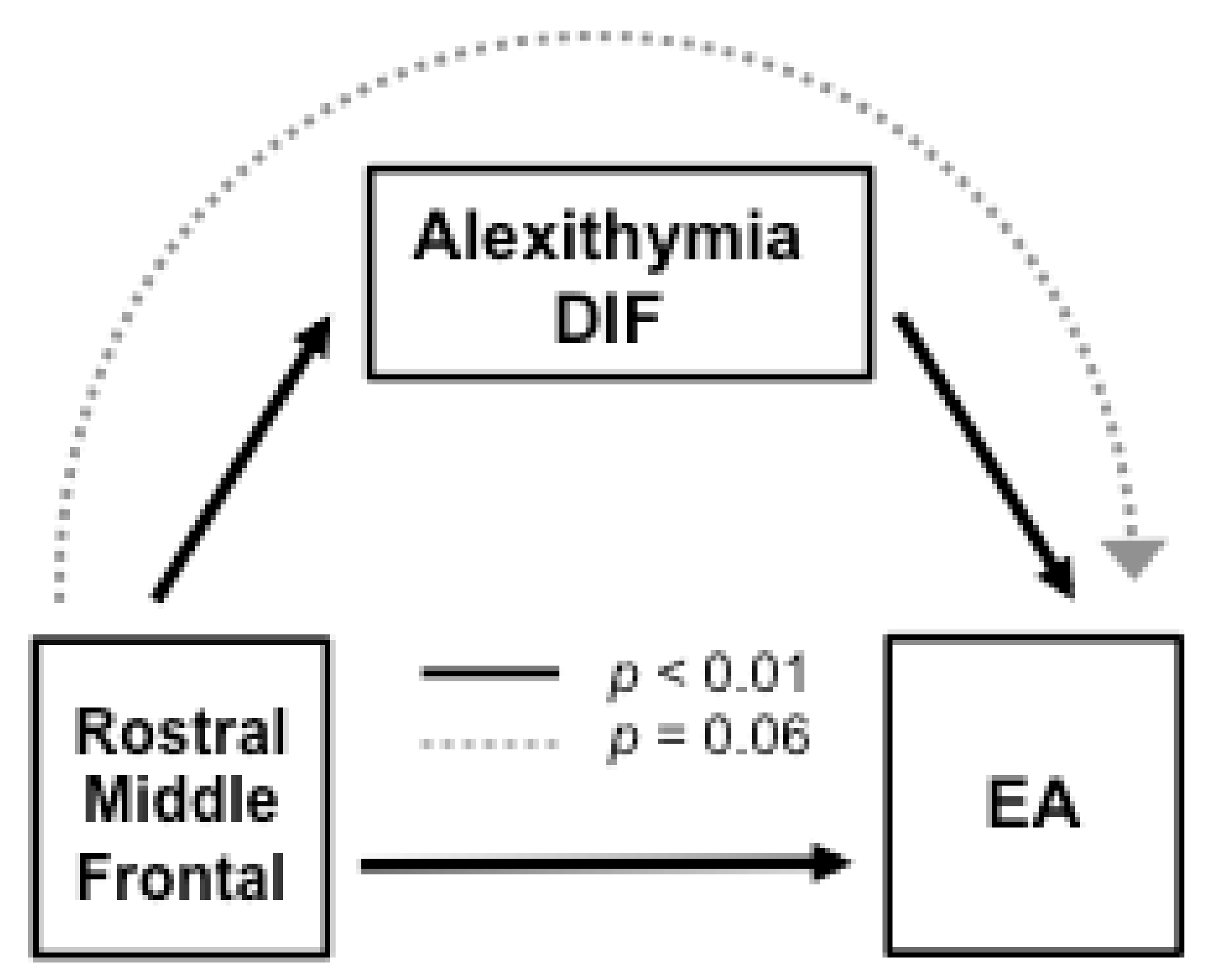
3.2. Role of Alexithymia in Cortical-EA Associations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petersen, A.C.; Joseph, J.; Feit, M. New Directions in Child Abuse and Neglect Research; National Academies Press (US): Washington, DC, USA, 2014. [Google Scholar] [CrossRef]
- Stoltenborgh, M.; Bakermans-Kranenburg, M.J.; van Ijzendoorn, M.H. The Neglect of Child Neglect: A Meta-Analytic Review of the Prevalence of Neglect. Soc. Psychiatry Psychiatr. Epidemiol. 2013, 48, 345–355. [Google Scholar] [CrossRef]
- Nemeroff, C.B. Paradise Lost: The Neurobiological and Clinical Consequences of Child Abuse and Neglect. Neuron 2016, 89, 892–909. [Google Scholar] [CrossRef] [PubMed]
- Teicher, M.H.; Samson, J.A.; Anderson, C.M.; Ohashi, K. The Effects of Childhood Maltreatment on Brain Structure, Function and Connectivity. Nat. Rev. Neurosci. 2016, 17, 652–666. [Google Scholar] [CrossRef]
- Feldman, R. The Adaptive Hum. Parental Brain: Implications for Children’s Social Development. Trends Neurosci. 2015, 38, 387–399. [Google Scholar] [CrossRef]
- León, I.; Rodrigo, M.J.; El-Deredy, W.; Modroño, C.; Hernández-Cabrera, J.A.; Quiñones, I. Limbic-Visual Attenuation to Crying Faces Underlies Neglectful Mothering. Sci. Rep. 2019, 9, 6373. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, M.J.; León, I.; García-Pentón, L.; Hernández-Cabrera, J.A.; Quiñones, I. Neglectful Maternal Caregiving Involves Altered Brain Volume in Empathy-Related Areas. Dev. Psychopathol. 2019, 1–10. [Google Scholar] [CrossRef]
- Niendam, T.A.; Laird, A.R.; Ray, K.L.; Dean, Y.M.; Glahn, D.C.; Carter, C.S. Meta-Analytic Evidence for a Superordinate Cognitive Control Network Subserving Diverse Executive Functions. Cogn. Affect. Behav. Neurosci. 2012, 12, 241–268. [Google Scholar] [CrossRef]
- Ochsner, K.N.; Silvers, J.A.; Buhle, J.T. Functional Imaging Studies of Emotion Regulation: A Synthetic Review and Evolving Model of the Cognitive Control of Emotion. Ann. N. Y. Acad. Sci. 2012, 1251, E1. [Google Scholar] [CrossRef]
- Crandall, A.; Deater-Deckard, K.; Riley, A.W. Maternal Emotion and Cognitive Control Capacities and Parenting: A Conceptual Framework. Dev. Rev. 2015, 36, 105–126. [Google Scholar] [CrossRef]
- Rutherford, H.J.; Wallace, N.S.; Laurent, H.K.; Mayes, L.C. Emotion Regulation in Parenthood. Dev. Rev. 2015, 36, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.; Strathearn, L.; Swain, J.E. The Maternal Brain and Its Plasticity in Hum.s. Horm. Behav. 2016, 77, 113–123. [Google Scholar] [CrossRef]
- Rodrigo, M.J.; León, I.; Quiñones, I.; Lage, A.; Byrne, S.; Bobes, M.A. Brain and Personality Bases of Insensitivity to Infant Cues in Neglectful Mothers: An Event-Related Potential Study. Dev. Psychopathol. 2011, 23, 163–176. [Google Scholar] [CrossRef]
