Pitch and Rhythm Perception and Verbal Short-Term Memory in Acute Traumatic Brain Injury
Abstract
1. Introduction
1.1. Neural Networks Underlying Pitch and Rhythm Discrimination
1.2. Acquired Amusia in Individuals with Traumatic Brain Injury and Stroke
1.3. The Question of Modularity in Short-Term Memory for Rhythm and Pitch
1.4. Goals of the Present Study
2. Materials and Methods
2.1. Participants
2.2. Measures and Procedure
2.2.1. Scale and Rhythm Tests of the Montreal Battery of Evaluation of Amusia (MBEA)
2.2.2. Digit Span
2.2.3. Extended Glasgow Outcome Scale (GOSE)
2.3. Image Acquisition
2.4. Statistical Analyses
- To evaluate the performance of TBI patients versus controls on pitch and rhythm processing, a quasi-experimental, paired-samples t-test design was used in a sample of TBI patients who were age- and education- matched with normal controls as independent variables, and the Scale test and Rhythm test total scores as dependent variables. An alpha level of 0.05 was used for all analyses.
- To determine whether there was a relationship between performance on the Scale and Rhythm tests of the MBEA, Pearson product-moment correlation coefficients were calculated.
- To determine whether damage predominantly located in the right or left hemisphere was associated with the hemispheric lateralization of pitch and rhythm discrimination, two 2-tailed independent samples t-tests were used to compare patients with left hemisphere damage to those with right hemisphere damage. In both, the independent variable was injury location, and the dependent variables were total score on the Scale test and the Rhythm test.
- To determine whether there was a relationship between performance on the Scale and Rhythm tests, and severity of brain injury, Pearson product-moment correlation coefficients were calculated.
- To determine associations between performance on the Scale and Rhythm tests and verbal short-term memory tests (Z scores representing longest digit span forward), Pearson product-moment correlation coefficients were calculated.
3. Results
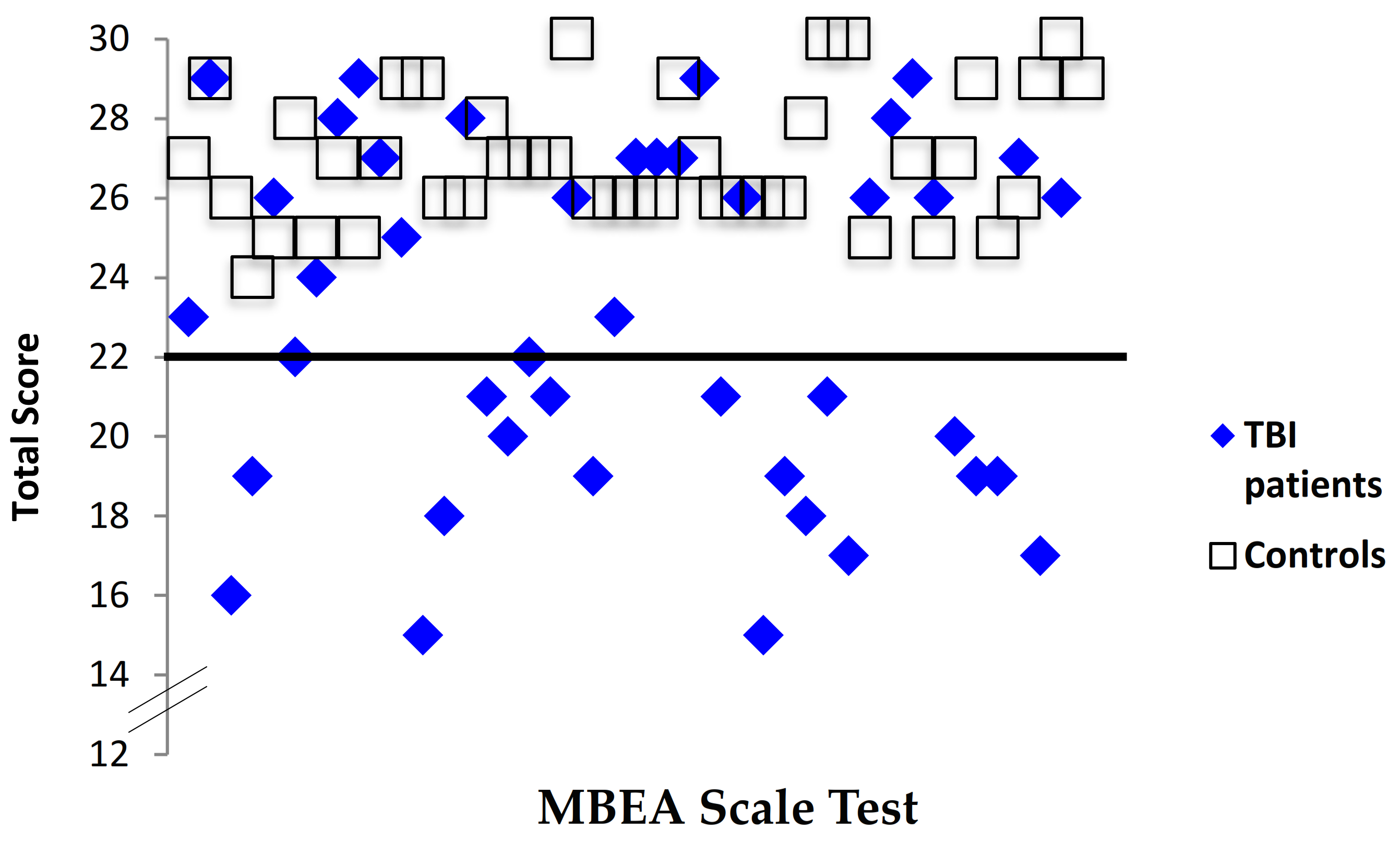
3.1. Data Integrity
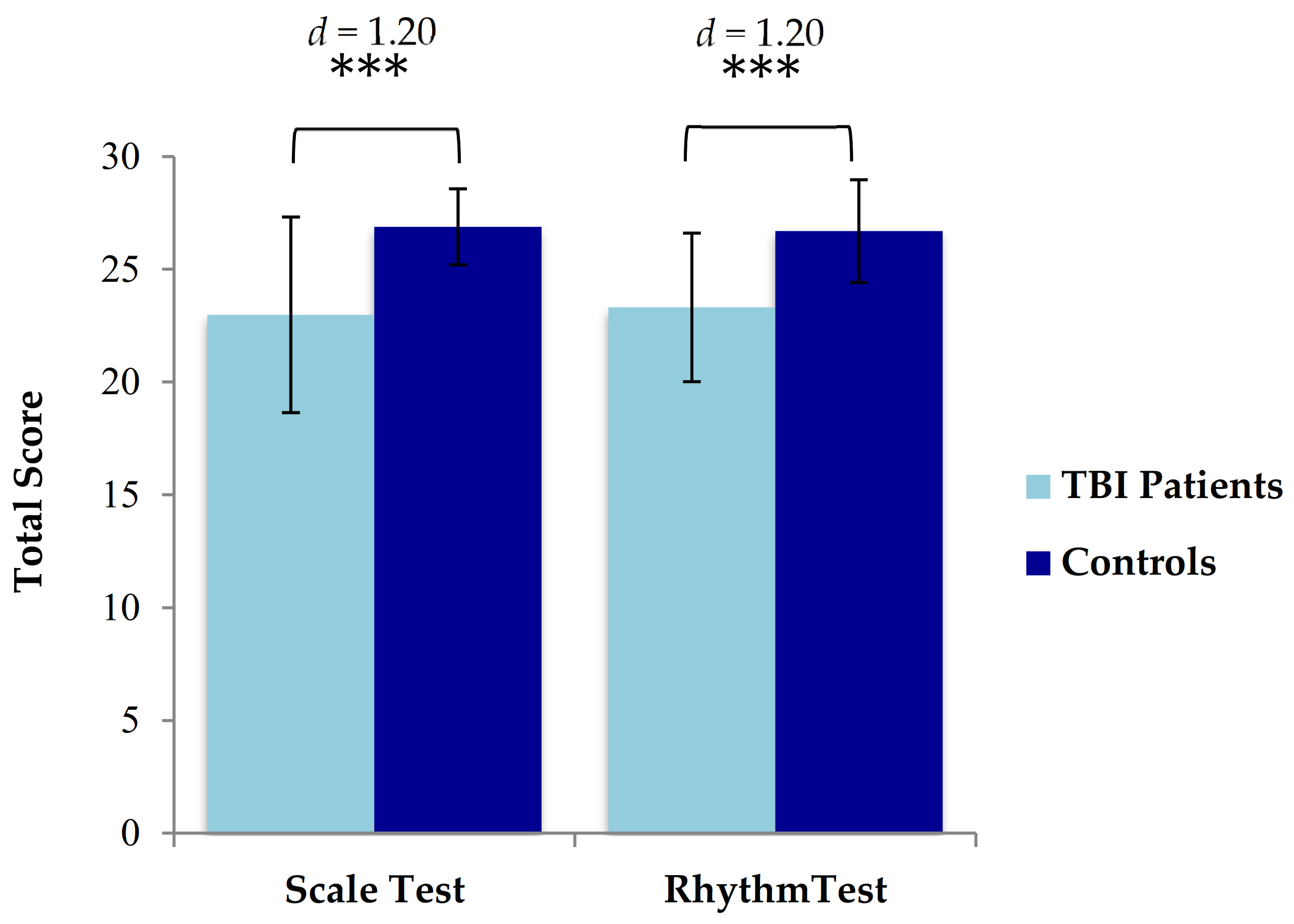
3.2. Comparison of Music Perception Scores in TBI Patients versus Controls
3.2.1. Scale Test
3.2.2. Rhythm Test
3.3. The Co-Occurrence of Pitch and Rhythm Deficits in Acquired Amusia
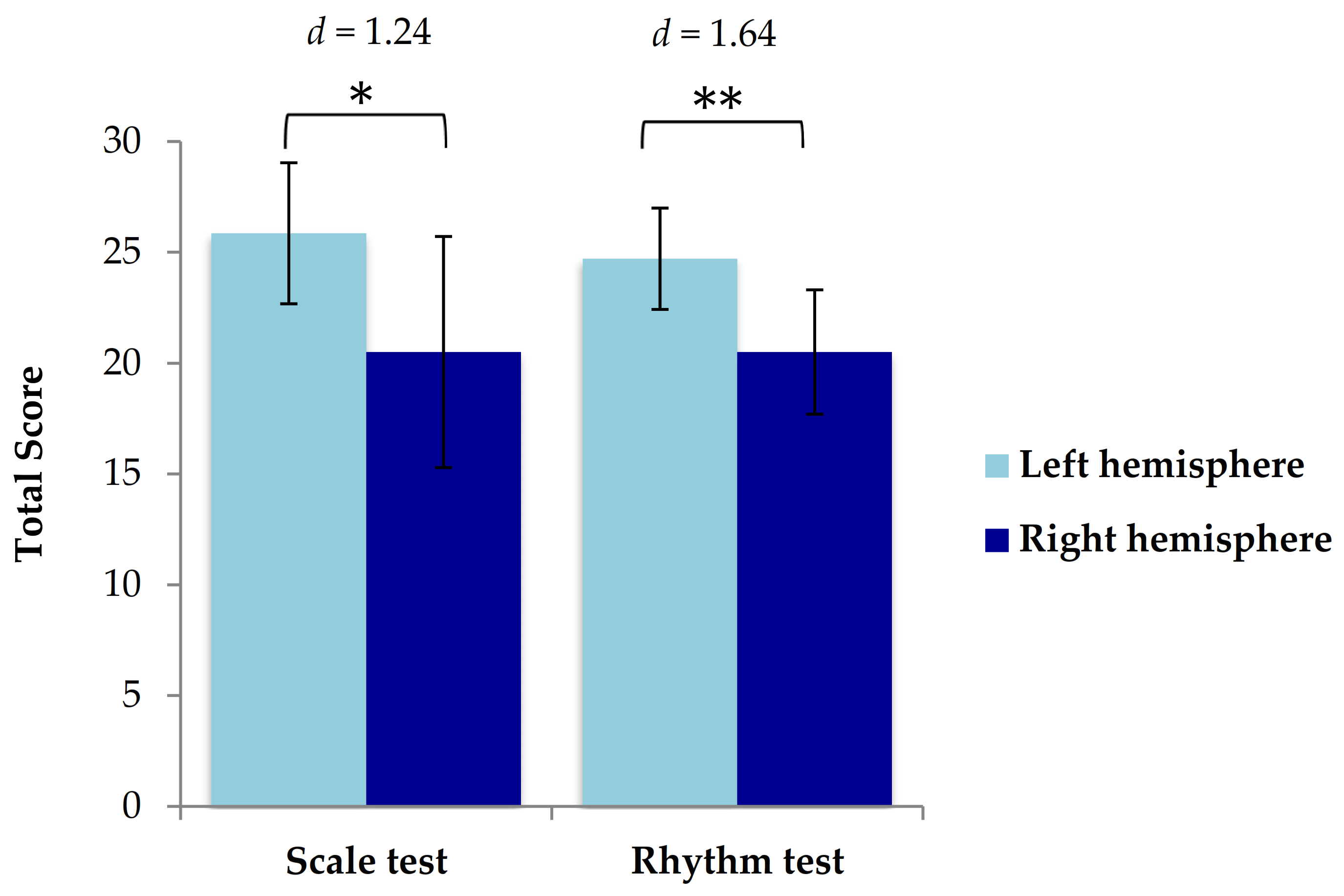
3.4. Neuroimaging and Performance on the Scale and Rhythm Tests of the MBEA
3.5. Severity of Brain Injury and Music Perception Deficits
3.6. Verbal Short-Term Memory and Music Perception Deficits
3.6.1. Longest Digit Span Forward
3.6.2. The Relationship between Music Perception Scores and Verbal Short-Term Memory
3.6.3. Pitch Processing and Verbal Short-Term Memory
3.6.4. Rhythm Processing and Verbal Short-Term Memory
3.6.5. Location of Injury, Music Perception Scores, and Verbal Short-Term Memory Scores
4. Discussion
4.1. Performance on the Scale and Rhythm Subtests of the Montreal Battery of Evaluation of Amusia (MBEA)
4.2. The Relationship of Pitch and Rhythm Processing in Acquired Amusia
4.3. The Lateralization of Pitch and Rhythm Deficits
4.4. The Relationship between Music Perception Scores and Verbal Short-Term Memory
4.4.1. Pitch Processing and Verbal Short-Term Memory
4.4.2. Rhythm Processing and Verbal Short-Term Memory
4.5. Implications of Acquired Music Perception Deficits on Music Interventions Following TBI
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tillmann, B.; Schulze, K.; Foxton, J.M. Congenital amusia: A short-term memory deficit for non-verbal, but not verbal sounds. Brain Cogn. 2009, 71, 259–264. [Google Scholar] [CrossRef]
