Effects of Music Intervention on Stress in Concussed and Non-Concussed Athletes †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
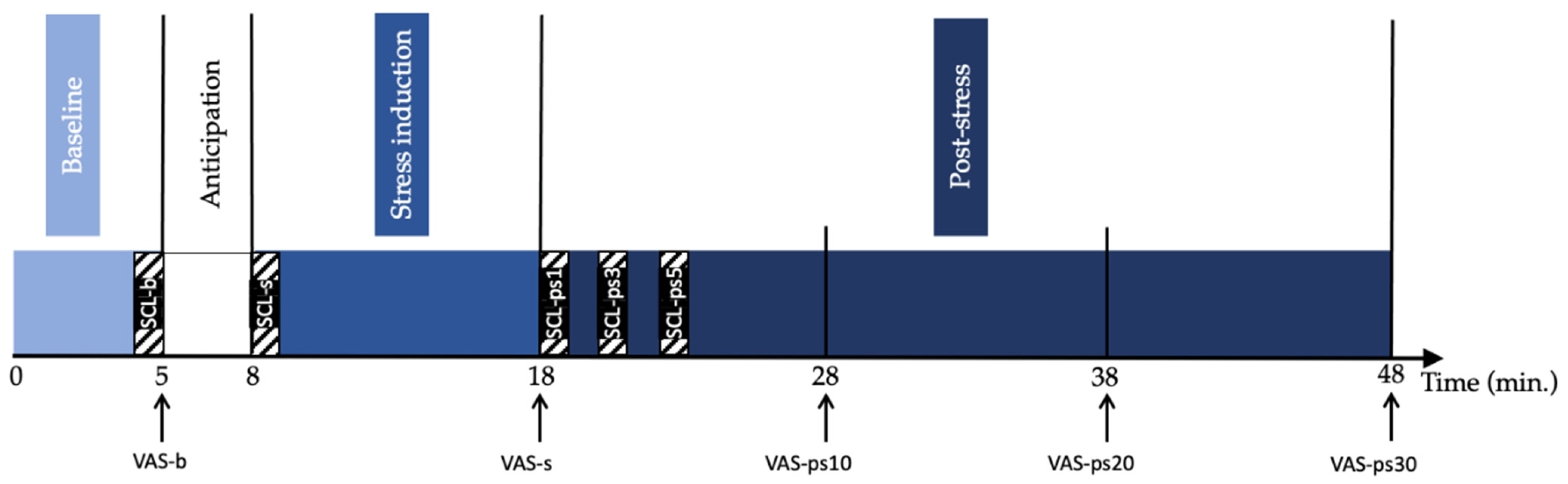
2.2. Study Procedure
2.3. Measures
2.3.1. Self-Reported Stress Measurements
2.3.2. Skin Conductance Level
2.4. Musical Material
2.5. Data Analysis
3. Results
3.1. Self-Reported Stress Levels (VAS)
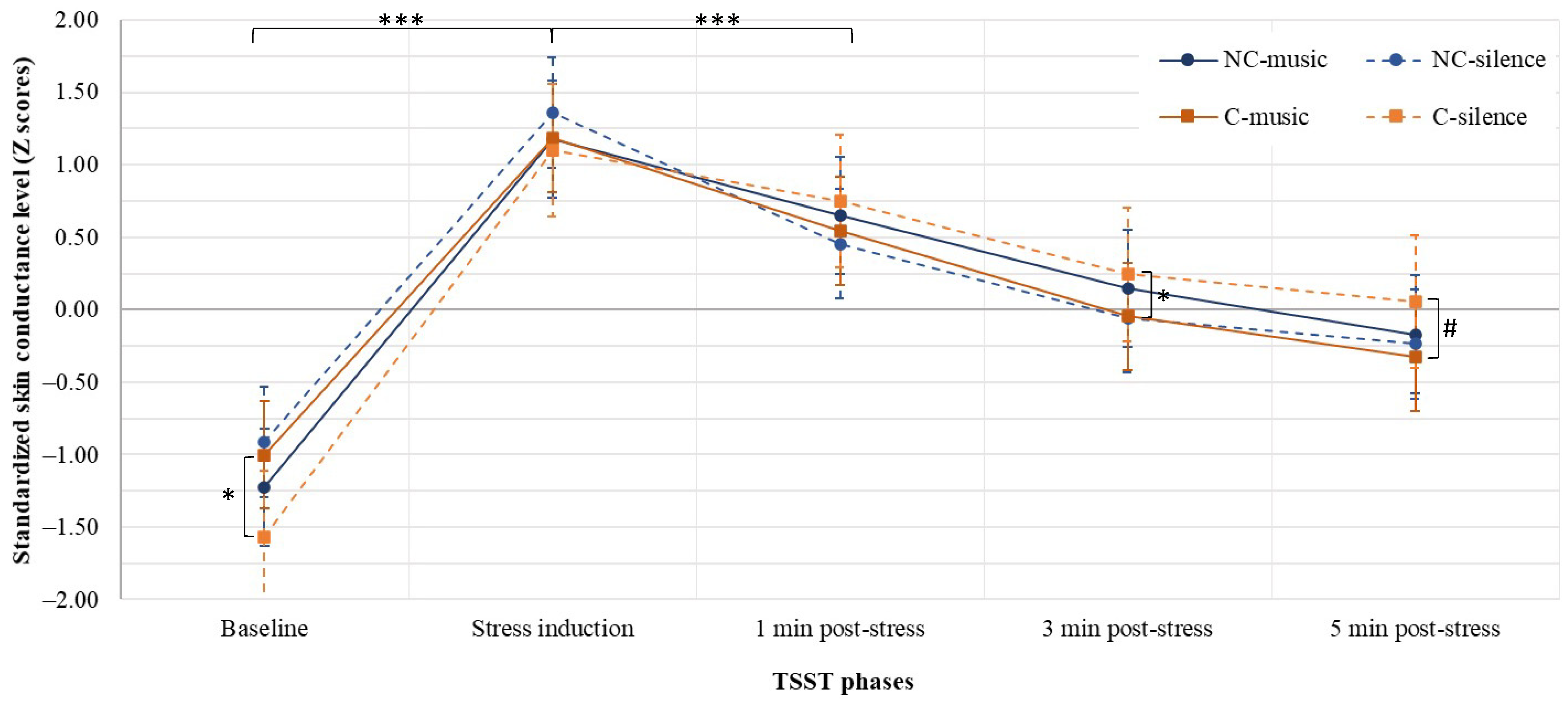
3.2. Skin Conductance Level (SCL)
3.2.1. Differences between Groups
3.2.2. Differences within Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCrory, P.; Meeuwisse, W.; Dvorak, J.; Aubry, M.; Bailes, J.; Broglio, S.; Cantu, R.C.; Cassidy, D.; Echemendia, R.J.; Castellani, R.J. Consensus statement on concussion in sport—The 5th international conference on concussion in sport held in Berlin, October 2016. Br. J. Sports Med. 2017, 51, 838–847. [Google Scholar]
- Laker, S.R. Epidemiology of concussion and mild traumatic brain injury. PM&R 2011, 3, S354–S358. [Google Scholar]
- Langlois, J.A.; Rutland-Brown, W.; Wald, M.M. The epidemiology and impact of traumatic brain injury: A brief overview. J. Head Trauma Rehabil. 2006, 21, 375–378. [Google Scholar] [CrossRef] [Green Version]
- Daneshvar, D.H.; Nowinski, C.J.; McKee, A.C.; Cantu, R.C. The epidemiology of sport-related concussion. Clin. Sports Med. 2011, 30, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harmon, K.G.; Drezner, J.A.; Gammons, M.; Guskiewicz, K.M.; Halstead, M.; Herring, S.A.; Kutcher, J.S.; Pana, A.; Putukian, M.; Roberts, W.O. American Medical Society for Sports Medicine position statement: Concussion in sport. Br. J. Sports. Med. 2013, 47, 15–26. [Google Scholar] [CrossRef] [Green Version]
- Davies, S.C.; Bird, B.M. Motivations for underreporting suspected concussion in college athletics. J. Clin. Sport Psychol. 2015, 9, 101–115. [Google Scholar] [CrossRef]
- Llewellyn, T.; Burdette, G.T.; Joyner, A.B.; Buckley, T.A. Concussion reporting rates at the conclusion of an intercollegiate athletic career. Clin. J. Sport. 2014, 24, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Echemendia, R.J.; Meeuwisse, W.; McCrory, P.; Davis, G.A.; Putukian, M.; Leddy, J.; Makdissi, M.; Sullivan, S.J.; Broglio, S.P.; Raftery, M. The sport concussion assessment tool 5th edition (SCAT5): Background and rationale. Br. J. Sports. Med. 2017, 51, 848–850. [Google Scholar]
- Willer, B.; Leddy, J.J. Management of concussion and post-concussion syndrome. Curr. Treat. Options Neurol. 2006, 8, 415–426. [Google Scholar] [CrossRef]