- Winkler, A.M.; Sabuncu, M.R.; Yeo, B.T.; Fischl, B.; Greve, D.N.; Kochunov, P.; Nichols, T.E.; Blangero, J.; Glahn, D.C. Measuring and Comparing Brain Cortical Surface Area and Other Areal Quantities. Neuroimage 2012, 61, 1428–1443. [Google Scholar] [CrossRef]
- Carmona, S.; Martínez-García, M.; Paternina-Die, M.; Barba-Müller, E.; Wierenga, L.M.; Alemán-Gómez, Y.; Pretus, C.; Marcos-Vidal, L.; Beumala, L.; Cortizo, R.; et al. Pregnancy and Adolescence Entail Similar Neuroanatomical Adaptations: A Comparative Analysis of Cerebral Morphometric Changes. Hum. Brain Mapp. 2019, 40, 2143–2152. [Google Scholar] [CrossRef]
- Hoekzema, E.; Barba-Müller, E.; Pozzobon, C.; Picado, M.; Lucco, F.; García-García, D.; Soliva, J.C.; Tobeña, A.; Desco, M.; Crone, E.A.; et al. Pregnancy Leads to Long-Lasting Changes in Hum. Brain Structure. Nat. Neurosci. 2017, 20, 287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, M.; Zhang, J.; Du, X.; Chen, Z. Brain Structural Plasticity Associated with Maternal Caregiving in Mothers: A Voxel-and Surface-Based Morphometry Study. Neurodegener. Dis. 2019, 19, 192–203. [Google Scholar] [CrossRef]
- Kim, P.; Dufford, A.J.; Tribble, R.C. Cortical Thickness Variation of the Maternal Brain in the First 6 Months Postpartum: Associations with Parental Self-Efficacy. Brain Struct. Funct. 2018, 223, 3267–3277. [Google Scholar] [CrossRef]
- Biringen, Z. Emotional Availability: Conceptualization and Research Findings. Am. J. Orthopsychiatry 2000, 70, 104–114. [Google Scholar] [CrossRef]
- Biringen, Z.; Derscheid, D.; Vliegen, N.; Closson, L.; Easterbrooks, M.A. Emotional Availability (EA): Theoretical Background, Empirical Research Using the EA Scales, and Clinical Applications. Dev. Rev. 2014, 34, 114–167. [Google Scholar] [CrossRef]
- Altenhofen, S.; Clyman, R.; Little, C.; Baker, M.; Biringen, Z. Attachment Security in Three-Year-Olds Who Entered Substitute Care in Infancy. Infant. Ment. Health J. 2013, 34, 435–445. [Google Scholar] [CrossRef]
- Beebe, B.; Steele, M. How Does Microanalysis of Mother—Infant Communication Inform Maternal Sensitivity and Infant Attachment? Attach. Hum. Dev. 2013, 15, 583–602. [Google Scholar] [CrossRef] [PubMed]
- Biro, S.; Alink, L.R.; Huffmeijer, R.; Bakermans-Kranenburg, M.J.; Van IJzendoorn, M.H. Attachment Quality Is Related to the Synchrony of Mother and Infant Monitoring Patterns. Attach. Hum. Dev. 2017, 19, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, M.J.; León, I.; Góngora, D.; Hernández-Cabrera, J.A.; Byrne, S.; Bobes, M.A. Inferior Frontoto-Temporo-Occipital Connectivity: A Missing Link between Maltreated Girls and Neglectful Mothers. Soc. Cogn. Affect. Neurosci. 2016, 11, 1658–1665. [Google Scholar] [CrossRef]