- Peretz, I.; Champod, A.S.; Hyde, K. Varieties of musical disorders. The Montreal Battery of Evaluation of Amusia. Ann. N. Y. Acad. Sci. 2003, 999, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Peretz, I.; Vuvan, D.T. Prevalence of congenital amusia. Eur. J. Hum. Genet. 2017, 25, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Sihvonen, A.J.; Ripolles, P.; Leo, V.; Rodriguez-Fornells, A.; Soinila, S.; Sarkamo, T. Neural Basis of Acquired Amusia and Its Recovery after Stroke. J. Neurosci. 2016, 36, 8872–8881. [Google Scholar] [CrossRef] [PubMed]
- Sihvonen, A.J.; Ripolles, P.; Rodriguez-Fornells, A.; Soinila, S.; Sarkamo, T. Revisiting the Neural Basis of Acquired Amusia: Lesion Patterns and Structural Changes Underlying Amusia Recovery. Front. Neurosci. 2017, 11, 426. [Google Scholar] [CrossRef]
- Sarkamo, T.; Tervaniemi, M.; Soinila, S.; Autti, T.; Silvennoinen, H.M.; Laine, M.; Hietanen, M. Amusia and cognitive deficits after stroke: Is there a relationship? Ann. N. Y. Acad. Sci. 2009, 1169, 441–445. [Google Scholar] [CrossRef]
- Steinke, W.R.; Cuddy, L.L.; Jakobson, L.S. Dissociations among functional subsystems governing melody recognition after right-hemisphere damage. Cogn. Neuropsychol. 2001, 18, 411–437. [Google Scholar] [CrossRef]
- Peretz, I. Processing of local and global musical information by unilateral brain-damaged patients. Brain 1990, 113 Pt 4, 1185–1205. [Google Scholar] [CrossRef] [PubMed]
- Hirel, C.; Nighoghossian, N.; Leveque, Y.; Hannoun, S.; Fornoni, L.; Daligault, S.; Bouchet, P.; Jung, J.; Tillmann, B.; Caclin, A. Verbal and musical short-term memory: Variety of auditory disorders after stroke. Brain Cogn. 2017, 113, 10–22. [Google Scholar] [CrossRef]
- Sihvonen, A.J.; Sarkamo, T.; Rodriguez-Fornells, A.; Ripolles, P.; Munte, T.F.; Soinila, S. Neural architectures of music-Insights from acquired amusia. Neurosci. Biobehav. Rev. 2019, 107, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Liegeois-Chauvel, C.; Peretz, I.; Babai, M.; Laguitton, V.; Chauvel, P. Contribution of different cortical areas in the temporal lobes to music processing. Brain 1998, 121 Pt 10, 1853–1867. [Google Scholar] [CrossRef]
- Zatorre, R.J. Discrimination and recognition of tonal melodies after unilateral cerebral excisions. Neuropsychologia 1985, 23, 31–41. [Google Scholar] [CrossRef]
- Zatorre, R.J. Pitch perception of complex tones and human temporal-lobe function. J. Acoust. Soc. Am. 1988, 84, 566–572. [Google Scholar] [CrossRef]
- McChesney-Atkins, S.; Davies, K.G.; Montouris, G.D.; Silver, J.T.; Menkes, D.L. Amusia after right frontal resection for epilepsy with singing seizures: Case report and review of the literature. Epilepsy Behav. 2003, 4, 343–347. [Google Scholar] [CrossRef]
- Peretz, I.; Kolinsky, R.; Tramo, M.; Labrecque, R.; Hublet, C.; Demeurisse, G.; Belleville, S. Functional dissociations following bilateral lesions of auditory cortex. Brain 1994, 117, 1283–1301. [Google Scholar] [CrossRef] [PubMed]
- Peretz, I. Can we lose memory for music? A case of music agnosia in a nonmusician. J. Cogn. Neurosci. 1996, 8, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Ayotte, J.; Peretz, I.; Rousseau, I.; Bard, C.; Bojanowski, M. Patterns of music agnosia associated with middle cerebral artery infarcts. Brain 2000, 123, 1926–1938. [Google Scholar] [CrossRef]
- Balzani, C.; Mariaud, A.-S.; Schön, D.; Cermolacce, M.; Vion-Dury, J. Changes in music listening in post-concussion syndrome after mild traumatic brain injury. Psychomusicol. Music. Mind Brain 2014, 24, 117. [Google Scholar] [CrossRef]
- Léard-Schneider, L.; Lévêque, Y. Perception of music and speech prosody after traumatic brain injury. Psyarxiv 2020. [Google Scholar] [CrossRef]
- Hattiangadi, N.; Pillion, J.P.; Slomine, B.; Christensen, J.; Trovato, M.K.; Speedie, L.J. Characteristics of auditory agnosia in a child with severe traumatic brain injury: A case report. Brain Lang. 2005, 92, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, N.; Jolicoeur, P.; Peretz, I. Impaired memory for pitch in congenital amusia. Ann. N. Y. Acad. Sci. 2009, 1169, 270–272. [Google Scholar] [CrossRef] [PubMed]
