Subjective Time in Dementia: A Critical Review
Abstract
:| Cognitive offloading: the use of physical action or external tools to alter the information processing requirements of a task and reduce internal cognitive demand. | Metacognition: cognition about cognition; the capacity to monitor, evaluate, and control one’s own cognitive processes. |
| Core network: a network of brain regions that show increased activity both when people remember past experiences and when they imagine future experiences. | Prospective memory: the ability to remember to carry out intentions in the future. |
| Delay discounting: the decline in the subjective value of an outcome with the delay to its receipt. | Retrospective time judgment: subjective timing judgments made at the end of an experiment without prior instructions to keep track of time. |
| Duration discrimination: deciding whether a comparison duration is shorter or longer than a presented duration. | Time estimation: tasks requiring participants to estimate how long stimuli were presented. |
| Episodic future thinking: the capacity to imagine or simulate experiences that might occur in one’s personal future. | Time perception: the ability to perceive, judge, and represent time intervals. |
| Episodic memory: recollection of personally experienced events situated within a unique spatial and temporal context. | Time production: the requirement to produce or generate experimenter-specified durations. |
| Intertemporal decision-making: making decisions with consequences that play out only over time, often involving trade-offs between sooner and later costs and benefits. | Time reproduction: the ability to reproduce specific durations presented by an experimenter. |
| Mental time travel: the capacity to mentally navigate through subjective time, including episodic memory and episodic foresight/future thinking. |