- Herrero-Roldán, S.; Byrne, S.; Rodrigo, M.J.; Hernández-Cabrera, J.A.; León, I. Sesgos en la evaluación del llanto infantil en la negligencia materna: El papel de la alexitimia. Rev. Estud. Investig. Psicol. Educ. 2019, 6, 24–36. [Google Scholar] [CrossRef]
- Herbert, C.; Herbert, B.M.; Ethofer, T.; Pauli, P. His or Mine? The Time Course of Self—Other Discrimination in Emotion Processing. Soc. Neurosci. 2011, 6, 277–288. [Google Scholar] [CrossRef]
- Mantani, T.; Okamoto, Y.; Shirao, N.; Okada, G.; Yamawaki, S. Reduced Activation of Posterior Cingulate Cortex during Imagery in Subjects with High Degrees of Alexithymia: A Functional Magnetic Resonance Imaging Study. Biol. Psychiatry 2005, 57, 982–990. [Google Scholar] [CrossRef]
- Taylor, G.J. Recent Developments in Alexithymia Theory and Research. Can. J. Psychiatry 2000, 45, 134–142. [Google Scholar] [CrossRef]
- Lenzi, D.; Trentini, C.; Pantano, P.; Macaluso, E.; Lenzi, G.L.; Ammaniti, M. Attachment Models Affect Brain Responses in Areas Related to Emotions and Empathy in Nulliparous Women. Hum. Brain Mapp. 2013, 34, 1399–1414. [Google Scholar] [CrossRef]
- Lewis, G.J.; Dickie, D.A.; Cox, S.R.; Karama, S.; Evans, A.C.; Starr, J.M.; Bastin, M.E.; Wardlaw, J.M.; Deary, I.J. Widespread Associations between Trait Conscientiousness and Thickness of Brain Cortical Regions. Neuroimage 2018, 176, 22–28. [Google Scholar] [CrossRef]
- Miglin, R.; Bounoua, N.; Goodling, S.; Sheehan, A.; Spielberg, J.M.; Sadeh, N. Cortical Thickness Links Impulsive Personality Traits and Risky Behavior. Brain Sci. 2019, 9, 373. [Google Scholar] [CrossRef]
- Barnett, D.; Manly, J.T.; Cicchetti, D. Defining Child Maltreatment: The Interface between Policy and Research. Child. Abuse Child. Dev. Soc. Policy 1993, 8, 7–73. [Google Scholar]
- Dale, A.M. Optimal Experimental Design for Event-Related FMRI. Hum. Brain Mapp. 1999, 8, 109–114. [Google Scholar] [CrossRef]
- Fischl, B.; Dale, A.M. Measuring the Thickness of the Human Cerebral Cortex from Magnetic Resonance Images. Proc. Natl. Acad. Sci. USA 2000, 97, 11050–11055. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, L.; Bobes, J.; Gibert, J.; Soto, M.; Soto, O. 1.1. MINI Entrevista Neuropsiquiátrica Internacional (MINI International Neuropsychiatric Interview, MINI). Instrum. Detección Orientación Diagnóstica 2000, 1–25. [Google Scholar]