- Williamson, V.J.; Stewart, L. Memory for pitch in congenital amusia: Beyond a fine-grained pitch discrimination problem. Memory 2010, 18, 657–669. [Google Scholar] [CrossRef]
- Foxton, J.M.; Dean, J.L.; Gee, R.; Peretz, I.; Griffiths, T.D. Characterization of deficits in pitch perception underlying ’tone deafness’. Brain 2004, 127, 801–810. [Google Scholar] [CrossRef]
- Schaal, N.K.; Pfeifer, J.; Krause, V.; Pollok, B. From amusic to musical?--Improving pitch memory in congenital amusia with transcranial alternating current stimulation. Behav. Brain Res. 2015, 294, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Albouy, P.; Mattout, J.; Bouet, R.; Maby, E.; Sanchez, G.; Aguera, P.E.; Daligault, S.; Delpuech, C.; Bertrand, O.; Caclin, A.; et al. Impaired pitch perception and memory in congenital amusia: The deficit starts in the auditory cortex. Brain 2013, 136, 1639–1661. [Google Scholar] [CrossRef]
- Tillmann, B.; Leveque, Y.; Fornoni, L.; Albouy, P.; Caclin, A. Impaired short-term memory for pitch in congenital amusia. Brain Res. 2016, 1640, 251–263. [Google Scholar] [CrossRef]
- Peretz, I. Neurobiology of Congenital Amusia. Trends. Cogn. Sci. 2016, 20, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Foxton, J.M.; Nandy, R.K.; Griffiths, T.D. Rhythm deficits in ’tone deafness’. Brain Cogn. 2006, 62, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Pfeuty, M.; Peretz, I. Abnormal pitch—Time interference in congenital amusia: Evidence from an implicit test. Atten. Percept. Psychophys. 2010, 72, 763–774. [Google Scholar] [CrossRef]
- Peretz, I.; Kolinsky, R. Boundaries of separability between melody and rhythm in music discrimination: A neuropsychological perspective. Q. J. Exp. Psychol. A 1993, 46, 301–325. [Google Scholar] [CrossRef]
- Di Pietro, M.; Laganaro, M.; Leemann, B.; Schnider, A. Receptive amusia: Temporal auditory processing deficit in a professional musician following a left temporo-parietal lesion. Neuropsychologia 2004, 42, 868–877. [Google Scholar] [CrossRef]
- Mavlov, L. Amusia due to rhythm agnosia in a musician with left hemisphere damage: A non-auditory supramodal defect. Cortex 1980, 16, 331–338. [Google Scholar] [CrossRef]
- Vuvan, D.T.; Paquette, S.; Mignault Goulet, G.; Royal, I.; Felezeu, M.; Peretz, I. The Montreal Protocol for Identification of Amusia. Behav. Res. Methods 2018, 50, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Albouy, P.; Peretz, I.; Bermudez, P.; Zatorre, R.J.; Tillmann, B.; Caclin, A. Specialized neural dynamics for verbal and tonal memory: fMRI evidence in congenital amusia. Hum. Brain Mapp. 2019, 40, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Rosslau, K.; Steinwede, D.; Schroder, C.; Herholz, S.C.; Lappe, C.; Dobel, C.; Altenmuller, E. Clinical investigations of receptive and expressive musical functions after stroke. Front. Psychol. 2015, 6, 768. [Google Scholar] [CrossRef] [PubMed]
- Sarkamo, T.; Tervaniemi, M.; Soinila, S.; Autti, T.; Silvennoinen, H.M.; Laine, M.; Hietanen, M.; Pihko, E. Auditory and cognitive deficits associated with acquired amusia after stroke: A magnetoencephalography and neuropsychological follow-up study. PLoS ONE 2010, 5, e15157. [Google Scholar] [CrossRef] [PubMed]
- Hyde, K.L.; Zatorre, R.J.; Griffiths, T.D.; Lerch, J.P.; Peretz, I. Morphometry of the amusic brain: A two-site study. Brain 2006, 129, 2562–2570. [Google Scholar] [CrossRef]
- Hyde, K.L.; Lerch, J.P.; Zatorre, R.J.; Griffiths, T.D.; Evans, A.C.; Peretz, I. Cortical thickness in congenital amusia: When less is better than more. J. Neurosci. 2007, 27, 13028–13032. [Google Scholar] [CrossRef]
- Loui, P.; Alsop, D.; Schlaug, G. Tone deafness: A new disconnection syndrome? J. Neurosci. 2009, 29, 10215–10220. [Google Scholar] [CrossRef]
