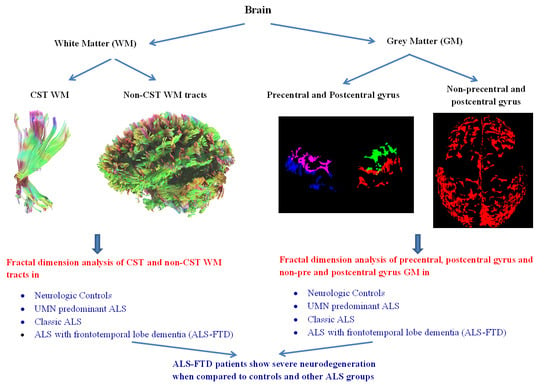
Corticospinal Tract and Related Grey Matter Morphometric Shape Analysis in ALS Phenotypes: A Fractal Dimension Study
Abstract
1. Introduction
2. Methods
2.1. Demographics
2.2. Imaging Protocol
2.3. Data Processing
2.4. White Matter Fractal Dimension Analysis
2.5. Grey Matter Fractal Dimension Analysis
2.6. Statistical Methods
2.7. Clinical Correlations
3. Results
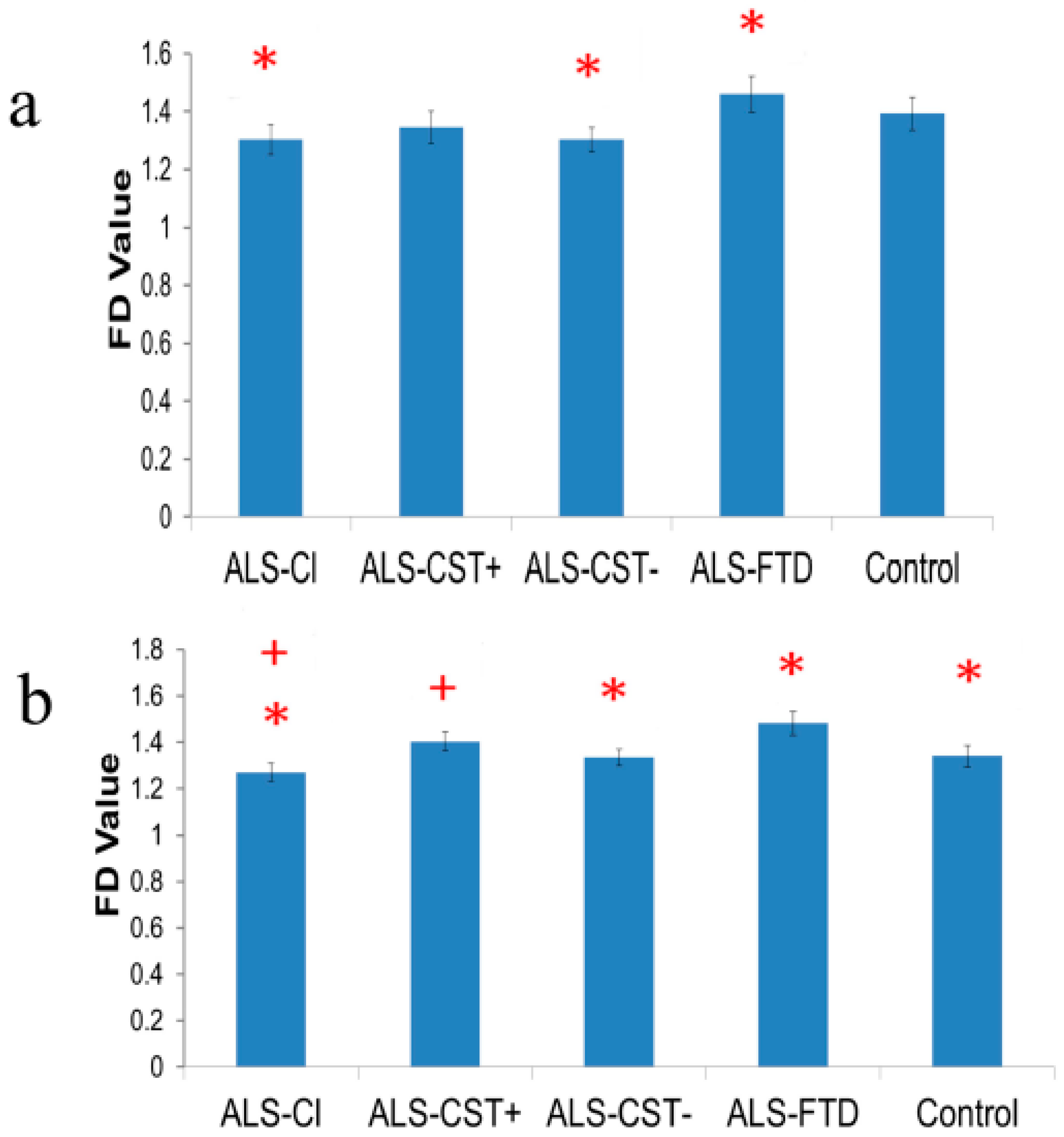
3.1. Fractal Dimension of Primary Motor Cortex-Originating Corticospinal Tract Fibers
3.2. Fractal Dimension of Primary Motor Cortex Grey Matter
3.3. Fractal Dimension of Primary Sensory Cortex-Originating Corticospinal Tract Fibers
3.4. Fractal Dimension of Primary Sensory Cortex Grey Matter
3.5. Fractal Dimension of Non-Primary Motor and Non-Primary Sensory Cortex Grey Matter
3.6. Fractal Dimension of Non-Corticospinal Tract White Matter Fiber Tracts
3.7. Correlation between Clinical and FD Measures
3.8. Demographics and Clinical Measure Comparisons between Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abe, O.; Yamada, H.; Masutani, Y.; Aoki, S.; Kunimatsu, A.; Yamasue, H.; Fukuda, R.; Kasai, K.; Hayashi, N.; Masumoto, T.; et al. Amyotrophic lateral sclerosis: Diffusion tensor tractography and voxel-based analysis. NMR Biomed. 2004, 17, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.-H.; Lee, K.-W.; Sung, J.-J.; Chang, K.-H.; Song, I.C. Diffusion tensor MRI as a diagnostic tool of upper motor neuron involvement in amyotrophic lateral sclerosis. J. Neurol. Sci. 2004, 227, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Dean, D.; Liu, J.Z.; Sahgal, V.; Wang, X.; Yue, G.H. Quantifying degeneration of white matter in normal aging using fractal dimension. Neurobiol. Aging 2007, 28, 1543–1555. [Google Scholar] [CrossRef]
- Bede, P.; Hardiman, O. Lessons of ALS imaging: Pitfalls and future directions—A critical review. Neuroimage Clin. 2014, 4, 436–443. [Google Scholar] [CrossRef]
- Holmes, G. The pathology of amyotrophic lateral sclerosis. Rev. Neurol. Psychiatry 1909, 7, 693–725. [Google Scholar]
- Hecht, M.J.; Fellner, C.; Schmid, A.; Neundörfer, B.; Fellner, F.A. Cortical T2 signal shortening in amyotrophic lateral sclerosis is not due to iron deposits. Neuroradiology 2005, 47, 805–808. [Google Scholar] [CrossRef]
- Zhang, L.; Ulug, A.M.; Zimmerman, R.D.; Lin, M.T.; Rubin, M.; Beal, M.F. The diagnostic utility of FLAIR imaging in clinically verified amyotrophic lateral sclerosis. J. Magn. Reson. Imaging 2003, 17, 521–527. [Google Scholar] [CrossRef]
- Mitsumoto, H.; Chad, D.A.; Pioro, E.P. Amyotrophic Lateral Sclerosis. Contemporary Neurology Series; F.A. Davis Company: Philadelphia, PA, USA, 1998; Volume 49. [Google Scholar]
- Graham, J.M.; Papadakis, N.; Evans, J.; Widjaja, E.; Romanowski, C.A.; Paley, M.N.; Wallis, L.I.; Wilkinson, I.D.; Shaw, P.J.; Griffiths, P.D. Diffusion tensor imaging for the assessment of upper motor neuron integrity in ALS. Neurology 2004, 63, 2111–2119. [Google Scholar] [CrossRef]