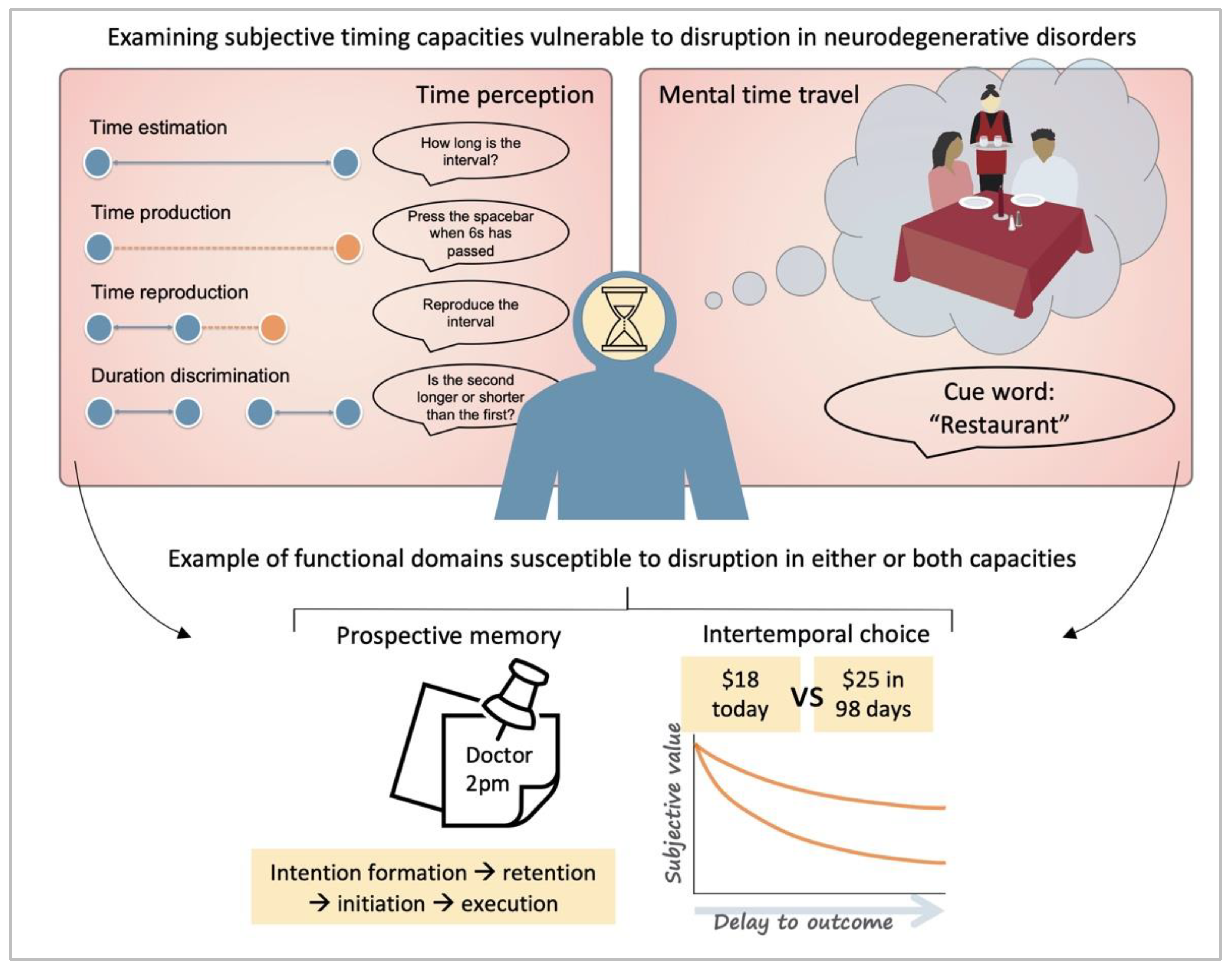
1. Time Perception
1.1. The Foundations of Time Perception
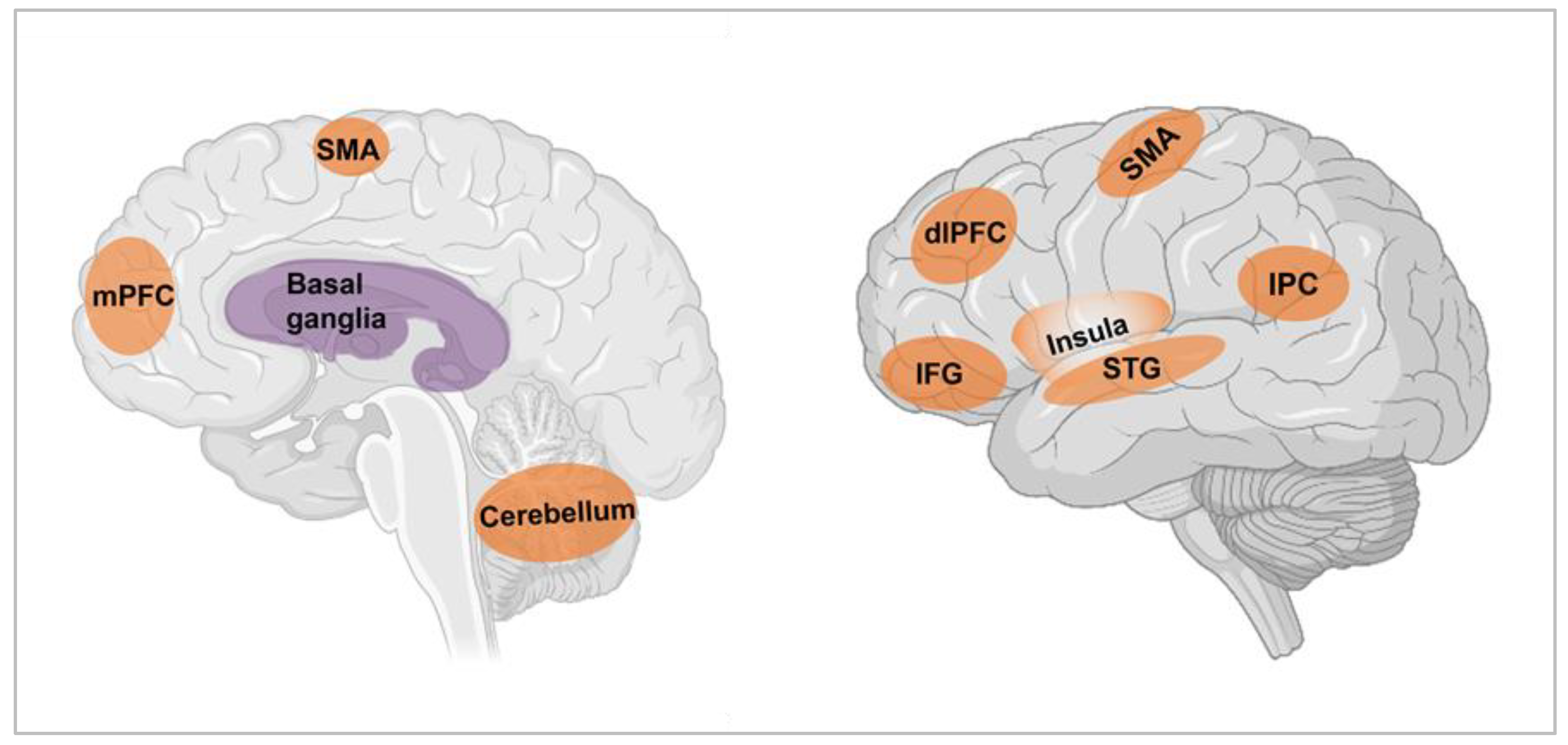
1.2. Neural Substrates of Time Perception
2. Mental Time Travel
2.1. The Foundations of Mental Time Travel
2.2. Neural Substrates of Mental Time Travel
3. Why Study Subjective Time in Neurodegenerative Disorders?
Clinical Reports of Subjective Timing Difficulties in Dementia
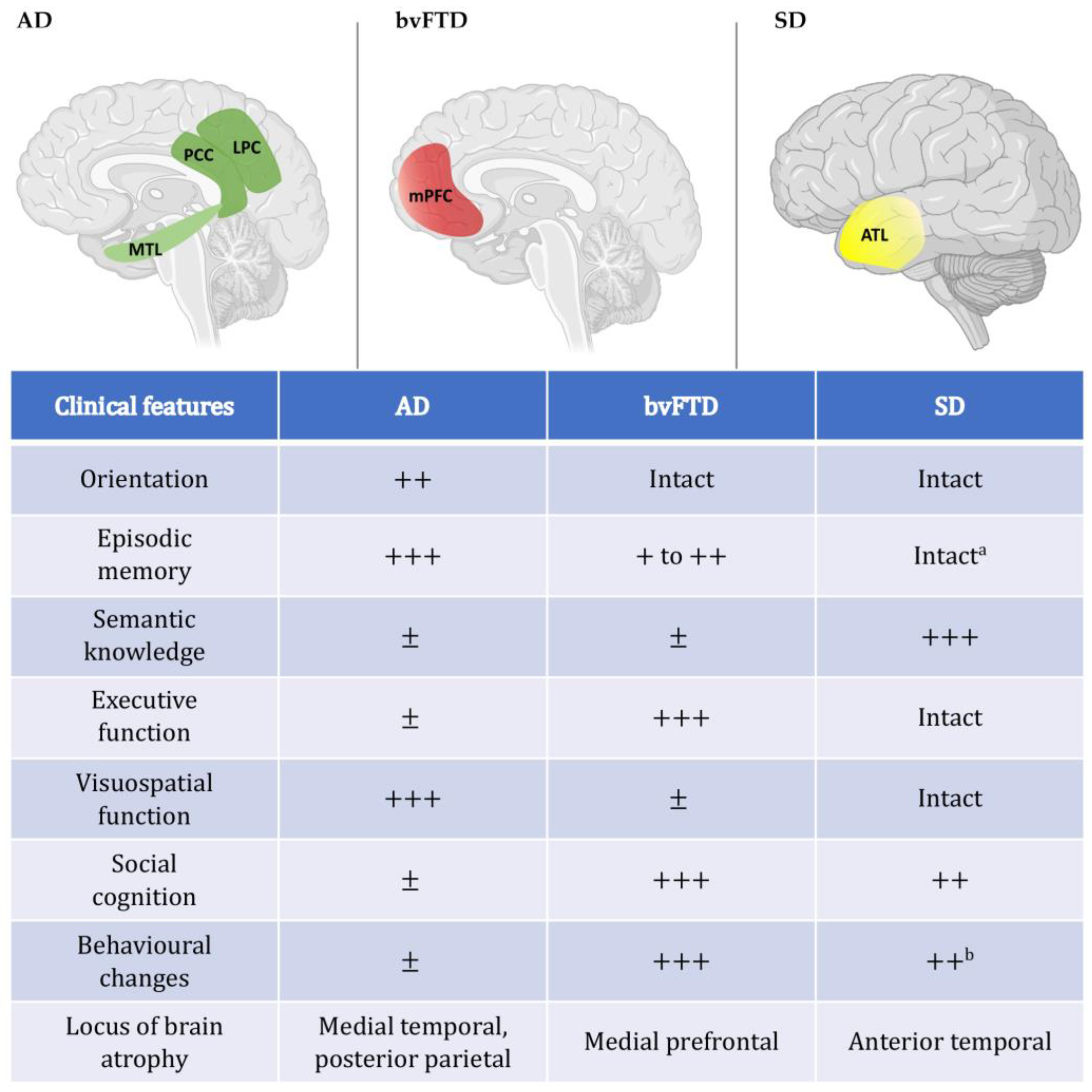
4. Time Perception in Dementia
4.1. Prospective Timing in Alzheimer’s Disease
4.2. Retrospective Timing in Alzheimer’s Disease
4.3. Prospective Timing in Frontotemporal Dementia
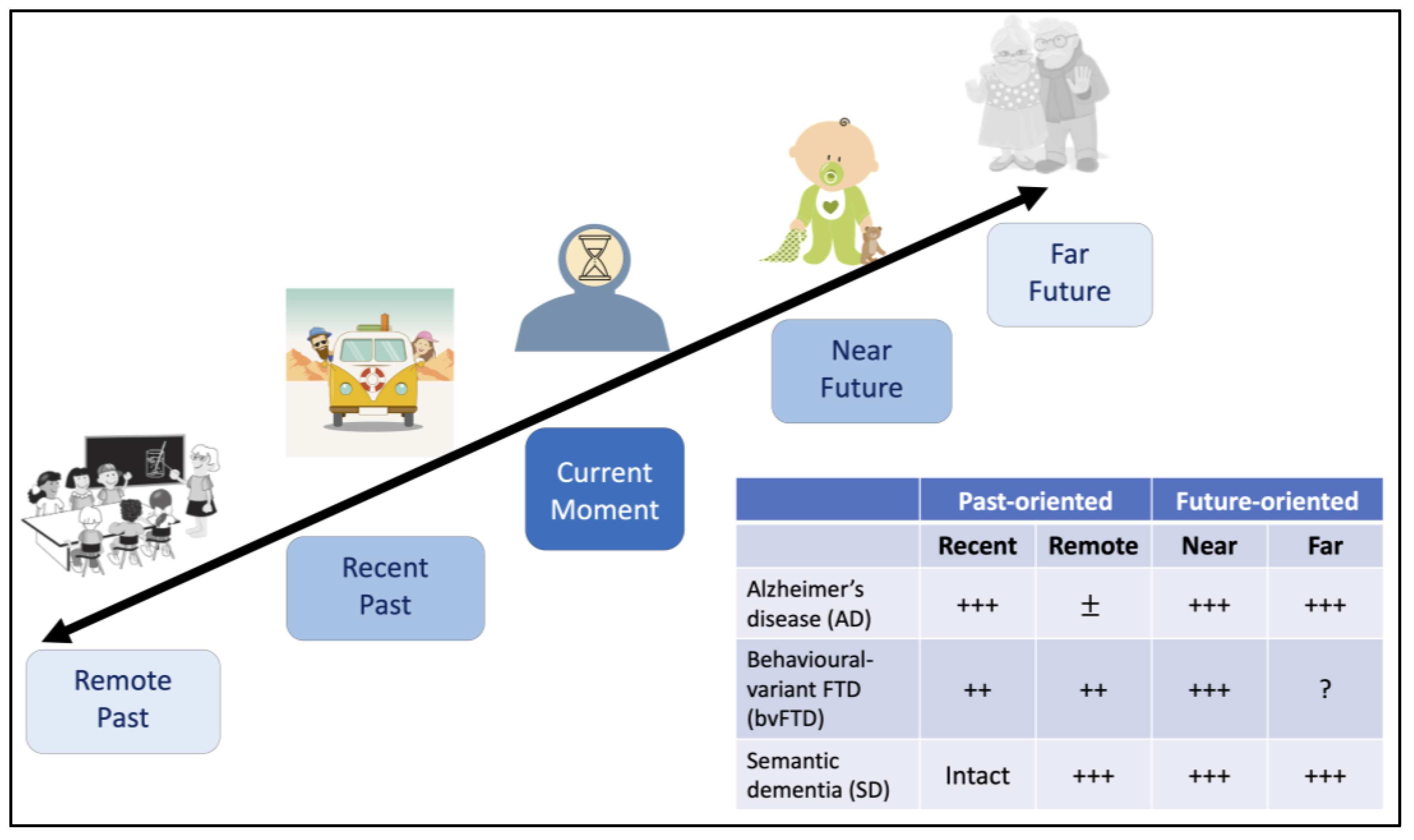
5. Disrupted Capacity for Mental Time Travel in Dementia
5.1. Revisiting the Past
5.2. Imagining the Future
6. Functional Relevance of Subjective Time Disturbances in Dementia
6.1. Disruptions in Intertemporal Choice
6.2. Prospective Memory Impairments
7. Improving Measurement and Assessment
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bulley, A.; Irish, M. The functions of prospection—Variations in health and disease. Front. Psychol. 2018, 9, 2328. [Google Scholar] [CrossRef]
- Buonomano, D. Your Brain Is a Time Machine: The Neuroscience and Physics of Time; WW Norton & Company: New York, NY, USA, 2017. [Google Scholar]
- Schacter, D.L.; Benoit, R.G.; Szpunar, K.K. Episodic future thinking: Mechanisms and functions. Curr. Opin. Behav. Sci. 2017, 17, 41–50. [Google Scholar] [CrossRef]
- Suddendorf, T.; Corballis, M.C. The evolution of foresight: What is mental time travel, and is it unique to humans? Behav. Brain Sci. 2007, 30, 299–351. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, M. The inner sense of time: How the brain creates a representation of duration. Nat. Rev. Neurosci. 2013, 14, 217–223. [Google Scholar] [CrossRef]
- Klein, S.B. The self and its brain. Soc. Cogn. 2012, 30, 474–518. [Google Scholar] [CrossRef]
- Strikwerda-Brown, C.; Grilli, M.D.; Andrews-Hanna, J.; Irish, M. “All is not lost”-Rethinking the nature of memory and the self in dementia. Ageing Res. Rev. 2019, 54, 100932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allman, M.J.; Teki, S.; Griffiths, T.D.; Meck, W.H. Properties of the internal clock: First- and second-order principles of subjective time. Annu. Rev. Psychol. 2014, 65, 743–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grondin, S. Timing and time perception: A review of recent behavioral and neuroscience findings and theoretical directions. Atten. Percept. Psychophys. 2010, 72, 561–582. [Google Scholar] [CrossRef]
- Ben Malek, H.; D’Argembeau, A.; Alle, M.C.; Meyer, N.; Danion, J.M.; Berna, F. Temporal processing of past and future autobiographical events in patients with schizophrenia. Sci. Rep. 2019, 9, 13858. [Google Scholar] [CrossRef] [Green Version]
- Thoenes, S.; Oberfeld, D. Meta-analysis of time perception and temporal processing in schizophrenia: Differential effects on precision and accuracy. Clin. Psychol. Rev. 2017, 54, 44–64. [Google Scholar] [CrossRef]
- Vicario, C.M.; Felmingham, K.L. Slower time estimation in Post-traumatic stress disorder. Sci. Rep. 2018, 8, 392. [Google Scholar] [CrossRef] [Green Version]
- Allan, L.G. The perception of time. Percept. Psychophys. 1979, 26, 340–354. [Google Scholar] [CrossRef] [Green Version]
- Risko, E.F.; Gilbert, S.J. Cognitive Offloading. Trends Cogn. Sci. 2016, 20, 676–688. [Google Scholar] [CrossRef]
- Block, R.A.; Zakay, D. Prospective and retrospective duration judgments: A meta-analytic review. Psychon. Bull. Rev. 1997, 4, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Grondin, S. Sensory modalities and temporal processing. In Time and Mind II: Information Processing Perspectives; Helfrich, H., Ed.; Hogrefe & Huber: Gottingen, Germany, 2003. [Google Scholar]
- Love, S.A.; Petrini, K.; Cheng, A.; Pollick, F.E. A psychophysical investigation of differences between synchrony and temporal order judgments. PLoS ONE 2013, 8, e54798. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, R. Threshold models of temporal-order judgments evaluated by a ternary response task. Percept. Psychophys. 1987, 42, 224–239. [Google Scholar] [CrossRef] [Green Version]
- Hoerl, C.; McCormack, T. Thinking in and about time: A dual systems perspective on temporal cognition. Behav. Brain Sci. 2018, 42, e244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nani, A.; Manuello, J.; Liloia, D.; Duca, S.; Costa, T.; Cauda, F. The neural correlates of time: A meta-analysis of neuroimaging studies. J. Cogn. Neurosci. 2019, 31, 1796–1826. [Google Scholar] [CrossRef] [PubMed]
- Ortuno, F.; Guillen-Grima, F.; Lopez-Garcia, P.; Gomez, J.; Pla, J. Functional neural networks of time perception: Challenge and opportunity for schizophrenia research. Schizophr. Res. 2011, 125, 129–135. [Google Scholar] [CrossRef]
- Schwartze, M.; Rothermich, K.; Kotz, S.A. Functional dissociation of pre-SMA and SMA-proper in temporal processing. NeuroImage 2012, 60, 290–298. [Google Scholar] [CrossRef]
- Wiener, M.; Turkeltaub, P.; Coslett, H.B. The image of time: A voxel-wise meta-analysis. NeuroImage 2010, 49, 1728–1740. [Google Scholar] [CrossRef] [PubMed]
- Mauk, M.D.; Buonomano, D.V. The neural basis of temporal processing. Annu. Rev. Neurosci. 2004, 27, 307–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, P.A.; Miall, R.C. A right hemispheric prefrontal system for cognitive time measurement. Behav. Process. 2006, 71, 226–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radua, J.; Del Pozo, N.O.; Gomez, J.; Guillen-Grima, F.; Ortuno, F. Meta-analysis of functional neuroimaging studies indicates that an increase of cognitive difficulty during executive tasks engages brain regions associated with time perception. Neuropsychologia 2014, 58, 14–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Kock, R.; Gladhill, K.A.; Ali, M.N.; Joiner, W.M.; Wiener, M. How movements shape the perception of time. Trends Cogn. Sci. 2021, 25, 950–963. [Google Scholar] [CrossRef]
- Wittmann, M.; Simmons, A.N.; Aron, J.L.; Paulus, M.P. Accumulation of neural activity in the posterior insula encodes the passage of time. Neuropsychologia 2010, 48, 3110–3120. [Google Scholar] [CrossRef] [Green Version]
- Craig, A.D. How do you feel—Now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009, 10, 59–70. [Google Scholar] [CrossRef]
- Bueti, D.; van Dongen, E.V.; Walsh, V. The role of superior temporal cortex in auditory timing. PLoS ONE 2008, 3, e2481. [Google Scholar] [CrossRef] [Green Version]
- Wiener, M.; Hamilton, R.; Turkeltaub, P.; Matell, M.S.; Coslett, H.B. Fast forward: Supramarginal gyrus stimulation alters time measurement. J. Cogn. Neurosci. 2010, 22, 23–31. [Google Scholar] [CrossRef] [Green Version]
- Merchant, H.; Harrington, D.L.; Meck, W.H. Neural basis of the perception and estimation of time. Annu. Rev. Neurosci. 2013, 36, 313–336. [Google Scholar] [CrossRef]