- Chen, J.L.; Kumar, S.; Williamson, V.J.; Scholz, J.; Griffiths, T.D.; Stewart, L. Detection of the arcuate fasciculus in congenital amusia depends on the tractography algorithm. Front. Psychol. 2015, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Hyde, K.L.; Zatorre, R.J.; Peretz, I. Functional MRI evidence of an abnormal neural network for pitch processing in congenital amusia. Cereb. Cortex 2011, 21, 292–299. [Google Scholar] [CrossRef]
- Jerde, T.A.; Childs, S.K.; Handy, S.T.; Nagode, J.C.; Pardo, J.V. Dissociable systems of working memory for rhythm and melody. Neuroimage 2011, 57, 1572–1579. [Google Scholar] [CrossRef] [PubMed]
- Lagrois, M.E.; Peretz, I. The co-occurrence of pitch and rhythm disorders in congenital amusia. Cortex 2019, 113, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Samson, S.; Ehrle, N.; Baulac, M. Cerebral substrates for musical temporal processes. Ann. N. Y. Acad. Sci. 2001, 930, 166–178. [Google Scholar] [CrossRef]
- Phillips-Silver, J.; Toiviainen, P.; Gosselin, N.; Peretz, I. Amusic does not mean unmusical: Beat perception and synchronization ability despite pitch deafness. Cogn. Neuropsychol. 2013, 30, 311–331. [Google Scholar] [CrossRef] [PubMed]
- Levitin, D.J.; Grahn, J.A.; London, J. The Psychology of Music: Rhythm and Movement. Annu. Rev. Psychol. 2018, 69, 51–75. [Google Scholar] [CrossRef] [PubMed]
- Penhune, V.B.; Zatorre, R.J.; Feindel, W.H. The role of auditory cortex in retention of rhythmic patterns as studied in patients with temporal lobe removals including Heschl’s gyrus. Neuropsychologia 1999, 37, 315–331. [Google Scholar] [CrossRef]
- Piras, F.; Piras, F.; Ciullo, V.; Danese, E.; Caltagirone, C.; Spalletta, G. Time dysperception perspective for acquired brain injury. Front. Neurol. 2014, 4, 217. [Google Scholar] [CrossRef] [PubMed]
- Menon, D.K.; Schwab, K.; Wright, D.W.; Maas, A.I. Position statement: Definition of traumatic brain injury. Arch. Phys. Med. Rehabil. 2010, 91, 1637–1640. [Google Scholar] [CrossRef]
- Arciniegas, D.B.; Held, K.; Wagner, P. Cognitive impairment following traumatic brain injury. Curr. Treat. Options Neurol. 2002, 4, 43–57. [Google Scholar] [CrossRef]
- Brown, S.; Martinez, M.J. Activation of premotor vocal areas during musical discrimination. Brain Cogn. 2007, 63, 59–69. [Google Scholar] [CrossRef]
- Baddeley, A. Working memory. Current Biol. 2010, 20, R136–R140. [Google Scholar] [CrossRef]
- Cowan, N. What are the differences between long-term, short-term, and working memory? Prog. Brain Res. 2008, 169, 323–338. [Google Scholar] [PubMed]
- Schulze, K.; Koelsch, S. Working memory for speech and music. Ann. N. Y. Acad. Sci. 2012, 1252, 229–236. [Google Scholar] [CrossRef]
- Chan, A.S.; Ho, Y.C.; Cheung, M.C. Music training improves verbal memory. Nature 1998, 396, 128. [Google Scholar] [CrossRef] [PubMed]
- Schendel, Z.A.; Palmer, C. Suppression effects on musical and verbal memory. Mem. Cognit. 2007, 35, 640–650. [Google Scholar] [CrossRef][Green Version]
- Talamini, F.; Altoe, G.; Carretti, B.; Grassi, M. Musicians have better memory than nonmusicians: A meta-analysis. PLoS ONE 2017, 12, e0186773. [Google Scholar] [CrossRef]
- Deutsch, D. Tones and numbers: Specificity of interference in immediate memory. Science 1970, 168, 1604–1605. [Google Scholar] [CrossRef]
- Salamé, P.; Baddeley, A. Effects of background music on phonological short-term memory. Q. J. Exp. Psychol. Sect. A 1989, 41, 107–122. [Google Scholar] [CrossRef]
- Defilippi, A.C.N.; Garcia, R.B.; Galera, C. Irrelevant sound interference on phonological and tonal working memory in musicians and nonmusicians. Psicologia Reflexão Crítica 2019, 32, 2. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.L.; Zalewski, C.; Brewer, C.; Lucker, J.; Drayna, D. Widespread auditory deficits in tune deafness. Ear. Hear. 2009, 30, 63. [Google Scholar] [CrossRef]