- Jacob, S.; Finsterbusch, J.; Weishaupt, J.H.; Khorram-Sefat, D.; Frahm, J.; Ehrenreich, H. Diffusion tensor imaging for long-term follow-up of corticospinal tract degeneration in amyotrophic lateral sclerosis. Neuroradiology 2003, 45, 598–600. [Google Scholar] [CrossRef]
- Bede, P.; Hardiman, O. Longitudinal structural changes in ALS: A three time-point imaging study of white and gray matter de-generation. Amyotroph. Lateral Scler. Front. Degener. 2018, 19, 232–241. [Google Scholar] [CrossRef]
- Li, J.; Pan, P.; Song, W.; Huang, R.; Chen, K.; Shang, H. A meta-analysis of diffusion tensor imaging studies in amyotrophic lateral sclerosis. Neurobiol. Aging 2012, 33, 1833–1838. [Google Scholar] [CrossRef] [PubMed]
- Ellis, C.M.; Simmons, A.; Jones, D.K.; Bland, J.; Dawson, J.M.; Horsfield, M.A.; Williams, S.C.R.; Leigh, P.N. Diffusion tensor MRI assesses corticospinal tract damage in ALS. Neurology 1999, 53, 1051. [Google Scholar] [CrossRef]
- Sach, M.; Winkler, G.; Glauche, V.; Liepert, J.; Heimbach, B.; Koch, M.A.; Büchel, C.; Weiller, C. Diffusion tensor MRI of early upper motor neuron involvement in amyotrophic lateral sclerosis. Brain 2004, 127, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Schimrigk, S.K.; Bellenberg, B.; Schlüter, M.; Stieltjes, B.; Drescher, R.; Rexilius, J.; Lukas, C.; Hahn, H.K.; Przuntek, H.; Köster, O. Diffusion tensor imaging-based fractional anisotropy quantification in the corticospinal tract of patients with amyotrophic lateral sclerosis using a probabilistic mixture model. Am. J. Neuroradiol. 2007, 28, 724–730. [Google Scholar]
- Lillo, P.; Mioshi, E.; Burrell, J.R.; Kiernan, M.C.; Hodges, J.R.; Hornberger, M. Grey and white matter changes across the amyotrophic lateral sclerosis-frontotemporal dementia continuum. PLoS ONE 2012, 7, e43993. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, V.; Pioro, E.P. Differential involvement of corticospinal tract (CST) fibers in UMN-predominant ALS patients with or without CST hyperintensity: A diffusion tensor tractography study. Neuroimage Clin. 2017, 14, 574–579. [Google Scholar] [CrossRef]
- Rajagopalan, V.; Pioro, E.P. 2-Deoxy-2-[18F]fluoro-d-glucose positron emission tomography, cortical thickness and white matter graph network abnormalities in brains of patients with amyotrophic lateral sclerosis and frontotemporal dementia suggest early neuronopathy rather than axonopathy. Eur. J. Neurol. 2020, 27, 1904–1912. [Google Scholar]
- Verstraete, E.; Veldink, J.H.; Hendrikse, J.; Schelhaas, H.J.; Heuvel, M.P.V.D.; Berg, L.H.V.D. Structural MRI reveals cortical thinning in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 2011, 83, 383–388. [Google Scholar] [CrossRef]
- Schuster, C.; Kasper, E.; Machts, J.; Bittner, D.; Kaufmann, J.; Benecke, R.; Teipel, S.; Vielhaber, S.; Prudlo, J. Focal thinning of the motor cortex mirrors clinical features of amyotrophic lateral sclerosis and their phenotypes: A neuroimaging study. J. Neurol. 2013, 260, 2856–2864. [Google Scholar] [CrossRef] [PubMed]