- Wiener, M.; Matell, M.S.; Coslett, H.B. Multiple mechanisms for temporal processing. Front. Integr. Neurosci. 2011, 5, 31. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.B.; Giampietro, V.; Brammer, M.; Halari, R.; Simmons, A.; Rubia, K. Functional development of fronto-striato-parietal networks associated with time perception. Front. Hum. Neurosci. 2011, 5, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, R.M.; Devenney, E.M.; Irish, M.; Ittner, A.; Naismith, S.; Ittner, L.M.; Rohrer, J.D.; Halliday, G.M.; Eisen, A.; Hodges, J.R.; et al. Neuronal network disintegration: Common pathways linking neurodegenerative diseases. J. Neurol. Neurosurg. Psychiatry 2016, 87, 1234–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irish, M.; Piguet, O.; Hodges, J.R. Self-projection and the default network in frontotemporal dementia. Nat. Rev. Neurol. 2012, 8, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.B.; Steindam, C. The role of subjective temporality in future-oriented mental time travel. In Seeing the Future: Theoretical Perspectives on Future-Oriented Mental Time Travel; Michaelian, K., Klein, S.B., Szpunar, K.K., Eds.; Oxford University Press: New York, NY, USA, 2016; pp. 135–152. [Google Scholar]
- Atance, C.M.; O’Neill, D.K. Episodic future thinking. Trends Cogn. Sci. 2001, 5, 533–539. [Google Scholar] [CrossRef]
- Tulving, E. Episodic memory: From mind to brain. Annu. Rev. Psychol. 2002, 53, 1–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irish, M.; Piguet, O. The pivotal role of semantic memory in remembering the past and imagining the future. Front. Behav. Neurosci. 2013, 7, 27. [Google Scholar] [CrossRef] [Green Version]
- Schacter, D.L.; Addis, D.R.; Buckner, R.L. Remembering the past to imagine the future: The prospective brain. Nat. Rev. Neurosci. 2007, 8, 657–661. [Google Scholar] [CrossRef]
- Friedman, W. Time in autobiographical memory. Soc. Cogn. 2004, 22, 591–605. [Google Scholar] [CrossRef]
- Mahr, J.B.; Greene, J.D.; Schacter, D.L. A long time ago, in a galaxy far, far away: How temporal are episodic contents? Conscious Cogn 2021, 96, 103224. [Google Scholar] [CrossRef] [PubMed]
- Redshaw, J. Does metarepresentation make human mental time travel unique? Wiley Interdiscip. Rev. Cogn. Sci. 2014, 5, 519–531. [Google Scholar] [CrossRef]
- Bulley, A.; Henry, J.D.; Suddendorf, T. Thinking about threats: Memory and prospection in human threat management. Conscious Cogn. 2017, 49, 53–69. [Google Scholar] [CrossRef]
- Miloyan, B.; Bulley, A.; Suddendorf, T. Anxiety: Here and beyond. Emot. Rev. 2019, 11, 39–49. [Google Scholar] [CrossRef]
- Suddendorf, T.; Bulley, A.; Miloyan, B. Prospection and natural selection. Curr. Opin. Behav. Sci. 2018, 24, 26–31. [Google Scholar] [CrossRef]
- Bulley, A.; Redshaw, J.; Suddendorf, T. The future-directed functions of the imagination: From prediction to metaforesight. In The Cambridge Handbook of the Imagination; Abraham, A., Ed.; Cambridge University Press: Cambridge, UK, 2020; pp. 425–443. [Google Scholar]
- Buckner, R.L.; Carroll, D.C. Self-projection and the brain. Trends Cogn. Sci. 2007, 11, 49–57. [Google Scholar] [CrossRef]
- Hassabis, D.; Kumaran, D.; Maguire, E.A. Using imagination to understand the neural basis of episodic memory. J. Neurosci. 2007, 27, 14365–14374. [Google Scholar] [CrossRef] [PubMed]
- Schacter, D.L.; Addis, D.R. The cognitive neuroscience of constructive memory: Remembering the past and imagining the future. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2007, 362, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Schacter, D.L.; Addis, D.R. On the nature of medial temporal lobe contributions to the constructive simulation of future events. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 1245–1253. [Google Scholar] [CrossRef] [Green Version]
- Eichenbaum, H. Time (and space) in the hippocampus. Curr. Opin. Behav. Sci. 2017, 17, 65–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranganath, C.; Hsieh, L.T. The hippocampus: A special place for time. Ann. N. Y. Acad. Sci. 2016, 1369, 93–110. [Google Scholar] [CrossRef]
- McCormick, C.; Ciaramelli, E.; De Luca, F.; Maguire, E.A. Comparing and Contrasting the Cognitive Effects of Hippocampal and Ventromedial Prefrontal Cortex Damage: A Review of Human Lesion Studies. Neuroscience 2018, 374, 295–318. [Google Scholar] [CrossRef]
- Gilboa, A.; Marlatte, H. Neurobiology of schemas and schema-mediated memory. Trends Cogn. Sci. 2017, 21, 618–631. [Google Scholar] [CrossRef] [PubMed]
- Benoit, R.G.; Szpunar, K.K.; Schacter, D.L. Ventromedial prefrontal cortex supports affective future simulation by integrating distributed knowledge. Proc. Natl. Acad. Sci. USA 2014, 111, 16550–16555. [Google Scholar] [CrossRef] [Green Version]
- Hassabis, D.; Spreng, R.N.; Rusu, A.A.; Robbins, C.A.; Mar, R.A.; Schacter, D.L. Imagine all the people: How the brain creates and uses personality models to predict behavior. Cereb. Cortex 2014, 24, 1979–1987. [Google Scholar] [CrossRef] [Green Version]
- Northoff, G.; Bermpohl, F. Cortical midline structures and the self. Trends Cogn. Sci. 2004, 8, 102–107. [Google Scholar] [CrossRef]
- Demblon, J.; Bahri, M.A.; D’Argembeau, A. Neural correlates of event clusters in past and future thoughts: How the brain integrates specific episodes with autobiographical knowledge. NeuroImage 2016, 127, 257–266. [Google Scholar] [CrossRef]
- Addis, D.R.; Wong, A.T.; Schacter, D.L. Remembering the past and imagining the future: Common and distinct neural substrates during event construction and elaboration. Neuropsychologia 2007, 45, 1363–1377. [Google Scholar] [CrossRef] [Green Version]
- Nyberg, L.; Kim, A.S.; Habib, R.; Levine, B.; Tulving, E. Consciousness of subjective time in the brain. Proc. Natl. Acad. Sci. USA 2010, 107, 22356–22359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szpunar, K.K.; Watson, J.M.; McDermott, K.B. Neural substrates of envisioning the future. Proc. Natl. Acad. Sci. USA 2007, 104, 642–647. [Google Scholar] [CrossRef] [Green Version]
- Gilmore, A.W.; Nelson, S.M.; McDermott, K.B. A parietal memory network revealed by multiple MRI methods. Trends Cogn. Sci. 2015, 19, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Aminoff, E.; Gronau, N.; Bar, M. The parahippocampal cortex mediates spatial and nonspatial associations. Cereb. Cortex 2007, 17, 1493–1503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mullally, S.L.; Maguire, E.A. A new role for the parahippocampal cortex in representing space. J. Neurosci. 2011, 31, 7441–7449. [Google Scholar] [CrossRef] [Green Version]
- Thakral, P.P.; Madore, K.P.; Schacter, D.L. The core episodic simulation network dissociates as a function of subjective experience and objective content. Neuropsychologia 2020, 136, 107263. [Google Scholar] [CrossRef]
- Ramanan, S.; Piguet, O.; Irish, M. Rethinking the role of the angular gyrus in remembering the past and imagining the future: The contextual integration model. Neuroscientist 2018, 24, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Strikwerda-Brown, C.; Mothakunnel, A.; Hodges, J.R.; Piguet, O.; Irish, M. External details revisited—A new taxonomy for coding ‘non-episodic’ content during autobiographical memory retrieval. J. Neuropsychol. 2018, 13, 371–397. [Google Scholar] [CrossRef]
- Seeley, W.W.; Crawford, R.K.; Zhou, J.; Miller, B.L.; Greicius, M.D. Neurodegenerative diseases target large-scale human brain networks. Neuron 2009, 62, 42–52. [Google Scholar] [CrossRef] [Green Version]
- El Haj, M.; Kapogiannis, D. Time distortions in Alzheimer’s disease: A systematic review and theoretical integration. NPJ Aging Mech. Dis. 2016, 2, 16016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrington, D.L.; Castillo, G.N.; Greenberg, P.A.; Song, D.D.; Lessig, S.; Lee, R.R.; Rao, S.M. Neurobehavioral mechanisms of temporal processing deficits in Parkinson’s disease. PLoS ONE 2011, 6, e17461. [Google Scholar] [CrossRef] [Green Version]