- Fischer, J.; Mathieson, C. The history of the Glasgow Coma Scale: Implications for practice. Crit. Care Nurs. Q. 2001, 23, 52–58. [Google Scholar] [CrossRef]
- Heilman, K.M.; Safran, A.; Geschwind, N. Closed head trauma and aphasia. J. Neurol. Neurosurg. Psychiatry 1971, 34, 265–269. [Google Scholar] [CrossRef][Green Version]
- Centers for Disease Control and Prevention. Report to congress on traumatic brain injury in the United States: Epidemiology and rehabilitation. Natl. Cent. Inj. Prev. Control 2015, 2, 1–72. [Google Scholar]
- Levin, H.S.; Boake, C.; Song, J.; McCauley, S.; Contant, C.; Diaz-Marchan, P.; Brundage, S.; Goodman, H.; Kotrla, K.J. Validity and sensitivity to change of the extended Glasgow Outcome Scale in mild to moderate traumatic brain injury. J. Neurotrauma 2001, 18, 575–584. [Google Scholar] [CrossRef]
- Wechsler, D. Wechsler Memory Scale (WMS-III); Psychological Corporation: San Antonio, TX, USA, 1997; Volume 14. [Google Scholar]
- Lichtenberger, E.O.; Kaufman, A.S. Essentials of WAIS-IV assessment. In Essentials of Psychological Assessment Series, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2013; p. 1. [Google Scholar]
- Wilson, J.L.; Pettigrew, L.E.; Teasdale, G.M. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: Guidelines for their use. J. Neurotrauma 1998, 15, 573–585. [Google Scholar] [CrossRef]
- Sharp, A.L.; Huang, B.Z.; Tang, T.; Shen, E.; Melnick, E.R.; Venkatesh, A.K.; Kanter, M.H.; Gould, M.K. Implementation of the Canadian CT Head Rule and Its Association With Use of Computed Tomography Among Patients With Head Injury. Ann. Emerg. Med. 2018, 71, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Kline, R.B. Principles and Practice of Structural Equation Modeling, 4th ed.; Kenny, D., Little, T., Eds.; Guilford Press: New York, NY, USA, 2016; pp. 74–77. [Google Scholar]
- Peretz, I.; Ayotte, J.; Zatorre, R.J.; Mehler, J.; Ahad, P.; Penhune, V.B.; Jutras, B. Congenital amusia: A disorder of fine-grained pitch discrimination. Neuron 2002, 33, 185–191. [Google Scholar] [CrossRef]
- Peretz, I.; Coltheart, M. Modularity of music processing. Nat. Neurosci. 2003, 6, 688–691. [Google Scholar] [CrossRef]
- Zatorre, R.J.; Belin, P. Spectral and temporal processing in human auditory cortex. Cereb. Cortex 2001, 11, 946–953. [Google Scholar] [CrossRef]
- Zatorre, R.J. Functional specialization of human auditory cortex for musical processing. Brain 1998, 121 Pt 10, 1817–1818. [Google Scholar] [CrossRef][Green Version]
- Milner, B. Laterality effects in audition. Interhemispheric Relat. Cereb. Domin. 1962, 177–195. [Google Scholar]
- Terao, Y.; Mizuno, T.; Shindoh, M.; Sakurai, Y.; Ugawa, Y.; Kobayashi, S.; Nagai, C.; Furubayashi, T.; Arai, N.; Okabe, S.; et al. Vocal amusia in a professional tango singer due to a right superior temporal cortex infarction. Neuropsychologia 2006, 44, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Fries, W.; Swihart, A.A. Disturbance of rhythm sense following right hemisphere damage. Neuropsychologia 1990, 28, 1317–1323. [Google Scholar] [CrossRef]
- Zatorre, R.J.; Evans, A.C.; Meyer, E. Neural mechanisms underlying melodic perception and memory for pitch. J. Neurosci. 1994, 14, 1908–1919. [Google Scholar] [CrossRef]
- Gaab, N.; Gaser, C.; Zaehle, T.; Jancke, L.; Schlaug, G. Functional anatomy of pitch memory—An fMRI study with sparse temporal sampling. Neuroimage 2003, 19, 1417–1426. [Google Scholar] [CrossRef]
- Schuppert, M.; Munte, T.F.; Wieringa, B.M.; Altenmuller, E. Receptive amusia: Evidence for cross-hemispheric neural networks underlying music processing strategies. Brain 2000, 123 Pt 3, 546–559. [Google Scholar] [CrossRef]
- Hulkower, M.B.; Poliak, D.B.; Rosenbaum, S.B.; Zimmerman, M.E.; Lipton, M.L. A decade of DTI in traumatic brain injury: 10 years and 100 articles later. AJNR Am. J. Neuroradiol. 2013, 34, 2064–2074. [Google Scholar] [CrossRef]