- Makdissi, M.; Cantu, R.C.; Johnston, K.M.; McCrory, P.; Meeuwisse, W.H. The difficult concussion patient: What is the best approach to investigation and management of persistent (>10 days) postconcussive symptoms? Br. J. Sports. Med. 2013, 47, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Conder, R.; Conder, A.A. Neuropsychological and psychological rehabilitation interventions in refractory sport-related post-concussive syndrome. Brain Inj. 2015, 29, 249–262. [Google Scholar] [CrossRef]
- Marshall, S.; Bayley, M.; McCullagh, S.; Velikonja, D.; Berrigan, L.; Ouchterlony, D.; Weegar, K. Updated clinical practice guidelines for concussion/mild traumatic brain injury and persistent symptoms. Brain Inj. 2015, 29, 688–700. [Google Scholar] [CrossRef]
- Broshek, D.K.; De Marco, A.P.; Freeman, J.R. A review of post-concussion syndrome and psychological factors associated with concussion. Brain Inj. 2015, 29, 228–237. [Google Scholar] [CrossRef]
- Ponsford, J.; Willmott, C.; Rothwell, A.; Cameron, P.; Kelly, A.-M.; Nelms, R.; Curran, C.; Ng, K. Factors influencing outcome following mild traumatic brain injury in adults. J. Int. Neuropsychol. Soc. 2000, 6, 568–579. [Google Scholar] [CrossRef]
- Ontario Neurotrauma Foundation. Guidelines for Concussions/Mild Traumatic Brain Injury and Persistent Symptoms; Ontario Neurotrauma Foundation: Toronto, ON, Canada, 2018. [Google Scholar]
- Lupien, S.J. Brains under stress. Can. J. Psychiatry 2009, 54, 4–5. [Google Scholar] [CrossRef]
- American Psychiatric Association (Ed.) Diagnostic and Statistical Manuel of Mental Disorders (DSM-5); American Psychiatric Association: Arlington, VA, USA, 2013; Volume 70. [Google Scholar]
- Spielberger, C.D. Anxiety: Current Trends in Theory and Research; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- de Witte, M.; Spruit, A.; van Hooren, S.; Moonen, X.; Stams, G.-J. Effects of music interventions on stress-related outcomes: A systematic review and two meta-analyses. Health Psychol. Rev. 2020, 14, 294–324. [Google Scholar] [CrossRef] [PubMed]
- Murison, R. The Neurobiology of Stress. In Neuroscience of Pain, Stress, and Emotion, 1st ed.; Al’Absi, M., Flaten, M., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 29–49. [Google Scholar]
- Critchley, H.D. Electrodermal responses: What happens in the brain. Neuroscientist 2002, 8, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Cacioppo, J.T.; Tassinary, L.G.; Berntson, G. Handbook of Psychophysiology; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Grapperon, J.; Pignol, A.-C.; Vion-Dury, J. La mesure de la réaction électrodermale. L’Encéphale 2012, 38, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Bloom, G.; Horton, A.; McCrory, P.; Johnston, K. Sport psychology and concussion: New impacts to explore. Br. J. Sports Med. 2004, 38, 519–521. [Google Scholar] [CrossRef] [Green Version]
- Wiese-Bjornstal, D.M.; White, A.C.; Russell, H.C.; Smith, A.M. Psychology of sport concussions. Kinesiol. Rev. 2015, 4, 169–189. [Google Scholar] [CrossRef]