- Easterbrooks, M.A.; Biringen, Z. The Emotional Availability Scales: Methodological Refinements of the Construct and Clinical Implications Related to Gender and at-Risk Interactions. Infant Ment. Health J. 2005, 26, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Bressi, C.; Taylor, G.; Parker, J.; Bressi, S.; Brambilla, V.; Aguglia, E.; Allegranti, I.; Bongiorno, A.; Giberti, F.; Bucca, M.; et al. Cross Validation of the Factor Structure of the 20-Item Toronto Alexithymia Scale: An Italian Multicenter Study. J. Psychosom. Res. 1996, 41, 551–559. [Google Scholar] [CrossRef]
- Taylor, G.J.; Bagby, R.M.; Parker, J.D. The 20-Item Toronto Alexithymia Scale: IV. Reliability and Factorial Validity in Different Languages and Cultures. J. Psychosom. Res. 2003, 55, 277–283. [Google Scholar] [CrossRef]
- Greve, D.N.; Fischl, B. False Positive Rates in Surface-Based Anatomical Analysis. Neuroimage 2018, 171, 6–14. [Google Scholar] [CrossRef]
- Desikan, R.S.; Ségonne, F.; Fischl, B.; Quinn, B.T.; Dickerson, B.C.; Blacker, D.; Buckner, R.L.; Dale, A.M.; Maguire, R.P.; Hyman, B.T.; et al. An Automated Labeling System for Subdividing the Hum. Cerebral Cortex on MRI Scans into Gyral Based Regions of Interest. Neuroimage 2006, 31, 968–980. [Google Scholar] [CrossRef]
- Mielke, E.L.; Neukel, C.; Bertsch, K.; Reck, C.; Möhler, E.; Herpertz, S.C. Maternal Sensitivity and the Empathic Brain: Influences of Early Life Maltreatment. J. Psychiatr. Res. 2016, 77, 59–66. [Google Scholar] [CrossRef]
- Vaillancourt, K.; Pawlby, S.; Fearon, R.P. History of Childhood Abuse and Mother—Infant Interaction: A Systematic Review of Observational Studies. Infant Ment. Health J. 2017, 38, 226–248. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Rosseel, Y. Lavaan: An R package for structural equation modeling and more. Version 0.5–12 (BETA). J. Stat. Softw. 2012, 48, 1–36. [Google Scholar] [CrossRef]
- Milham, M.; Banich, M.; Webb, A.; Barad, V.; Cohen, N.; Wszalek, T.; Kramer, A. The Relative Involvement of Anterior Cingulate and Prefrontal Cortex in Attentional Control Depends on Nature of Conflict. Cogn. Brain Res. 2001, 12, 467–473. [Google Scholar] [CrossRef]
- Rudebeck, P.H.; Murray, E.A. The Orbitofrontal Oracle: Cortical Mechanisms for the Prediction and Evaluation of Specific Behavioral Outcomes. Neuron 2014, 84, 1143–1156. [Google Scholar] [CrossRef] [PubMed]
- Bounoua, N.; Miglin, R.; Spielberg, J.M.; Sadeh, N. Childhood Assaultive Trauma and Physical Aggression: Links with Cortical Thickness in Prefrontal and Occipital Cortices. Neuroimage Clin. 2020, 27, 102321. [Google Scholar] [CrossRef]
- Henschel, S.; de Bruin, M.; Möhler, E. Self-Control and Child Abuse Potential in Mothers with an Abuse History and Their Preschool Children. J. Child. Fam. Stud. 2014, 23, 824–836. [Google Scholar] [CrossRef]