- Aboitiz, F. Brain connections: Interhemispheric fiber systems and anatomical brain asymmetries in humans. Biol. Res. 1992, 25, 51–61. [Google Scholar]
- Heiss, W.-D.; Thiel, A. A proposed regional hierarchy in recovery of post-stroke aphasia. Brain Lang. 2006, 98, 118–123. [Google Scholar] [CrossRef]
- Duque, J.; Hummel, F.; Celnik, P.; Murase, N.; Mazzocchio, R.; Cohen, L.G. Transcallosal inhibition in chronic subcortical stroke. Neuroimage 2005, 28, 940–946. [Google Scholar] [CrossRef]
- Marin, M.M.; Gingras, B.; Stewart, L. Perception of musical timbre in congenital amusia: Categorization, discrimination and short-term memory. Neuropsychologia 2012, 50, 367–378. [Google Scholar] [CrossRef]
- Moreau, P.; Jolicœur, P.; Peretz, I. Pitch discrimination without awareness in congenital amusia: Evidence from event-related potentials. Brain Cogn. 2013, 81, 337–344. [Google Scholar] [CrossRef]
- Johnsrude, I.S.; Penhune, V.B.; Zatorre, R.J. Functional specificity in the right human auditory cortex for perceiving pitch direction. Brain 2000, 123 Pt 1, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Buchsbaum, B.R.; Olsen, R.K.; Koch, P.; Berman, K.F. Human dorsal and ventral auditory streams subserve rehearsal-based and echoic processes during verbal working memory. Neuron 2005, 48, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Fiez, J.A.; Raife, E.A.; Balota, D.A.; Schwarz, J.P.; Raichle, M.E.; Petersen, S.E. A positron emission tomography study of the short-term maintenance of verbal information. J. Neurosci. 1996, 16, 808–822. [Google Scholar] [CrossRef] [PubMed]
- Ravizza, S.M.; Delgado, M.R.; Chein, J.M.; Becker, J.T.; Fiez, J.A. Functional dissociations within the inferior parietal cortex in verbal working memory. Neuroimage 2004, 22, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Buchsbaum, B.R.; D’Esposito, M. The search for the phonological store: From loop to convolution. J. Cogn. Neurosci. 2008, 20, 762–778. [Google Scholar] [CrossRef]
- Omigie, D.; Müllensiefen, D.; Stewart, L. The experience of music in congenital amusia. Music Perception An. Interdiscip. J. 2012, 30, 1–18. [Google Scholar] [CrossRef]
- McDonald, C.; Stewart, L. Uses and functions of music in congenital amusia. Music Percept. 2008, 25, 345–355. [Google Scholar] [CrossRef]
- Lew, H.L.; Jerger, J.F.; Guillory, S.B.; Henry, J.A. Auditory dysfunction in traumatic brain injury. J. Rehabil. Res. Dev. 2007, 44, 921–928. [Google Scholar] [CrossRef]
- Landon, J.; Shepherd, D.; Stuart, S.; Theadom, A.; Freundlich, S. Hearing every footstep: Noise sensitivity in individuals following traumatic brain injury. Neuropsychol. Rehabil. 2012, 22, 391–407. [Google Scholar] [CrossRef]
- Shepherd, D.; Landon, J.; Kalloor, M.; Barker-Collo, S.; Starkey, N.; Jones, K.; Ameratunga, S.; Theadom, A. The association between health-related quality of life and noise or light sensitivity in survivors of a mild traumatic brain injury. Qual. Life Res. 2020, 29, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Gilbertson, S.; Aldridge, D. Music Therapy and Traumatic Brain Injury: A Light on a Dark Night; Jessica Kingsley Publishers: London, UK, 2008. [Google Scholar]
- Ruud, E. Music and the quality of life. Nord. J. Music. Ther. 1997, 6, 86–97. [Google Scholar] [CrossRef]
- Goulet, G.M.; Moreau, P.; Robitaille, N.; Peretz, I. Congenital amusia persists in the developing brain after daily music listening. PLoS ONE 2012, 7, e36860. [Google Scholar] [CrossRef]
- Peretz, I.; Gosselin, N.; Tillmann, B.; Cuddy, L.L.; Gagnon, B.; Trimmer, C.G.; Paquette, S.; Bouchard, B. On-line identification of congenital amusia. Music Percept. 2008, 25, 331–343. [Google Scholar] [CrossRef]