- Yang, J.; Peek-Asa, C.; Covassin, T.; Torner, J.C. Post-concussion symptoms of depression and anxiety in division I collegiate athletes. Dev. Neuropsychol. 2015, 40, 18–23. [Google Scholar] [CrossRef]
- van der Horn, H.J.; Liemburg, E.J.; Aleman, A.; Spikman, J.M.; van der Naalt, J. Brain networks subserving emotion regulation and adaptation after mild traumatic brain injury. J. Neurotrauma 2016, 33, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Abaji, J.P.; Curnier, D.; Moore, R.D.; Ellemberg, D. Persisting effects of concussion on heart rate variability during physical exertion. J. Neurotrauma 2016, 33, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, M.G.; Mainwaring, L.; Senthinathan, A.; Churchill, N.; Thomas, S.; Richards, D. Psychological and physiological markers of stress in concussed athletes across recovery milestones. J. Head Trauma Rehabil. 2017, 32, E38–E48. [Google Scholar] [CrossRef]
- Gall, B.; Parkhouse, W.; Goodman, D. Heart rate variability of recently concussed athletes at rest and exercise. Med. Sci. Sports Exerc. 2004, 36, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Gall, B.; Parkhouse, W.; Goodman, D. Exercise following a sport induced concussion. Br. J. Sports Med. 2004, 38, 773–777. [Google Scholar] [CrossRef] [Green Version]
- Senthinathan, A.; Mainwaring, L.M.; Hutchison, M. Heart rate variability of athletes across concussion recovery milestones: A preliminary study. Clin. J. Sport Med. 2017, 27, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.W.; Kontos, A.P.; Reynolds, E.; Murawski, C.D.; Fu, F.H. A comprehensive, targeted approach to the clinical care of athletes following sport-related concussion. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 235–246. [Google Scholar] [CrossRef]
- Kontos, A.P.; Deitrick, J.M.; Reynolds, E. Mental health implications and consequences following sport-related concussion. Br. J. Sports Med. 2016, 50, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Laukka, P.; Quick, L. Emotional and motivational uses of music in sports and exercise: A questionnaire study among athletes. Psychol. Music 2013, 41, 198–215. [Google Scholar] [CrossRef]
- Kühlmann, A.; de Rooij, A.; Kroese, L.; van Dijk, M.; Hunink, M.; Jeekel, J. Meta-analysis evaluating music interventions for anxiety and pain in surgery. Br. J. Surg. 2018, 105, 773–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terry, P.C.; Karageorghis, C.I.; Curran, M.L.; Martin, O.V.; Parsons-Smith, R.L. Effects of music in exercise and sport: A meta-analytic review. Psychol. Bull. 2020, 146, 91. [Google Scholar] [CrossRef] [Green Version]
- Pelletier, C.L. The effect of music on decreasing arousal due to stress: A meta-analysis. J. Music Ther. 2004, 41, 192–214. [Google Scholar] [CrossRef]