- Fairhall, S.L.; Ishai, A. Neural Correlates of Object Indeterminacy in Art Compositions. Conscious. Cogn. 2008, 17, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Nagy, K.; Greenlee, M.W.; Kovács, G. The Lateral Occipital Cortex in the Face Perception Network: An Effective Connectivity Study. Front. Psychol. 2012, 3, 141. [Google Scholar] [CrossRef]
- León, I.; Rodrigo, M.J.; Quiñones, I.; Hernández, J.A.; Lage, A.; Padrón, I.; Bobes, M.A. Electrophysiological Responses to Affective Stimuli in Neglectful Mothers. PLoS ONE 2014, 9, e87808. [Google Scholar] [CrossRef]
- Seeley, W.W.; Crawford, R.K.; Zhou, J.; Miller, B.L.; Greicius, M.D. Neurodegenerative Diseases Target Large-Scale Hum. Brain Networks. Neuron 2009, 62, 42–52. [Google Scholar] [CrossRef]
- Buckner, R.L.; Snyder, A.Z.; Sanders, A.L.; Raichle, M.E.; Morris, J.C. Functional Brain Imaging of Young, Nondemented, and Demented Older Adults. J. Cogn. Neurosci. 2000, 12, 24–34. [Google Scholar] [CrossRef]
- Belathur Suresh, M.; Fischl, B.; Salat, D.H.; Initiative (ADNI), A.D.N. Factors Influencing Accuracy of Cortical Thickness in the Diagnosis of Alzheimer’s Disease. Hum. Brain Mapp. 2018, 39, 1500–1515. [Google Scholar] [CrossRef] [PubMed]
- Goldstone, A.; Willoughby, A.R.; de Zambotti, M.; Franzen, P.L.; Kwon, D.; Pohl, K.M.; Pfefferbaum, A.; Sullivan, E.V.; Müller-Oehring, E.M.; Prouty, D.E.; et al. The Mediating Role of Cortical Thickness and Gray Matter Volume on Sleep Slow-Wave Activity during Adolescence. Brain Struct. Funct. 2018, 223, 669–685. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.L.; Tamnes, C.K. Methods and Considerations for Longitudinal Structural Brain Imaging Analysis across Development. Dev. Cogn. Neurosci. 2014, 9, 172–190. [Google Scholar] [CrossRef]
- Azhari, A.; Leck, W.; Gabrieli, G.; Bizzego, A.; Rigo, P.; Setoh, P.; Bornstein, M.H.; Esposito, G. Parenting Stress Undermines Mother-Child Brain-to-Brain Synchrony: A Hyperscanning Study. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Japee, S.; Holiday, K.; Satyshur, M.D.; Mukai, I.; Ungerleider, L.G. A Role of Right Middle Frontal Gyrus in Reorienting of Attention: A Case Study. Front. Syst. Neurosci. 2015, 9, 23. [Google Scholar] [CrossRef]
- Taylor, G.J.; Bagby, R.M. New Trends in Alexithymia Research. Psychother. Psychosom. 2004, 73, 68–77. [Google Scholar] [CrossRef]

| Neglect Group n = 24 | Control Group n = 21 | Comparisons χ2/t | |
|---|---|---|---|
| Sociodemographic profile | |||
| Mean age of the mother | 29.1 (7.1) | 33.6 (3.2) | −2.82 ** |
| Number of children | 2.08 (0.8) | 1.57 (0.6) | 2.24 * |
| Mean age of target child | 2.7 (1.5) | 2.3 (1.9) | 0.82 |
| Rural areas (%) | 87.5 | 80.9 | 0.04 |
| Level of education (%): | 2.53 | ||