- Pfeifer, J.; Hamann, S. Revising the diagnosis of congenital amusia with the Montreal Battery of Evaluation of Amusia. Front. Hum. Neurosci. 2015, 9, 161. [Google Scholar] [CrossRef]
- Henry, M.J.; McAuley, J.D. On the prevalence of congenital amusia. Music Percept. 2010, 27, 413–418. [Google Scholar] [CrossRef]
- Bigler, E.D.; Maxwell, W.L. Neuroimaging and neuropathology of TBI. NeuroRehabilitation 2011, 28, 63–74. [Google Scholar] [CrossRef]
- Bigler, E.D.; Maxwell, W.L. Neuropathology of mild traumatic brain injury: Relationship to neuroimaging findings. Brain Imaging Behav. 2012, 6, 108–136. [Google Scholar] [CrossRef]
- Bigler, E.D. Neuropathology of mild traumatic brain injury: Correlation to neurocognitive and neurobehavioral findings. In Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2015. [Google Scholar]
- Mac Donald, C.L.; Dikranian, K.; Bayly, P.; Holtzman, D.; Brody, D. Diffusion tensor imaging reliably detects experimental traumatic axonal injury and indicates approximate time of injury. J. Neurosci. 2007, 27, 11869–11876. [Google Scholar] [CrossRef] [PubMed]
- Silver, J.M.; McAllister, T.W.; Arciniegas, D.B.; American Psychiatric Association. Textbook of Traumatic Brain Injury, 3rd ed.; American Psychiatric Association Publishing: Washington, DC, USA, 2019; p. 953. [Google Scholar]
- Ingram, L. “In Tune” What effect does gender have on the ability to identify the difference of pitch between two tones? Trans. Kans. Acad. Sci. 2004, 107, 165–169. [Google Scholar] [CrossRef]



| Variable | |
|---|---|
| Age at injury (M ± SD) | 51.1 ± 18.5 |
| Sex | |
| Male | 36 (86%) |
| Female | 6 (14%) |
| Musical Training (in years; M ± SD) | 1.2 ± 2.6 |
| <3 years | 24 (57%) |
| ≥3 years | 5 (12%) |
| Accident Variables | |
| TBI Etiology | |
| Motor vehicle accident | 16 (38%) |
| Fall | 15 (35%) |
| Assault | 3 (7%) |
| Suicide attempt | 2 (5%) |
| Sports | 2 (5%) |
| Other | 4 (9%) |
| TBI Severity | |
| mild | 34 (79%) |
| moderate | 3 (7%) |
| severe | 5 (17%) |
| LOC | |
| No | 14 (33%) |
| Yes | 28 (67%) |
| PTA | |
| No | 11(26%) |
| Yes | 31(74%) |
| LOS (in days; M ± SD) | 14.70 (9.36) |
| Delay (days from accident to evaluation); M ± SD) | 6.7 ± 4.6 |
| Patient Characteristics | Tests | |||||||
|---|---|---|---|---|---|---|---|---|
| Age | Gender | Severity | Hemi | Description of Injury | Scale (Z) | Rhythm (Z) | Digit Span Fwd (Z) | |
| P1 | 23 | M | moderate | -- | −2.28 | −1.62 | −0.09 | |
| P2 | 50 | M | mild complex | no mass effect | 1.25 | 0.57 | −0.69 | |
| P3 | 63 | F | mild | -- | −6.40 | −1.62 | −2.08 | |
| P4 | 65 | M | moderate | B | left holohemispheric SDH (20 mm); midline shift 10 mm; previous surgical resection of right temporal lobe in 1967. | −4.64 | −2.93 | -- |
| P5 | 61 | M | mild | no acute intracranial findings | −0.52 | −1.62 | -- | |
| P6 | 35 | M | moderate | B | multiple small foci of hemorrhagic contusions in the white matter of pre-SMA and SMA of frontal lobes | −2.87 | −2.93 | −1.50 |
| P7 | 37 | M | mild | no acute intracranial findings | −1.69 | −3.81 | −1.50 | |
| P8 | 64 | M | mild complex | no mass effect | 0.66 | 0.57 | −0.54 | |
| P9 | 62 | M | mild | -- | 1.25 | −0.74 | -- | |
| P10 | 62 | M | severe | L | holohemispheric SDH (27 mm), midline shift 6 mm left to right | 0.07 | 0.14 | 0.23 |
| P11 | 40 | M | mild | no acute intracranial findings | −1.11 | −1.62 | -- | |
| P12 | 78 | F | moderate | R | frontal lobe intraparenchymal hematoma (32 mm) | −6.99 | −3.37 | −1.00 |
| P13 | 63 | M | mild complex | B | SDH in right parietal lobe (15 mm) and left frontal lobe (13 mm). | −5.22 | −2.06 | -- |
| P14 | 43 | M | mild complex | no mass effect | 0.66 | 0.57 | 0.17 | |
| P15 | 72 | M | mild complex | R | hemorrhagic contusion in the pre-SMA of the right superior frontal gyrus (12 mm) | −3.46 | −3.81 | -- |
| P16 | 17 | M | mild complex | no mass effect | −4.05 | −0.74 | -- | |