- Jones, C.R.; Jahanshahi, M. Motor and perceptual timing in Parkinson’s disease. Adv. Exp. Med. Biol. 2014, 829, 265–290. [Google Scholar] [CrossRef]
- Marinho, V.; Oliveira, T.; Rocha, K.; Ribeiro, J.; Magalhaes, F.; Bento, T.; Pinto, G.R.; Velasques, B.; Ribeiro, P.; Di Giorgio, L.; et al. The dopaminergic system dynamic in the time perception: A review of the evidence. Int. J. Neurosci. 2018, 128, 262–282. [Google Scholar] [CrossRef]
- Bernardinis, M.; Atashzar, S.F.; Jog, M.S.; Patel, R.V. Differential temporal perception abilities in Parkinson’s disease patients based on timing magnitude. Sci. Rep. 2019, 9, 19638. [Google Scholar] [CrossRef] [Green Version]
- Teri, L.; Borson, S.; Kiyak, H.A.; Yamagishi, M. Behavioral disturbance, cognitive dysfunction, and functional skill Prevalence and relationship in Alzheimer’s disease. J. Am. Geriatr. Soc. 1989, 37, 109–116. [Google Scholar]
- Levy, B.; Dreier, T. Preservation of temporal skills in Alzheimer’s Disease. Percept. Mot. Skills. 1997, 85, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Jonveaux, T.R.; Batt, M.; Empereur, F.; Braun, M.; Trognon, A. Evaluation of temporality semantic knowledge in normal aging and in mild and moderate stages of Alzheimer’s disease. Encephale 2015, 41, 137–143. [Google Scholar] [CrossRef]
- El Haj, M.; Moroni, C.; Samson, S.; Fasotti, L.; Allain, P. Prospective and retrospective time perception are related to mental time travel: Evidence from Alzheimer’s disease. Brain Cogn. 2013, 83, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Yew, B.; Alladi, S.; Shailaja, M.; Hodges, J.R.; Hornberger, M. Lost and forgotten? Orientation versus memory in Alzheimer’s disease and frontotemporal dementia. J. Alzheimers Dis. 2013, 33, 473–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyatsanza, S.; Shetty, T.; Gregory, C.; Lough, S.; Dawson, K.; Hodges, J.R. A study of stereotypic behaviours in Alzheimer’s disease and frontal and temporal variant frontotemporal dementia. J. Neurol. Neurosurg. Psychiatry 2003, 74, 1398–1402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Callaghan, C.; Shine, J.M.; Hodges, J.R.; Andrews-Hanna, J.R.; Irish, M. Hippocampal atrophy and intrinsic brain network dysfunction relate to alterations in mind wandering in neurodegeneration. Proc. Natl. Acad. Sci. USA 2019, 116, 3316–3321. [Google Scholar] [CrossRef] [Green Version]
- O’Callaghan, C.; Irish, M. Candidate mechanisms of spontaneous cognition as revealed by dementia syndromes. In The Oxford Handbook of Spontaneous Thought: Mind-Wandering, Creativity, and Dreaming; Christoff, K., Fox, K.C., Eds.; Oxford University Press: New York, NY, USA, 2018; pp. 493–508. [Google Scholar] [CrossRef]
- Irish, M.; Piolino, P. Impaired capacity for prospection in the dementias—Theoretical and clinical implications. Br. J. Clin. Psychol. 2016, 55, 49–68. [Google Scholar] [CrossRef]
- Hodges, J.R.; Patterson, K. Semantic dementia: A unique clinicopathological syndrome. Lancet Neurol. 2007, 6, 1004–1014. [Google Scholar] [CrossRef]
- Snowden, J.S.; Bathgate, D.; Varma, A.; Blackshaw, A.; Gibbons, Z.C.; Neary, D. Distinct behavioural profiles in frontotemporal dementia and semantic dementia. J. Neurol. Neurosurg. Psychiatry 2001, 70, 323–332. [Google Scholar] [CrossRef] [Green Version]
- Requena-Komuro, M.C.; Marshall, C.R.; Bond, R.L.; Russell, L.L.; Greaves, C.; Moore, K.M.; Agustus, J.L.; Benhamou, E.; Sivasathiaseelan, H.; Hardy, C.J.D.; et al. Altered time awareness in dementia. Front Neurol. 2020, 11, 291. [Google Scholar] [CrossRef] [Green Version]
- El Haj, M.; Omigie, D.; Moroni, C. Time reproduction during high and low attentional tasks in Alzheimer’s Disease. “A watched kettle never boils”. Brain Cogn. 2014, 88, 1–5. [Google Scholar] [CrossRef]
- Nichelli, P.; Venneri, A.; Molinari, M.; Tavani, F.; Grafman, J. Precision and accuracy of subjective time estimation in different memory disorders. Cogn. Brain Res. 1993, 1, 87–93. [Google Scholar] [CrossRef]
- Rueda, A.D.; Schmitter-Edgecombe, M. Time estimation abilities in mild cognitive impairment and Alzheimer’s disease. Neuropsychology 2009, 23, 178–188. [Google Scholar] [CrossRef]
- Carrasco, M.C.; Guillem, M.J.; Redolat, R. Estimation of short temporal intervals in Alzheimer’s disease. Exp. Aging Res. 2000, 26, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Caselli, L.; Iaboli, L.; Nichelli, P. Time estimation in mild Alzheimer’s disease patients. Behav. Brain Funct. 2009, 5, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papagno, C.; Allegra, A.; Cardaci, M. Time estimation in Alzheimer’s disease and the role of the central executive. Brain Cogn. 2004, 54, 18–23. [Google Scholar] [CrossRef]
- Heinik, J. Accuracy of estimation of time-intervals in psychogeriatric outpatients. Int. Psychogeriatr. 2012, 24, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Mioni, G.; Roman-Caballero, R.; Clerici, J.; Capizzi, M. Prospective and retrospective timing in mild cognitive impairment and Alzheimer’s disease patients: A systematic review and meta-analysis. Behav. Brain Res. 2021, 410, 113354. [Google Scholar] [CrossRef]
- Wiener, M.; Coslett, H.B. Disruption of temporal processing in a subject with probable frontotemporal dementia. Neuropsychologia 2008, 46, 1927–1939. [Google Scholar] [CrossRef] [Green Version]
- Henley, S.M.; Downey, L.E.; Nicholas, J.M.; Kinnunen, K.M.; Golden, H.L.; Buckley, A.; Mahoney, C.J.; Crutch, S.J. Degradation of cognitive timing mechanisms in behavioural variant frontotemporal dementia. Neuropsychologia 2014, 65, 88–101. [Google Scholar] [CrossRef] [Green Version]
- Kamminga, J.; O’Callaghan, C.; Hodges, J.R.; Irish, M. Differential prospective memory profiles in frontotemporal dementia syndromes. J. Alzheimers Dis. 2014, 38, 669–679. [Google Scholar] [CrossRef]
- Irish, M.; Hodges, J.R. Autobiographical memory in Alzheimer’s disease and frontotemporal dementia: Practical and theoretical implications. In Research Progress in Alzheimer’s Disease and Dementia; Sun, M.K., Ed.; Nova Science Publishers: New York, NY, USA, 2012; pp. 51–75. [Google Scholar]
- Conway, M.A. Sensory-perceptual episodic memory and its context: Autobiographical memory. Philos. Trans. R Soc. Lond. B Biol. Sci. 2001, 356, 1375–1384. [Google Scholar] [CrossRef]
- Tulving, E. Memory and consciousness. Can. Psychol./Psychol. Can. 1985, 26, 1–12. [Google Scholar] [CrossRef]
- Kumfor, F.; Teo, D.; Miller, L.; Lah, S.; Mioshi, E.; Hodges, J.R.; Piguet, O.; Irish, M. Examining the relationship between autobiographical memory impairment and carer burden in dementia syndromes. J. Alzheimers Dis. 2016, 51, 237–248. [Google Scholar] [CrossRef]
- Ribot, T. Les Maladies de la Mémoire; Germer Baillière: Paris, France, 1881. [Google Scholar]
- Kopelman, M.D.; Wilson, B.A.; Baddeley, A.D. The autobiographical memory interview: A new assessment of autobiographical and personal semantic memory in amnesic patients. J. Clin. Exp. Neuropsychol. 1989, 11, 724–744. [Google Scholar] [CrossRef] [PubMed]
- Greene, J.D.; Hodges, J.R.; Baddeley, A.D. Autobiographical memory and executive function in early dementia of Alzheimer type. Neuropsychologia 1995, 33, 1647–1670. [Google Scholar] [CrossRef]