- Chafin, S.; Roy, M.; Gerin, W.; Christenfeld, N. Music can facilitate blood pressure recovery from stress. Br. J. Health Psychol. 2004, 9, 393–403. [Google Scholar] [CrossRef]
- De la Torre-Luque, A.; Díaz-Piedra, C.; Buela-Casal, G. Effects of preferred relaxing music after acute stress exposure: A randomized controlled trial. Psychol. Music 2017, 45, 795–813. [Google Scholar] [CrossRef]
- Ilie, G.; Rehana, R. Effects of individual music playing and music listening on acute stress recovery. Les effets du jeu et de l’écoute musicale sur le rétablissement d’un individu a la suite d’un stress aigu. Can. J. Music Ther. 2013, 19, 23. [Google Scholar]
- Khalfa, S.; Dalla Bella, S.; Roy, M.; Peretz, I.; Lupien, S.J. Effects of relaxing music on salivary cortisol level after psychological stress. Ann. N. Y. Acad. Sci. 2003, 999, 374–376. [Google Scholar] [CrossRef]
- Lee, K.S.; Jeong, H.C.; Yim, J.E.; Jeon, M.Y. Effects of music therapy on the cardiovascular and autonomic nervous system in stress-induced university students: A randomized controlled trial. J. Altern. Complementary Med. 2016, 22, 59–65. [Google Scholar] [CrossRef]
- Sandstrom, G.M.; Russo, F.A. Music hath charms: The effects of valence and arousal on recovery following an acute stressor. Music Med. 2010, 2, 137–143. [Google Scholar] [CrossRef]
- Jiang, J.; Rickson, D.; Jiang, C. The mechanism of music for reducing psychological stress: Music preference as a mediator. Arts Psychother. 2016, 48, 62–68. [Google Scholar] [CrossRef]
- Yamamoto, M.; Naga, S.; Shimizu, J. Positive musical effects on two types of negative stressful conditions. Psychol. Music. 2007, 35, 249–275. [Google Scholar] [CrossRef]
- Chanda, M.L.; Levitin, D.J. The neurochemistry of music. Trends Cogn. Sci. 2013, 17, 179–193. [Google Scholar] [CrossRef] [Green Version]
- Koelsch, S.; Jäncke, L. Music and the heart. Eur. Heart J. 2015, 36, 3043–3049. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, L.; Mas-Herrero, E.; Zatorre, R.J.; Ripollés, P.; Gomez-Andres, A.; Alicart, H.; Olivé, G.; Marco-Pallarés, J.; Antonijoan, R.M.; Valle, M. Dopamine modulates the reward experiences elicited by music. Proc. Natl. Acad. Sci. USA 2019, 116, 3793–3798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koelsch, S. Brain correlates of music-evoked emotions. Nat. Rev. Neurosci. 2014, 15, 170–180. [Google Scholar] [CrossRef]
- Blood, A.J.; Zatorre, R.J. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proc. Natl. Acad. Sci. USA 2001, 98, 11818–11823. [Google Scholar] [CrossRef] [Green Version]
- Zatorre, R.J.; Salimpoor, V.N. From perception to pleasure: Music and its neural substrates. Proc. Natl. Acad. Sci. USA 2013, 110, 10430–10437. [Google Scholar] [CrossRef] [Green Version]