| P17 | 73 | M | mild complex | no mass effect | −2.87 | −0.30 | -- | |
| P18 | 55 | M | mild complex | no mass effect | −3.46 | −2.06 | −1.31 | |
| P19 | 52 | M | mild complex | R | right parietal (4 mm) and right temporal (3 mm) SDHs | −0.52 | −1.62 | −1.46 |
| P20 | 71 | M | moderate | R | frontal SDH (10 mm) and residual hypodensities in the temporal lobe, following evacuation for a holohemispheric SDH | −4.64 | −3.37 | −0.64 |
| P21 | 43 | M | severe | B | multiple small foci of hemorrhagic contusions involving the subcortical white matter of both frontal lobes | −2.28 | −2.06 | −0.67 |
| P22 | 55 | M | mild complex | L | hemorrhagic contusion in the parahippocampal formation (6 mm). | 0.07 | −2.50 | −1.31 |
| P23 | 44 | M | mild | no acute intracranial findings | 0.07 | 0.14 | −1.50 | |
| P24 | 56 | F | mild complex | L | hemorrhagic contusions in the inferior frontal and prefrontal cortex | 0.07 | 0.57 | −2.08 |
| P25 | 60 | M | mild complex | L | hemorrhagic contusion in the left frontoparietal area (8 mm) | 1.25 | −1.18 | −0.54 |
| P26 | 27 | F | mild | -- | −3.46 | −1.18 | -- | |
| P27 | 51 | M | moderate | L | holospheric hematoma (9 mm) with left to right midline shift (3 mm) | −0.52 | −1.18 | −0.69 |
| P28 | 58 | M | moderate | R | hemorrhage in the pallidum and putamen (15 mm). | −6.99 | −3.37 | −1.31 |
| P29 | 48 | M | severe | -- | −4.64 | −2.06 | −1.46 | |
| P30 | 87 | F | mild complex | no mass effect | −5.22 | −2.93 | -- | |
| P31 | 33 | F | mild | no acute intracranial findings | −3.46 | −4.25 | -- | |
| P32 | 18 | F | mild | no acute intracranial findings | −5.81 | −3.37 | −2.18 | |
| P33 | 21 | M | mild complex | no mass effect | −0.52 | −2.50 | −1.00 | |
| P34 | 26 | M | mild | no acute intracranial findings | 0.66 | 0.14 | 0.00 | |
| P35 | 20 | F | mild | -- | 1.25 | 1.01 | −1.00 | |
| P36 | 34 | F | mild | no acute intracranial findings | −0.52 | 1.01 | 1.43 | |
| P37 | 65 | M | mild complex | no mass effect | −4.05 | −1.18 | −0.57 | |
| P38 | 77 | M | moderate | L | large holohemispheric mixed density subdural hematoma (13 mm ) with left to right midline shift (9 mm) | −4.64 | −1.18 | −1.91 |
| P39 | 56 | M | mild complex | B | small frontal hemorrhagic contusions in the SMA (6 mm on right, and 7 mm on left) | −4.64 | −0.30 | −1.31 |
| P40 | 52 | M | mild complex | R | holohemispheric SDH (18 mm), right to left midline shift (7 mm) | 0.07 | −0.74 | 0.08 |
| P41 | 84 | M | mild complex | no mass effect | −5.81 | −1.62 | −0.85 | |
| P42 | 45 | M | mild complex | L | inferior frontal SDH (4 mm) | −0.52 | −0.74 | −0.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anderson, K.S.; Gosselin, N.; Sadikot, A.F.; Laguë-Beauvais, M.; Kang, E.S.H.; Fogarty, A.E.; Marcoux, J.; Dagher, J.; de Guise, E. Pitch and Rhythm Perception and Verbal Short-Term Memory in Acute Traumatic Brain Injury. Brain Sci. 2021, 11, 1173. https://doi.org/10.3390/brainsci11091173
Anderson KS, Gosselin N, Sadikot AF, Laguë-Beauvais M, Kang ESH, Fogarty AE, Marcoux J, Dagher J, de Guise E. Pitch and Rhythm Perception and Verbal Short-Term Memory in Acute Traumatic Brain Injury. Brain Sciences. 2021; 11(9):1173. https://doi.org/10.3390/brainsci11091173
Chicago/Turabian StyleAnderson, Kirsten S., Nathalie Gosselin, Abbas F. Sadikot, Maude Laguë-Beauvais, Esther S. H. Kang, Alexandra E. Fogarty, Judith Marcoux, Jehane Dagher, and Elaine de Guise. 2021. "Pitch and Rhythm Perception and Verbal Short-Term Memory in Acute Traumatic Brain Injury" Brain Sciences 11, no. 9: 1173. https://doi.org/10.3390/brainsci11091173
APA StyleAnderson, K. S., Gosselin, N., Sadikot, A. F., Laguë-Beauvais, M., Kang, E. S. H., Fogarty, A. E., Marcoux, J., Dagher, J., & de Guise, E. (2021). Pitch and Rhythm Perception and Verbal Short-Term Memory in Acute Traumatic Brain Injury. Brain Sciences, 11(9), 1173. https://doi.org/10.3390/brainsci11091173