- Irish, M.; Cunningham, C.J.; Walsh, J.B.; Coakley, D.; Lawlor, B.A.; Robertson, I.H.; Coen, R.F. Investigating the enhancing effect of music on autobiographical memory in mild Alzheimer’s disease. Dement Geriatr. Cogn. Disord. 2006, 22, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Irish, M.; Lawlor, B.A.; O’Mara, S.M.; Coen, R.F. Impaired capacity for autonoetic reliving during autobiographical event recall in mild Alzheimer’s disease. Cortex 2011, 47, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Piolino, P.; Desgranges, B.; Belliard, S.; Matuszewski, V.; Lalevee, C.; De la Sayette, V.; Eustache, F. Autobiographical memory and autonoetic consciousness: Triple dissociation in neurodegenerative diseases. Brain 2003, 126, 2203–2219. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Svoboda, E.; Hay, J.F.; Winocur, G.; Moscovitch, M. Aging and autobiographical memory: Dissociating episodic from semantic retrieval. Psychol. Aging 2002, 17, 677–689. [Google Scholar] [CrossRef]
- Barnabe, A.; Whitehead, V.; Pilon, R.; Arsenault-Lapierre, G.; Chertkow, H. Autobiographical memory in mild cognitive impairment and Alzheimer’s disease: A comparison between the Levine and Kopelman interview methodologies. Hippocampus 2012, 22, 1809–1825. [Google Scholar] [CrossRef] [PubMed]
- Irish, M.; Hornberger, M.; El Wahsh, S.; Lam, B.Y.; Lah, S.; Miller, L.; Hsieh, S.; Hodges, J.R.; Piguet, O. Grey and white matter correlates of recent and remote autobiographical memory retrieval--insights from the dementias. PLoS ONE 2014, 9, e113081. [Google Scholar] [CrossRef]
- Irish, M.; Hornberger, M.; Lah, S.; Miller, L.; Pengas, G.; Nestor, P.J.; Hodges, J.R.; Piguet, O. Profiles of recent autobiographical memory retrieval in semantic dementia, behavioural-variant frontotemporal dementia, and Alzheimer’s disease. Neuropsychologia 2011, 49, 2694–2702. [Google Scholar] [CrossRef]
- Irish, M.; Landin-Romero, R.; Mothakunnel, A.; Ramanan, S.; Hsieh, S.; Hodges, J.R.; Piguet, O. Evolution of autobiographical memory impairments in Alzheimer’s disease and frontotemporal dementia—A longitudinal neuroimaging study. Neuropsychologia 2018, 110, 14–25. [Google Scholar] [CrossRef]
- El Haj, M.; Antoine, P.; Nandrino, J.L.; Kapogiannis, D. Autobiographical memory decline in Alzheimer’s disease, a theoretical and clinical overview. Ageing Res. Rev. 2015, 23, 183–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irish, M.; Lawlor, B.A.; O’Mara, S.M.; Coen, R.F. Exploring the recollective experience during autobiographical memory retrieval in amnestic mild cognitive impairment. J. Int. Neuropsychol. Soc. 2010, 16, 546–555. [Google Scholar] [CrossRef] [Green Version]
- Irish, M.; Kamminga, J.; Addis, D.R.; Crain, S.; Thornton, R.; Hodges, J.R.; Piguet, O. ‘Language of the past’—Exploring past tense disruption during autobiographical narration in neurodegenerative disorders. J. Neuropsychol. 2016, 10, 295–316. [Google Scholar] [CrossRef]
- Rascovsky, K.; Hodges, J.R.; Knopman, D.; Mendez, M.F.; Kramer, J.H.; Neuhaus, J.; van Swieten, J.C.; Seelaar, H.; Dopper, E.G.; Onyike, C.U.; et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011, 134, 2456–2477. [Google Scholar] [CrossRef]
- Matuszewski, V.; Piolino, P.; de la Sayette, V.; Lalevee, C.; Pelerin, A.; Dupuy, B.; Viader, F.; Eustache, F.; Desgranges, B. Retrieval mechanisms for autobiographical memories: Insights from the frontal variant of frontotemporal dementia. Neuropsychologia 2006, 44, 2386–2397. [Google Scholar] [CrossRef]
- Piolino, P.; Chetelat, G.; Matuszewski, V.; Landeau, B.; Mezenge, F.; Viader, F.; de la Sayette, V.; Eustache, F.; Desgranges, B. In search of autobiographical memories: A PET study in the frontal variant of frontotemporal dementia. Neuropsychologia 2007, 45, 2730–2743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas-Anterion, C.; Jacquin, K.; Laurent, B. Differential mechanisms of impairment of remote memory in Alzheimer’s and frontotemporal dementia. Dement. Geriatr. Cogn. Disord. 2000, 11, 100–106. [Google Scholar] [CrossRef]
- Graham, K.S.; Hodges, J.R. Differentiating the roles of the hippocampus complex and the neocortex in long-term memory storage: Evidence from the study of semantic dementia and Alzheimer’s disease. Neuropsychology 1997, 11, 77. [Google Scholar] [CrossRef] [PubMed]
- Ivanoiu, A.; Cooper, J.M.; Shanks, M.F.; Venneri, A. Patterns of impairment in autobiographical memory in the degenerative dementias constrain models of memory. Neuropsychologia 2006, 44, 1936–1955. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.E.; Miller, B.L.; Kramer, J.H. Patterns of autobiographical memory loss in dementia. Int. J. Geriatr. Psychiatry 2005, 20, 809–815. [Google Scholar] [CrossRef]
- Adlam, A.L.; Patterson, K.; Hodges, J.R. “I remember it as if it were yesterday”: Memory for recent events in patients with semantic dementia. Neuropsychologia 2009, 47, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, M.C.; Black, S.E.; Miller, B.; Moscovitch, M.; Levine, B. Autobiographical memory in semantic dementia: Implications for theories of limbic-neocortical interaction in remote memory. Neuropsychologia 2006, 44, 2421–2429. [Google Scholar] [CrossRef] [Green Version]
- Renoult, L.; Irish, M.; Moscovitch, M.; Rugg, M.D. From knowing to remembering: The semantic-episodic distinction. Trends Cogn. Sci. 2019, 23, 1041–1057. [Google Scholar] [CrossRef]
- La Corte, V.; Piolino, P. On the role of personal semantic memory and temporal distance in episodic future thinking: The TEDIFT model. Front Hum. Neurosci. 2016, 10, 385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suddendorf, T.; Addis, D.R.; Corballis, M.C. Mental time travel and the shaping of the human mind. Philos. Trans. R Soc. Lond. B Biol. Sci. 2009, 364, 1317–1324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, D.T.; Wilson, T.D. Prospection: Experiencing the future. Science 2007, 317, 1351–1354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suddendorf, T.; Corballis, M.C. Mental time travel and the evolution of the human mind. Genet. Soc. Gen. Psychol. Monogr. 1997, 123, 133–167. [Google Scholar]
- Bulley, A. The history and future of human prospection. Evol. Stud. Imaginative Cult. 2018, 2, 75–94. [Google Scholar] [CrossRef] [Green Version]
- Addis, D.R.; Sacchetti, D.C.; Ally, B.A.; Budson, A.E.; Schacter, D.L. Episodic simulation of future events is impaired in mild Alzheimer’s disease. Neuropsychologia 2009, 47, 2660–2671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Haj, M.; Antoine, P.; Kapogiannis, D. Flexibility decline contributes to similarity of past and future thinking in Alzheimer’s disease. Hippocampus 2015, 25, 1447–1455. [Google Scholar] [CrossRef] [Green Version]
- El Haj, M.; Antoine, P.; Kapogiannis, D. Similarity between remembering the past and imagining the future in Alzheimer’s disease: Implication of episodic memory. Neuropsychologia 2015, 66, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Irish, M.; Hodges, J.R.; Piguet, O. Episodic future thinking is impaired in the behavioural variant of frontotemporal dementia. Cortex 2013, 49, 2377–2388. [Google Scholar] [CrossRef]
- Irish, M.; Addis, D.R.; Hodges, J.R.; Piguet, O. Exploring the content and quality of episodic future simulations in semantic dementia. Neuropsychologia 2012, 50, 3488–3495. [Google Scholar] [CrossRef]