- Labbé, E.; Schmidt, N.; Babin, J.; Pharr, M. Coping with stress: The effectiveness of different types of music. Appl. Psychophysiol. Biofeedback 2007, 32, 163–168. [Google Scholar] [CrossRef]
- Dileo, C.; Bradt, J. Music therapy: Applications to stress management. In Principles and Practice of Stress Management, 3rd ed.; Guilford: New York, NY, USA, 2007. [Google Scholar]
- Kemper, K.J.; Danhauer, S.C. Music as therapy. South Med. J. 2005, 98, 282–288. [Google Scholar] [CrossRef]
- Wearne, T.A.; Lucien, A.; Trimmer, E.M.; Logan, J.A.; Rushby, J.; Wilson, E.; Filipčíková, M.; McDonald, S. Anxiety sensitivity moderates the subjective experience but not the physiological response to psychosocial stress. Int. J. Psychophysiol. 2019, 141, 76–83. [Google Scholar] [CrossRef]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Lesage, F.-X.; Berjot, S.; Deschamps, F. Psychometric properties of the French versions of the Perceived Stress Scale. Int. J. Occup. Med. Environ. Health 2012, 25, 178–184. [Google Scholar] [CrossRef]
- Bergeron, J.; Landry, M.; Bélanger, D. The development and validation of a French form of the State-Trait Anxiety Inventory. Cross-Cult. Anxiety 1976, 1, 41–50. [Google Scholar]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R.E. Manual for the State-Trait Anxiety Inventory; Consulting Psychologists Press: Palo Alto, CA, USA, 1970. [Google Scholar]
- De Beaumont, L.; Lassonde, M.; Leclerc, S.; Théoret, H. Long-term and cumulative effects of sports concussion on motor cortex inhibition. Neurosurgery 2007, 61, 329–337. [Google Scholar] [CrossRef]
- King, N.; Crawford, S.; Wenden, F.; Moss, N.; Wade, D. The rivermead post concussion symptoms questionnaire: A measure of symptoms commonly experienced after head injury and its reliability. J. Neurol. 1995, 242, 587–592. [Google Scholar] [CrossRef]
- Thompson, C.; Davies, P.; Herrmann, L.; Summers, M.; Potter, S. Approaches to establishing validated cut-off scores on the Rivermead post-concussion symptoms questionnaire (RPQ). Brain Inj. 2016, 30, 770. [Google Scholar]
- Kirschbaum, C.; Pirke, K.-M.; Hellhammer, D.H. The ‘Trier Social Stress Test’–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 1993, 28, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Contrada, R.; Baum, A. The Handbook of Stress Science: Biology, Psychology, and Health; Springer: New York, NY, USA, 2011. [Google Scholar]
- Tat, D.; Pelletier, G.; Massicotte, E.; Gosselin, N. The Relation Between Perceived Physiological Arousal and Valence during Relaxing Music Listening; Tenth anniversary symposium of the International Laboratory for Brain, Music and Sound Research: Montreal, QC, Canada, 2015. [Google Scholar]