- Walhout, R.; Westeneng, H.-J.; Verstraete, E.; Hendrikse, J.; Veldink, J.H.; Heuvel, M.P.V.D.; Berg, L.H.V.D. Cortical thickness in ALS: Towards a marker for upper motor neuron involvement. J. Neurol. Neurosurg. Psychiatry 2014, 86, 288–294. [Google Scholar] [CrossRef]
- Donatelli, G.; Retico, A.; Ienco, E.C.; Cecchi, P.; Costagli, M.; Frosini, D.; Biagi, L.; Tosetti, M.; Siciliano, G.; Cosottini, M. Semiautomated evaluation of the primary motor cortex in patients with amyotrophic lateral sclerosis at 3T. Am. J. Neuroradiol. 2017, 39, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Cluskey, S.; Ramsden, D.B. Mechanisms of neurodegeneration in amyotrophic lateral sclerosis. J. Clin. Pathol. Mol. Pathol. 2001, 54, 386–392. [Google Scholar]
- Metwalli, N.S.; Benatar, M.; Nair, G.; Usher, S.; Hu, X.; Carew, J.D. Utility of axial and radial diffusivity from diffusion tensor MRI as markers of neurodegeneration in amyotrophic lateral sclerosis. Brain Res. 2010, 1348, 156–164. [Google Scholar] [CrossRef]
- Good, C.D.; Johnsrude, I.S.; Ashburner, J.; Henson, R.N.; Friston, K.J.; Frackowiak, R.S. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage 2001, 14, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Esteban, F.J.; Sepulcre, J.; De Miras, J.R.; Navas, J.; De Mendizábal, N.V.; Goñi, J.; Quesada, J.M.; Bejarano, B.; Villoslada, P. Fractal dimension analysis of grey matter in multiple sclerosis. J. Neurol. Sci. 2009, 282, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Esteban, F.J.; Sepulcre, J.; De Mendizábal, N.V.; Goñi, J.; Navas, J.; De Miras, J.R.; Bejarano, B.; Masdeu, J.C.; Villoslada, P. Fractal dimension and white matter changes in multiple sclerosis. Neuroimage 2007, 36, 543–549. [Google Scholar] [CrossRef]
- Wu, Y.-T.; Shyu, K.-K.; Jao, C.-W.; Wang, Z.-Y.; Soong, B.-W.; Wu, H.-M.; Wang, P.-S. Fractal dimension analysis for quantifying cerebellar morphological change of multiple system atrophy of the cerebellar type (MSA-C). Neuroimage 2010, 49, 539–551. [Google Scholar] [CrossRef] [PubMed]
- King, R.D.; Brown, B.; Hwang, M.; Jeon, T.; George, A.T. Fractal dimension analysis of the cortical ribbon in mild Alzheimer’s disease. Neuroimage 2010, 53, 471–479. [Google Scholar] [CrossRef]
- Rajagopalan, V.; Liu, Z.; Allexandre, D.; Zhang, L.; Wang, X.-F.; Pioro, E.P.; Yue, G.H. Brain white matter shape changes in amyotrophic lateral sclerosis (ALS): A fractal dimension study. PLoS ONE 2013, 8, e73614. [Google Scholar] [CrossRef]
- Jenkinson, M. Fast, automated, N-dimensional phase-unwrapping algorithm. Magn. Reson. Med. 2003, 49, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, M. Improving the registration of B0-disorted EPI images using calculated cost function weights. In Proceedings of the 10th International Conference on Functional Mapping of the Human Brain, Budapest, Hungary, 13–17 June 2004. [Google Scholar]