- Irish, M.; Eyre, N.; Dermody, N.; O’Callaghan, C.; Hodges, J.R.; Hornberger, M.; Piguet, O. Neural Substrates of Semantic Prospection—Evidence from the Dementias. Front Behav. Neurosci. 2016, 10, 96. [Google Scholar] [CrossRef] [Green Version]
- Burgess, P.W.; Veitch, E.; de Lacy Costello, A.; Shallice, T. The cognitive and neuroanatomical correlates of multitasking. Neuropsychologia 2000, 38, 848–863. [Google Scholar] [CrossRef]
- Irish, M. Semantic memory as the essential scaffold for future-oriented mental time travel. In Seeing the Future: Theoretical Perspectives on Future-Oriented Mental Time Travel; Michaelian, K., Klein, S.B., Szpunar, K.K., Eds.; Oxford University Press: New York, NY, USA, 2016; pp. 389–408. [Google Scholar]
- Irish, M. On the interaction between episodic and semantic representations–constructing a unified account of imagination. In The Cambridge Handbook of Imagination; Abraham, A., Ed.; Cambridge University Press: New York, NY, USA, 2020; pp. 447–465. [Google Scholar]
- Duval, C.; Desgranges, B.; de La Sayette, V.; Belliard, S.; Eustache, F.; Piolino, P. What happens to personal identity when semantic knowledge degrades? A study of the self and autobiographical memory in semantic dementia. Neuropsychologia 2012, 50, 254–265. [Google Scholar] [CrossRef]
- Irish, M.; Addis, D.R.; Hodges, J.R.; Piguet, O. Considering the role of semantic memory in episodic future thinking: Evidence from semantic dementia. Brain 2012, 135, 2178–2191. [Google Scholar] [CrossRef] [Green Version]
- Viard, A.; Piolino, P.; Belliard, S.; de La Sayette, V.; Desgranges, B.; Eustache, F. Episodic future thinking in semantic dementia: A cognitive and FMRI study. PLoS ONE 2014, 9, e111046. [Google Scholar] [CrossRef] [Green Version]
- Irish, M.; Mothakunnel, A.; Dermody, N.; Wilson, N.A.; Hodges, J.R.; Piguet, O. Damage to right medial temporal structures disrupts the capacity for scene construction-a case study. Hippocampus 2017, 27, 635–641. [Google Scholar] [CrossRef] [PubMed]
- La Corte, V.; Ferrieux, S.; Abram, M.; Bertrand, A.; Dubois, B.; Teichmann, M.; Piolino, P. The role of semantic memory in prospective memory and episodic future thinking: New insights from a case of semantic dementia. Memory 2021, 29, 943–962. [Google Scholar] [CrossRef]
- Loewenstein, G.; Elster, J. Choice Over Time; Russell Sage Foundation: New York, NY, USA, 1992. [Google Scholar]
- Kable, J.W. Valuation, intertemporal choice, and self-control. In Neuroeconomics: Decision Making and the Brain, 2nd ed.; Glimcher, P.W., Fehr, E., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 173–192. [Google Scholar]
- Lempert, K.M.; Steinglass, J.E.; Pinto, A.; Kable, J.W.; Simpson, H.B. Can delay discounting deliver on the promise of RDoC? Psychol. Med. 2019, 49, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Redshaw, J.; Bulley, A. Future thinking in animals—Capacities and limits. In The Psychology of Thinking about the Future; Oettingen, G., Sevincer, A.T., Gollwitzer, P.M., Eds.; The Guildford Press: New York, NY, USA, 2018; pp. 31–51. [Google Scholar]
- Mischel, W.; Shoda, Y.; Rodriguez, M.I. Delay of gratification in children. Science 1989, 244, 933–938. [Google Scholar] [CrossRef] [Green Version]
- Amlung, M.; Marsden, E.; Holshausen, K.; Morris, V.; Patel, H.; Vedelago, L.; Naish, K.R.; Reed, D.D.; McCabe, R.E. Delay discounting as a transdiagnostic process in psychiatric disorders: A meta-analysis. JAMA Psychiatry 2019, 76, 1176–1186. [Google Scholar] [CrossRef] [PubMed]
- Angeletos, G.M.; Laibson, D.; Repetto, A.; Tobacman, J.; Weinberg, S. The hyperbolic consumption model: Calibration, simulation, and empirical evaluation. J. Econ. Perspect. 2001, 15, 47–68. [Google Scholar] [CrossRef] [Green Version]
- Bulley, A.; Pepper, G.V. Cross-country relationships between life expectancy, intertemporal choice and age at first birth. Evol. Hum. Behav. 2017, 38, 652–658. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.J.; DeBruine, L.M.; Jones, B.C. Individual-specific mortality is associated with how individuals evaluate future discounting decisions. Proc. Biol. Sci. 2018, 285, 20180304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wittmann, M.; Simmons, A.N.; Flagan, T.; Lane, S.D.; Wackermann, J.; Paulus, M.P. Neural substrates of time perception and impulsivity. Brain Res. 2011, 1406, 43–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wittmann, M.; Paulus, M.P. Decision making, impulsivity and time perception. Trends Cogn. Sci. 2007, 12, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.K.; Zauberman, G. Perception of anticipatory time in temporal discounting. J. Neurosci. Psychol. Econ. 2009, 2, 91–101. [Google Scholar] [CrossRef] [Green Version]
- Baumann, A.A.; Odum, A.L. Impulsivity, risk taking, and timing. Behav. Processes 2012, 90, 408–414. [Google Scholar] [CrossRef] [Green Version]
- Urminsky, O.; Zauberman, G. The Psychology of Intertemporal Preferences. In The Wiley Blackwell Handbook of Judgment and Decision Making, II; Keren, G., Wu, G., Eds.; John Wiley & Sons, Incorporated: Hoboken, NJ, USA, 2015; pp. 141–181. [Google Scholar] [CrossRef]
- Kim, B.K.; Zauberman, G. Psychological time and intertemporal preference. Curr. Opin. Psychol. 2019, 26, 90–93. [Google Scholar] [CrossRef]
- Ebert, J.E.J.; Prelec, D. The fragility of time: Time-insensitivity and valuation of the near and far future. Manag. Sci. 2007, 53, 1423–1438. [Google Scholar] [CrossRef] [Green Version]
- Killeen, P.R. An additive-utility model of delay discounting. Psychol Rev. 2009, 116, 602–619. [Google Scholar] [CrossRef]
- Geng, Z.; Wu, X.; Wang, L.; Zhou, S.; Tian, Y.; Wang, K.; Wei, L. Reduced delayed reward selection by Alzheimer’s disease and mild cognitive impairment patients during intertemporal decision-making. J. Clin. Exp. Neuropsychol. 2020, 42, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Lempert, K.M.; Mechanic-Hamilton, D.J.; Xie, L.; Wisse, L.E.M.; de Flores, R.; Wang, J.; Das, S.R.; Yushkevich, P.A.; Wolk, D.A.; Kable, J.W. Neural and behavioral correlates of episodic memory are associated with temporal discounting in older adults. Neuropsychologia 2020, 146, 107549. [Google Scholar] [CrossRef]
- Thoma, M.V.; Maercker, A.; Forstmeier, S. Evidence for different trajectories of delay discounting in older adults with mild cognitive impairment and mild Alzheimer’s disease. J. Gerontol. B Psychol. Sci. Soc. Sci. 2017, 72, 956–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Haj, M.; Boutoleau-Bretonniere, C.; Allain, P. Memory of decisions: Relationship between decline of autobiographical memory and temporal discounting in Alzheimer’s disease. J. Clin. Exp. Neuropsychol. 2020, 42, 415–424. [Google Scholar] [CrossRef]
- El Haj, M.; Boutoleau-Bretonniere, C.; Moustafa, A.; Allain, P. The discounted future: Relationship between temporal discounting and future thinking in Alzheimer’s disease. Appl. Neuropsychol. Adult 2020, 1–7. Available online: https://pubmed.ncbi.nlm.nih.gov/32429783/ (accessed on 8 November 2021). [CrossRef]
- Beagle, A.J.; Zahir, A.; Borzello, M.; Kayser, A.S.; Hsu, M.; Miller, B.L.; Kramer, J.H.; Chiong, W. Amount and delay insensitivity during intertemporal choice in three neurodegenerative diseases reflects dorsomedial prefrontal atrophy. Cortex 2019, 124, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Bertoux, M.; de Souza, L.C.; Zamith, P.; Dubois, B.; Bourgeois-Gironde, S. Discounting of future rewards in behavioural variant frontotemporal dementia and Alzheimer’s disease. Neuropsychology 2015, 29, 933–939. [Google Scholar] [CrossRef]