- Birkett, M.A. The Trier Social Stress Test protocol for inducing psychological stress. J. Vis. Exp. 2011, 56, 3238. [Google Scholar] [CrossRef] [PubMed]
- Kudielka, B.M.; Hellhammer, D.H.; Kirschbaum, C. Ten Years of Research with the Trier Social Stress Test—Revisited; The Guilford Press: New York, NY, USA, 2007. [Google Scholar]
- Rimmele, U.; Seiler, R.; Marti, B.; Wirtz, P.H.; Ehlert, U.; Heinrichs, M. The level of physical activity affects adrenal and cardiovascular reactivity to psychosocial stress. Psychoneuroendocrinology 2009, 34, 190–198. [Google Scholar] [CrossRef] [Green Version]
- Campbell, J.; Ehlert, U. Acute psychosocial stress: Does the emotional stress response correspond with physiological responses? Psychoneuroendocrinology 2012, 37, 1111–1134. [Google Scholar] [CrossRef]
- Radstaak, M.; Geurts, S.A.; Brosschot, J.F.; Kompier, M.A. Music and psychophysiological recovery from stress. Psychosom. Med. 2014, 76, 529–537. [Google Scholar] [CrossRef]
- Villada, C.; Hidalgo, V.; Almela, M.; Salvador, A. Individual differences in the psychobiological response to psychosocial stress (Trier social stress test): The relevance of trait anxiety and coping styles. Stress Health 2016, 32, 90–99. [Google Scholar] [CrossRef]
- Chen, G.; Mishra, V.; Chen, C.-H. Temporal factors of listening to music on stress reduction. In Adjunct Proceedings of the 2019 ACM International Joint Conference on Pervasive and Ubiquitous Computing and Proceedings of the 2019 ACM International Symposium on Wearable Computers, London, UK, 9–13 September 2019; ACM: New York, NY, USA, 2019; pp. 907–914. [Google Scholar] [CrossRef]
- Knight, W.E.; Rickard, N.S. Relaxing music prevents stress-induced increases in subjective anxiety, systolic blood pressure, and heart rate in healthy males and females. J. Music. Ther. 2001, 38, 254–272. [Google Scholar] [CrossRef] [PubMed]
- Mücke, M.; Ludyga, S.; Colledge, F.; Gerber, M. Influence of regular physical activity and fitness on stress reactivity as measured with the trier social stress test protocol: A systematic review. Sports Med. 2018, 48, 2607–2622. [Google Scholar] [CrossRef]
- Kuan, G.; Morris, T.; Kueh, Y.C.; Terry, P.C. Effects of relaxing and arousing music during imagery training on dart-throwing performance, physiological arousal indices, and competitive state anxiety. Front. Psychol. 2018, 9, 14. [Google Scholar] [CrossRef] [Green Version]
- Karageorghis, C.I.; Jones, L.; Low, D.C. Relationship between exercise heart rate and music tempo preference. Res. Q. Exerc. Sport 2006, 77, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Karageorghis, C.I.; Jones, L.; Priest, D.-L.; Akers, R.I.; Clarke, A.; Perry, J.M.; Reddick, B.T.; Bishop, D.T.; Lim, H.B. Revisiting the relationship between exercise heart rate and music tempo preference. Res. Q. Exerc. Sport 2011, 82, 274–284. [Google Scholar] [CrossRef]