- Smith, S.M.; Jenkinson, M.; Woolrich, M.W.; Beckmann, C.F.; Behrens, T.E.; Johansen-Berg, H.; Bannister, P.R.; De Luca, M.; Drobnjak, I.; Flitney, D.E.; et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004, 23, S208–S219. [Google Scholar] [CrossRef]
- Leemans, A.; Jones, D.K. The B-matrix must be rotated when correcting for subject motion in DTI data. Magn. Reson. Med. 2009, 61, 1336–1349. [Google Scholar] [CrossRef] [PubMed]
- Sage, C.A.; Van Hecke, W.; Peeters, R.; Sijbers, J.; Robberecht, W.; Parizel, P.; Marchal, G.; Leemans, A.; Sunaert, S. Quantitative diffusion tensor imaging in amyotrophic lateral sclerosis: Revisited. Hum. Brain Mapp. 2009, 30, 3657–3675. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Van Zijl, P.C.; Kim, J.; Pearlson, G.D.; Mori, S. DtiStudio: Resource program for diffusion tensor computation and fiber bundle tracking. Comput. Methods Programs Biomed. 2006, 81, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Wakana, S.; Caprihan, A.; Panzenboeck, M.M.; Fallon, J.H.; Perry, M.; Gollub, R.L.; Hua, K.; Zhang, J.; Jiang, H.; Dubey, P.; et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage 2007, 36, 630–644. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, V.; Yue, G.H.; Pioro, E.P. Brain white matter diffusion tensor metrics from clinical 1.5T MRI distinguish between ALS phenotypes. J. Neurol. 2013, 260, 2532–2540. [Google Scholar] [CrossRef]
- Di Ieva, A. (Ed.) The Fractal Geometry of the Brain; Springer: New York, NY, USA, 2016. [Google Scholar]
- Zhang, Y.; Brady, M.; Smith, S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imaging 2001, 20, 45–57. [Google Scholar] [CrossRef]
- Jenkinson, M.; Smith, S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001, 5, 143–156. [Google Scholar] [CrossRef]
- Jenkinson, M.; Bannister, P.; Brady, M.; Smith, S. Improved optimisation for the robust and accurate linear registration and motion correction of brain images. Neuroimage 2002, 17, 825–841. [Google Scholar] [CrossRef]
- Russell, J.R.; Demyer, W. The quantitative corticoid origin of pyramidal axons of Macaca rhesus. With some remarks on the slow rate of axolysis. Neurology 1961, 11, 96–108. [Google Scholar] [CrossRef]
- Smith, M.C. Nerve fibre degeneration in the brain in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 1960, 23, 269–282. [Google Scholar] [CrossRef]
- Whitwell, J.L.; Avula, R.; Senjem, M.L.; Kantarci, K.; Weigand, S.D.; Samikoglu, A.; Edmonson, H.A.; Vemuri, P.; Knopman, D.S.; Boeve, B.F.; et al. Gray and white matter water diffusion in the syndromic variants of frontotemporal dementia. Neurology 2010, 74, 1279–1287. [Google Scholar] [CrossRef]
- Rajagopalan, V.; Pioro, E.P. Distinct patterns of cortical atrophy in ALS patients with or without dementia: An MRI VBM study. Amyotroph. Lateral Scler. Front. Degener. 2014, 15, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, V.; Allexandre, D.; Yue, G.H.; Pioro, E.P. Diffusion tensor imaging evaluation of corticospinal tract hyperintensity in upper motor neuron-predominant ALS Patients. J. Aging Res. 2011, 2011, 481745. [Google Scholar] [CrossRef]