| Primary | 70 | 47.6 | |
| Secondary | 16.6 | 28.5 | |
| >Secondary | 12.5 | 23.8 | |
| Single-parent family | 50 | 14 | 4.92 * |
| Financial help from institutions | 83 | 14 | 18.7 *** |
| Neglect risk profile | |||
| History abuse/neglect (%) | 67 | 14 | 10.5 ** |
| Intimate partner conflict (%) | 5 | 0 | 1.22 |
| Chronic physical illness (%) | 16 | 5 | 0.22 |
| Poor household management (%) | 88 | 0 | 24.4 *** |
| Disregard health/education needs (%) | 61 | 0 | 12.4 *** |
| Disregard emotion/cognitive needs (%) | 89 | 5 | 20.9 *** |
| Rigid/inconsistent norms (%) | 67 | 5 | 11.4 *** |
| Psychiatric Disorders factor | 0.67 (0.99) | −0.69 (0.23) | 6.35 *** |
| Neglect Group n = 24 | Control Group n = 21 | Comparisons | ||
|---|---|---|---|---|
| M (SD) | M (SD) | T (43) | δ | |
| Alexithymia (averaged score) | 3.10 (0.88) | 2.45 (0.79) | 2.56 * | 0.77 |
| Difficulty in Describing | 3.35 (1.30) | 2.59 (1.18) | 2.03 * | 0.60 |
| Feelings | ||||
| Difficulty in Identifying | 2.98 (1.31) | 2.18 (1.07) | 2.21 * | 0.66 |
| Feelings | ||||
| Externally Oriented Thinking | 3.05 (0.61) | 2.59 (0.56) | 2.63 ** | 0.78 |
| Emotional Availability (factor Score) | −0.62 (0.92) | 0.71 (0.48) | −6.18 *** | 1.78 |
| Cluster/Regions | Total Vertex | Cluster-Wise p-Value | Max x, y, z (mm) | Max −log10 (p-Value) |
|---|---|---|---|---|
| Control Group > Neglect Group (Cortical thickness) | ||||
| R. Rostral middle frontal, lateral and medial orbitofrontal | 1434 | 0.014 | 21.1, 51.5, −12.5 | 3.45 |
| Control Group < Neglect Group (Surface area) | ||||
| R. Lingual and lateral occipital | 1185 | 0.0002 | 7.4, −88.3, −11.3 | −3.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
León, I.; Rodrigo, M.J.; Quiñones, I.; Hernández-Cabrera, J.A.; García-Pentón, L. Distinctive Frontal and Occipitotemporal Surface Features in Neglectful Parenting. Brain Sci. 2021, 11, 387. https://doi.org/10.3390/brainsci11030387
León I, Rodrigo MJ, Quiñones I, Hernández-Cabrera JA, García-Pentón L. Distinctive Frontal and Occipitotemporal Surface Features in Neglectful Parenting. Brain Sciences. 2021; 11(3):387. https://doi.org/10.3390/brainsci11030387
Chicago/Turabian StyleLeón, Inmaculada, María José Rodrigo, Ileana Quiñones, Juan Andrés Hernández-Cabrera, and Lorna García-Pentón. 2021. "Distinctive Frontal and Occipitotemporal Surface Features in Neglectful Parenting" Brain Sciences 11, no. 3: 387. https://doi.org/10.3390/brainsci11030387
APA StyleLeón, I., Rodrigo, M. J., Quiñones, I., Hernández-Cabrera, J. A., & García-Pentón, L. (2021). Distinctive Frontal and Occipitotemporal Surface Features in Neglectful Parenting. Brain Sciences, 11(3), 387. https://doi.org/10.3390/brainsci11030387