- Chiong, W.; Wood, K.A.; Beagle, A.J.; Hsu, M.; Kayser, A.S.; Miller, B.L.; Kramer, J.H. Neuroeconomic dissociation of semantic dementia and behavioural variant frontotemporal dementia. Brain 2016, 139, 578–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coelho, S.; Guerreiro, M.; Chester, C.; Silva, D.; Maroco, J.; Paglieri, F.; de Mendonca, A. Delay discounting in mild cognitive impairment. J. Clin. Exp. Neuropsychol. 2017, 39, 336–346. [Google Scholar] [CrossRef]
- Lindbergh, C.A.; Puente, A.N.; Gray, J.C.; Mackillop, J.; Miller, L.S. Delay and probability discounting as candidate markers for dementia: An initial investigation. Arch. Clin. Neuropsychol. 2014, 29, 651–662. [Google Scholar] [CrossRef] [Green Version]
- Mariano, L.I.; O’Callaghan, C.; Guimaraes, H.C.; Gambogi, L.B.; da Silva, T.B.L.; Yassuda, M.S.; Amaral, J.S.; Caramelli, P.; Hornberger, M.; Teixeira, A.L.; et al. Disinhibition in frontotemporal dementia and Alzheimer’s disease: A neuropsychological and behavioural investigation. J. Int. Neuropsychol. Soc. 2020, 26, 163–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebreton, M.; Bertoux, M.; Boutet, C.; Lehericy, S.; Dubois, B.; Fossati, P.; Pessiglione, M. A critical role for the hippocampus in the valuation of imagined outcomes. PLoS Biol. 2013, 11, e1001684. [Google Scholar] [CrossRef]
- Manuel, A.L.; Roquet, D.; Landin-Romero, R.; Kumfor, F.; Ahmed, R.M.; Hodges, J.R.; Piguet, O. Interactions between decision-making and emotion in behavioral-variant frontotemporal dementia and Alzheimer’s disease. Soc. Cogn. Affect. Neurosci. 2020, 15, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Rung, J.M.; Madden, G.J. Experimental reductions of delay discounting and impulsive choice: A systematic review and meta-analysis. J. Exp. Psychol. Gen. 2018, 147, 1349–1381. [Google Scholar] [CrossRef]
- Ahmed, R.M.; Tse, N.Y.; Chen, Y.; Henning, E.; Hodges, J.R.; Kiernan, M.C.; Irish, M.; Farooqi, I.S.; Piguet, O. Neural correlates of fat preference in frontotemporal dementia: Translating insights from the obesity literature. Ann. Clin. Transl. Neurol. 2021, 8, 1318–1329. [Google Scholar] [CrossRef] [PubMed]
- Bulley, A.; Lempert, K.M.; Conwell, C.; Irish, M.; Schacter, D.L. Intertemporal choice reflects value comparison rather than self-control: Insights from confidence judgments. PsyArXiv 2021. Available online: https://osf.io/n8t5e/ (accessed on 8 November 2021). [CrossRef]
- Bakkour, A.; Palombo, D.J.; Zylberberg, A.; Kang, Y.H.; Reid, A.; Verfaellie, M.; Shadlen, M.N.; Shohamy, D. The hippocampus supports deliberation during value-based decisions. Elife 2019, 8, e46080. [Google Scholar] [CrossRef]
- Wong, S.; Irish, M.; Savage, G.; Hodges, J.R.; Piguet, O.; Hornberger, M. Strategic value-directed learning and memory in Alzheimer’s disease and behavioural-variant frontotemporal dementia. J. Neuropsychol. 2019, 13, 328–353. [Google Scholar] [CrossRef] [Green Version]
- Peters, J.; Buchel, C. The neural mechanisms of inter-temporal decision-making: Understanding variability. Trends Cogn. Sci. 2011, 15, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Sellitto, M.; Ciaramelli, E.; di Pellegrino, G. The neurobiology of intertemporal choice: Insight from imaging and lesion studies. Rev. Neurosci. 2011, 22, 565–574. [Google Scholar] [CrossRef]
- Boyer, P. Evolutionary economics of mental time travel? Trends Cogn. Sci. 2008, 12, 219–224. [Google Scholar] [CrossRef]
- Gabaix, X.; Laibson, D. Myopia and Discounting; National Bureau of Economic Research: Cambridge, MA, USA, 2017. [Google Scholar]
- Gershman, S.J.; Bhui, R. Rationally inattentive intertemporal choice. Nat. Commun. 2020, 11, 3365. [Google Scholar] [CrossRef] [PubMed]
- Kurth-Nelson, Z.; Bickel, W.; Redish, A.D. A theoretical account of cognitive effects in delay discounting. Eur. J. Neurosci. 2012, 35, 1052–1064. [Google Scholar] [CrossRef] [PubMed]
- Bulley, A.; Henry, J.; Suddendorf, T. Prospection and the present moment: The role of episodic foresight in intertemporal choices between immediate and delayed rewards. Rev. Gen. Psychol. 2016, 20, 29–47. [Google Scholar] [CrossRef] [Green Version]
- Bulley, A.; Schacter, D. Episodic future thinking, memory, and decision-making: From theory to application. In Memory in Science and Society; Oxford University Press: Oxford, UK, 2022. [Google Scholar]
- Bulley, A.; Schacter, D.L. Deliberating trade-offs with the future. Nat. Hum. Behav. 2020, 4, 238–247. [Google Scholar] [CrossRef]
- Rösch, S.; Stramaccia, D.; Benoit, R.G. Promoting farsighted decisions via episodic future thinking: A meta-analysis. PsyArXiv 2021. Available online: https://psyarxiv.com/53ju2/ (accessed on 8 November 2021). [CrossRef]
- Bulley, A.; Miloyan, B.; Pepper, G.V.; Gullo, M.J.; Henry, J.D.; Suddendorf, T. Cuing both positive and negative episodic foresight reduces delay discounting but does not affect risk-taking. Q J. Exp. Psychol. 2019, 72, 1998–2017. [Google Scholar] [CrossRef] [PubMed]
- Sasse, L.K.; Peters, J.; Brassen, S. Cognitive control modulates effects of episodic simulation on delay discounting in aging. Front Aging Neurosci. 2017, 9, 58. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Uhle, F.; Fliessbach, K.; Wagner, M.; Han, Y.; Weber, B.; Jessen, F. Reduced future-oriented decision making in individuals with subjective cognitive decline: A functional MRI study. Alzheimers Dement. 2017, 6, 222–231. [Google Scholar] [CrossRef]
- Palombo, D.J.; Keane, M.M.; Verfaellie, M. The medial temporal lobes are critical for reward-based decision making under conditions that promote episodic future thinking. Hippocampus 2015, 25, 345–353. [Google Scholar] [CrossRef] [Green Version]
- Palombo, D.J.; Keane, M.M.; Verfaellie, M. How do lesion studies elucidate the role of the hippocampus in intertemporal choice? Hippocampus 2015, 25, 407–408. [Google Scholar] [CrossRef]
- Kwan, D.; Craver, C.F.; Green, L.; Myerson, J.; Boyer, P.; Rosenbaum, R.S. Future decision-making without episodic mental time travel. Hippocampus 2012, 22, 1215–1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwan, D.; Craver, C.F.; Green, L.; Myerson, J.; Rosenbaum, R.S. Dissociations in future thinking following hippocampal damage: Evidence from discounting and time perspective in episodic amnesia. J. Exp. Psychol. Gen. 2013, 142, 1355–1369. [Google Scholar] [CrossRef] [PubMed]
- Kwan, D.; Craver, C.F.; Green, L.; Myerson, J.; Gao, F.Q.; Black, S.E.; Rosenbaum, R.S. Cueing the personal future to reduce discounting in intertemporal choice: Is episodic prospection necessary? Hippocampus 2015, 25, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Palombo, D.J.; Keane, M.M.; Verfaellie, M. Using future thinking to reduce temporal discounting: Under what circumstances are the medial temporal lobes critical? Neuropsychologia 2016, 89, 437–444. [Google Scholar] [CrossRef] [Green Version]
- Einstein, G.O.; McDaniel, M.A. Normal aging and prospective memory. J. Exp. Psychol. Learn Mem. Cogn. 1990, 16, 717–726. [Google Scholar] [CrossRef]
- McDaniel, M.A.; Einstein, G.O. The neuropsychology of prospective memory in normal aging: A componential approach. Neuropsychologia 2011, 49, 2147–2155. [Google Scholar] [CrossRef] [Green Version]