- Ellis, C.M.; Suckling, J.; Amaro, E.; Bullmore, E.T.; Simmons, A.; Williams, S.C.; Leigh, P.N. Volumetric analysis reveals corticospinal tract degeneration and extramotor involvement in ALS. Neurology 2001, 57, 1571–1578. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Adad, J.; El Mendili, M.-M.; Morizot-Koutlidis, R.; Lehéricy, S.; Meininger, V.; Blancho, S.; Rossignol, S.; Benali, H.; Pradat, P.-F. Involvement of spinal sensory pathway in ALS and specificity of cord atrophy to lower motor neuron degeneration. Amyotroph. Lateral Scler. Front. Degener. 2012, 14, 30–38. [Google Scholar] [CrossRef]
- Iglesias, C.; Sangari, S.; El Mendili, M.-M.; Benali, H.; Marchand-Pauvert, V.; Pradat, P.-F. Electrophysiological and spinal imaging evidences for sensory dysfunction in amyotrophic lateral sclerosis. BMJ Open 2015, 5, e007659. [Google Scholar] [CrossRef] [PubMed]
- El Mendili, M.M.; Querin, G.; Bede, P.; Pradat, P.-F. Spinal cord imaging in amyotrophic lateral sclerosis: Historical concepts—Novel techniques. Front. Neurol. 2019, 10, 350. [Google Scholar] [CrossRef] [PubMed]

| ALS-Cl | ALS-CST+ | ALS-CST− | ALS-FTD | Controls | Significance | |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age (year) | 57.5 ± 12.2 | 51.7 ± 11.6 | 59.4 ± 10.5 | 67.4 ±10.2 | 51.7 ± 15.7 | p = 0.007 |
| n | 19 | 17 | 25 | 14 | 14 | |
| Gender | 11 male, 8 female | 12 male, 5 female | 15 male, 10 female | 3 male, 11 female | 9 male, 5 female | p > 0.05 |
| ALSFRS-R | 37.0 ± 9.3 | 33 ± 7.8 | 35.9 ± 7.0 | 29 ± 6.9 | p = 0.047 | |
| Symptom duration (months) | 27.2 ± 26.5 | 15.4 ± 8.1 | 61.4 ± 61.5 | 38.4 ± 20.5 | p = 0.003 | |
| Disease progression rate | −0.7 ± 0.8 | −1.5 ± 1.8 | −0.4 ± 0.3 | −0.6 ± 0.30 | p = 0.009 | |
| El Escorial Score | 2.5 ± 0.9 | 1.8 ± 1.1 | 1.4 ± 0.8 | 2.3 ± 1.3 | p < 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajagopalan, V.; Pioro, E.P. Corticospinal Tract and Related Grey Matter Morphometric Shape Analysis in ALS Phenotypes: A Fractal Dimension Study. Brain Sci. 2021, 11, 371. https://doi.org/10.3390/brainsci11030371
Rajagopalan V, Pioro EP. Corticospinal Tract and Related Grey Matter Morphometric Shape Analysis in ALS Phenotypes: A Fractal Dimension Study. Brain Sciences. 2021; 11(3):371. https://doi.org/10.3390/brainsci11030371
Chicago/Turabian StyleRajagopalan, Venkateswaran, and Erik P. Pioro. 2021. "Corticospinal Tract and Related Grey Matter Morphometric Shape Analysis in ALS Phenotypes: A Fractal Dimension Study" Brain Sciences 11, no. 3: 371. https://doi.org/10.3390/brainsci11030371
APA StyleRajagopalan, V., & Pioro, E. P. (2021). Corticospinal Tract and Related Grey Matter Morphometric Shape Analysis in ALS Phenotypes: A Fractal Dimension Study. Brain Sciences, 11(3), 371. https://doi.org/10.3390/brainsci11030371