- Bailes, J.E.; Petraglia, A.L.; Omalu, B.I.; Nauman, E.; Talavage, T. Role of subconcussion in repetitive mild traumatic brain injury: A review. J. Neurosurg. 2013, 119, 1235–1245. [Google Scholar] [CrossRef] [PubMed]

| Variables | NCM (n = 27) | NCS (n = 24) | CM (n = 17) | CS (n = 16) | p-Value | |
|---|---|---|---|---|---|---|
| Demographic characteristics | Sex (women/men) | 14/13 | 9/15 | 7/10 | 7/9 | 0.767 |
| Age (years) | 20.89 (1.48) | 22.00 (2.96) | 22.76 (3.47) | 23.81 (4.98) | 0.235 | |
| Years of education | 16.83 (1.93) | 16.92 (2.50) | 16.65 (2.18) | 16.27 (2.52) | 0.833 | |
| Years of music training | 2.30 (3.01) | 1.67 (2.88) | 1.43 (2.36) | 2.13 (3.20) | 0.750 | |
| Sport practice | Type of sports (contact/team with reduced contact/aquatic/endurance/ ball-racket/balance-coordination) | 4/4/10/4/4/1 | 9/0/2/8/5/0 | 11/2/1/1/0/2 | 5/2/1/5/0/3 | - |
| Affective symptoms | PSS-10 (stress, max score = 40) | 13.22 (5.98) | 14.04 (4.89) | 14.06 (5.36) | 14.50 (6.61) | 0.899 |
| STAI-State (anxiety, max score = 80) | 32.07 (7.65) | 33.04 (7.49) | 33.24 (6.05) | 30.50 (5.03) | 0.634 | |
| STAI-Trait (anxiety, max score = 80) | 37.67 (6.93) | 37.92 (7.17) | 36.35 (6.05) | 35.63 (5.48) | 0.663 | |
| Concussion information | Number of previous concussions | - | - | 2.88 (1.69) | 1.88 (1.20) | 0.059 |
| Delay since latest concussion (months) | - | - | 33.80 (20.48) | 51.53 (54.08) | 0.250 | |
| Rivermead (persistent post-concussive symptoms) | - | - | 6.47 (4.97) | 6.93 (5.80) | 0.815 |
| NCM | CM | p-Value | |
|---|---|---|---|
| Valence | 71.89 (16.05) | 84.27 (14.91) | 0.019 * |
| Arousal | 22.00 (10.40) | 13.73 (12.29) | 0.026 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Léonard, C.; Desaulniers-Simon, J.M.; Tat, D.; De Beaumont, L.; Gosselin, N. Effects of Music Intervention on Stress in Concussed and Non-Concussed Athletes. Brain Sci. 2021, 11, 1501. https://doi.org/10.3390/brainsci11111501
Léonard C, Desaulniers-Simon JM, Tat D, De Beaumont L, Gosselin N. Effects of Music Intervention on Stress in Concussed and Non-Concussed Athletes. Brain Sciences. 2021; 11(11):1501. https://doi.org/10.3390/brainsci11111501
Chicago/Turabian StyleLéonard, Camille, Jeanne Marie Desaulniers-Simon, Diana Tat, Louis De Beaumont, and Nathalie Gosselin. 2021. "Effects of Music Intervention on Stress in Concussed and Non-Concussed Athletes" Brain Sciences 11, no. 11: 1501. https://doi.org/10.3390/brainsci11111501
APA StyleLéonard, C., Desaulniers-Simon, J. M., Tat, D., De Beaumont, L., & Gosselin, N. (2021). Effects of Music Intervention on Stress in Concussed and Non-Concussed Athletes. Brain Sciences, 11(11), 1501. https://doi.org/10.3390/brainsci11111501