- Henry, J.D. Prospective memory impairment in neurological disorders: Implications and management. Nat. Rev. Neurol. 2021, 17, 297–307. [Google Scholar] [CrossRef]
- Kliegel, M.; Altgassen, M.; Hering, A.; Rose, N.S. A process-model based approach to prospective memory impairment in Parkinson’s disease. Neuropsychologia 2011, 49, 2166–2177. [Google Scholar] [CrossRef]
- Block, R.A.; Gruber, R.P. Time perception, attention, and memory: A selective review. Acta Psychol. 2014, 149, 129–133. [Google Scholar] [CrossRef]
- Mackinlay, R.J.; Kliegel, M.; Mantyla, T. Predictors of time-based prospective memory in children. J. Exp. Child. Psychol. 2009, 102, 251–264. [Google Scholar] [CrossRef]
- Mioni, G.; Grondin, S.; McLennan, S.N.; Stablum, F. The role of time-monitoring behaviour in time-based prospective memory performance in younger and older adults. Memory 2020, 28, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Mioni, G.; Stablum, F. Monitoring behaviour in a time-based prospective memory task: The involvement of executive functions and time perception. Memory 2014, 22, 536–552. [Google Scholar] [CrossRef]
- van den Berg, E.; Kant, N.; Postma, A. Remember to buy milk on the way home! A meta-analytic review of prospective memory in mild cognitive impairment and dementia. J. Int. Neuropsychol. Soc. 2012, 18, 706–716. [Google Scholar] [CrossRef]
- Pirogovsky, E.; Woods, S.P.; Vincent Filoteo, J.; Gilbert, P.E. Prospective memory deficits are associated with poorer everyday functioning in Parkinson’s disease. J. Int. Neuropsychol. Soc. 2012, 18, 986–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, G.; Della Sala, S.; Logie, R.H.; Maylor, E.A. Prospective and retrospective memory in normal ageing and dementia: A questionnaire study. Memory 2000, 8, 311–321. [Google Scholar] [CrossRef]
- Kvavilashvili, L.; Niedzwienska, A.; Gilbert, S.J.; Markostamou, I. Deficits in spontaneous cognition as an early marker of Alzheimer’s disease. Trends Cogn. Sci. 2020, 24, 285–301. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Roquet, D.; Ahmed, R.M.; Hodges, J.R.; Piguet, O.; Irish, M. Examining prefrontal contributions to past- and future-oriented memory disturbances in daily life in dementia. Cortex 2021, 134, 307–319. [Google Scholar] [CrossRef]
- Dermody, N.; Hornberger, M.; Piguet, O.; Hodges, J.R.; Irish, M. Prospective memory impairments in Alzheimer’s disease and behavioral variant frontotemporal dementia: Clinical and neural correlates. J. Alzheimers Dis. 2016, 50, 425–441. [Google Scholar] [CrossRef] [Green Version]
- Altgassen, M.; Rendell, P.G.; Bernhard, A.; Henry, J.D.; Bailey, P.E.; Phillips, L.H.; Kliegel, M. Future thinking improves prospective memory performance and plan enactment in older adults. Q J. Exp. Psychol. 2015, 68, 192–204. [Google Scholar] [CrossRef]
- Brewer, G.A.; Knight, J.; Meeks, J.T.; Marsh, R.L. On the role of imagery in event-based prospective memory. Conscious Cogn. 2011, 20, 901–907. [Google Scholar] [CrossRef]
- Brewer, G.A.; Marsh, R.L. On the role of episodic future simulation in encoding of prospective memories. Cogn. Neurosci. 2010, 1, 81–88. [Google Scholar] [CrossRef]
- Kretschmer-Trendowicz, A.; Schnitzspahn, K.M.; Reuter, L.; Altgassen, M. Episodic future thinking improves children’s prospective memory performance in a complex task setting with real life task demands. Psychol. Res. 2019, 83, 514–525. [Google Scholar] [CrossRef]
- Mioni, G.; Bertucci, E.; Rosato, A.; Terrett, G.; Rendell, P.G.; Zamuner, M.; Stablum, F. Improving prospective memory performance with future event simulation in traumatic brain injury patients. Br. J. Clin. Psychol. 2017, 56, 130–148. [Google Scholar] [CrossRef] [PubMed]
- Neroni, M.A.; Gamboz, N.; Brandimonte, M.A. Does episodic future thinking improve prospective remembering? Conscious Cogn. 2014, 23, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Platt, B.; Kamboj, S.K.; Italiano, T.; Rendell, P.G.; Curran, H.V. Prospective memory impairments in heavy social drinkers are partially overcome by future event simulation. Psychopharmacology 2016, 233, 499–506. [Google Scholar] [CrossRef] [Green Version]
- Spreng, R.N.; Madore, K.P.; Schacter, D.L. Better imagined: Neural correlates of the episodic simulation boost to prospective memory performance. Neuropsychologia 2018, 113, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Shelton, J.T.; Lee, J.H.; Scullin, M.K.; Rose, N.S.; Rendell, P.G.; McDaniel, M.A. Improving prospective memory in healthy older adults and individuals with very mild Alzheimer’s disease. J. Am. Geriatr. Soc. 2016, 64, 1307–1312. [Google Scholar] [CrossRef] [Green Version]
- Beck, S.M.; Ruge, H.; Walser, M.; Goschke, T. The functional neuroanatomy of spontaneous retrieval and strategic monitoring of delayed intentions. Neuropsychologia 2014, 52, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Irish, M. “Loss of the Future”: Prospection Impairments in the Dementias. In Research Progress in Alzheimer’s Disease and Dementia; Nova Science Publishers: New York, NY, USA, 2016; Volume 6, pp. 1–26. [Google Scholar]
- Bulley, A.; McCarthy, T.; Gilbert, S.J.; Suddendorf, T.; Redshaw, J. Children devise and selectively use tools to offload cognition. Curr. Biol. 2020, 30, 3457–3464. [Google Scholar] [CrossRef]
- Scarampi, C.; Gilbert, S.J. Age differences in strategic reminder setting and the compensatory role of metacognition. Psychol. Aging. 2021, 36, 172–185. [Google Scholar] [CrossRef]
- Armitage, K.L.; Bulley, A.; Redshaw, J. Developmental origins of cognitive offloading. Proc. Biol. Sci. 2020, 287, 20192927. [Google Scholar] [CrossRef] [PubMed]
- Redshaw, J.; Vandersee, J.; Bulley, A.; Gilbert, S.J. Development of children’s use of external reminders for hard-to-remember intentions. Child Dev. 2018, 89, 2099–2108. [Google Scholar] [CrossRef] [Green Version]
- Davalos, D.; Mioni, G.; Grondin, S.; Ortuño, F. Time perception and dysfunction: Clinical and practical implications. Front. Hum. Neurosci. 2018, 12, 435. [Google Scholar] [CrossRef] [Green Version]
- Miloyan, B.; McFarlane, K.A. The measurement of episodic foresight: A systematic review of assessment instruments. Cortex 2019, 117, 351–370. [Google Scholar] [CrossRef] [PubMed]
- Conti, F.; Irish, M. Harnessing visual imagery and oculomotor behaviour to understand prospection. Trends Cogn. Sci. 2021, 25, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Strikwerda-Brown, C.; Shaw, S.R.; Hodges, J.R.; Piguet, O.; Irish, M. Examining the episodic-semantic interaction during future thinking—A reanalysis of external details. Mem. Cogn. 2021, 1–3. Available online: https://pubmed.ncbi.nlm.nih.gov/34401984/ (accessed on 8 November 2021). [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Bulley, A.; Irish, M. Subjective Time in Dementia: A Critical Review. Brain Sci. 2021, 11, 1502. https://doi.org/10.3390/brainsci11111502
Liu L, Bulley A, Irish M. Subjective Time in Dementia: A Critical Review. Brain Sciences. 2021; 11(11):1502. https://doi.org/10.3390/brainsci11111502
Chicago/Turabian StyleLiu, Lulu, Adam Bulley, and Muireann Irish. 2021. "Subjective Time in Dementia: A Critical Review" Brain Sciences 11, no. 11: 1502. https://doi.org/10.3390/brainsci11111502
APA StyleLiu, L., Bulley, A., & Irish, M. (2021). Subjective Time in Dementia: A Critical Review. Brain Sciences, 11(11), 1502. https://doi.org/10.3390/brainsci11111502






